Abstract
The Kaiser Permanente National Total Joint Replacement Registry (TJRR) is a national level database designed as a postmarket surveillance system for elective total hip and knee replacement. As of March 31, 2007, the TJRR recorded 16,945 primary total hip arthroplasties (THA), 2144 revisions (11.2%); and 30,815 total knee arthroplasties (TKA), 1794 revisions (5.5%). Statistically significant findings include: older age and higher American Society of Anesthesiology risk scores for revision THAs. Osteoarthritis is the most common diagnosis for THA and TKA, and aseptic loosening and instability are most common in revision THAs and TKAs. The TJRR has provided a mechanism for recalls, identified patients at risk for early revisions and changed practice by providing feedback to physicians.
Introduction
Within the US, over 600,000 total hip and total knee replacements are performed each year.1 By the year 2030, that number is projected to exceed 4 million.1 Annual hospital costs associated with these procedures are projected to exceed $65 billion by 2015.2 Although patients who undergo total joint arthroplasty (TJA) are often of retirement age, recent studies have shown that patients below age 65 represent 35–45% of all TJA recipients in the US.3,4 As TJA is marketed more as a lifestyle operation than as a final option to retain mobility for end-stage arthritis, the proportion of patients below age 65 may increase.
The projected increases in TJA demand and the costs associated with these procedures will challenge our already overburdened US health care system. One potential method to address this pending crisis is through comparative safety and clinical effectiveness research aimed at reducing the need for TJA revision surgery. Registries are one example of clinical effectiveness studies that can help surgeons and patients make informed decisions about which implant to use or in which patients the risks and potential costs of failure make surgery unwise. These studies can also identify the relative value of TJA over alternative treatments or the effectiveness of one implant brand or design over another. Although implants vary widely in cost, there is little evidence to support the use of new, more expensive designs instead of more established, traditional designs.
The goals of the Kaiser Permanente (KP) Total Joint Replacement Registry (TJRR) are: 1) to monitor revision, failure, and rates of key complications (eg, infection, venous thromboembolic disease such as blood clots and embolism, and mortality); 2) to identify patients at risk for poor clinical outcomes following TJA; 3) to identify the most effective techniques and implant devices (best practices and implant constructs); 4) to track implant usage and costs; and 5) to monitor and to support implant recalls and advisories in cooperation with the US Food and Drug Administration.
Methods
Data Collection & Sources
The TJRR uses an innovative observational method that collects uniform data at the point of care (collected by surgeons, medical assistants, and circulating registered nurses). The core of the TJRR is standardized total joint replacement preoperative, operative, and postoperative documentation forms that contain a small number of key data elements identified by consensus among all surgeons. The standardized forms replace traditional, nonuniform clinic and operative notes and provide information on patient demographics, implant characteristics, surgical techniques, and clinical outcomes.
Data from the standardized TJRR forms are supplemented with data from independent KP administrative data sources to provide a comprehensive database for TJA surveillance and monitoring (Figure 1). KP administrative databases such the Health Information Management System, Kaiser Anesthesia and Surgery Information System (KASIS), Management and Information Analysis Mortality and Membership datasets, and KP HealthConnect (electronic medical health record) inpatient, ambulatory, operating room management, and emergency department system modules provide additional data on hospital readmissions, patient comorbidities, complications, hospital length of stay, Health Plan (HP) membership, and mortality.
Figure 1.
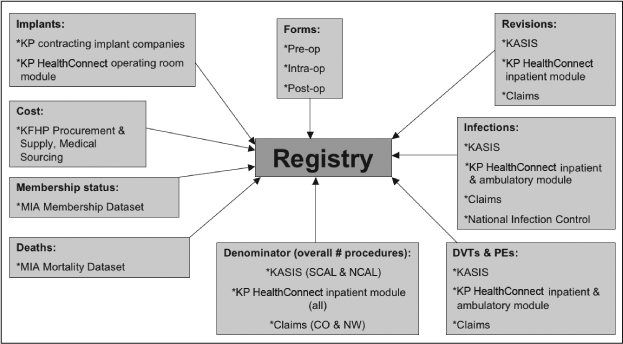
Registry data map.
Participating Regions: Colorado (CO), SCAL (Southern CA), NCAL (Northern CA), Hawaii (HI), Northwest (NW); KP = Kaiser Permanente; KFHP = Kaiser Foundation Health Plan; MIA = Management and Information Analysis Dept; KASIS = Kaiser Anesthesia and Surgery Information System.
Volume and Participation
The TJRR has been fully implemented in five of eight KP Regions (Southern California, Northern California, Hawaii, Northwest, and Colorado). More than 350 surgeons and 50 hospitals contribute to the database. Between April 2001 and March 2008, the registry recorded 68,000 joint replacement procedures.
Participation reports are offered to sites quarterly, at least 90 days after the closing of the current reporting quarter. Clinical support, site education, and mechanisms to enhance participation are also offered by the registry staff. Participation in the TJRR has consistently been over 95% since its inception. Participation in the registry is validated using independent operative administrative databases (KASIS and KP HealthConnect operating room management systems).
Outcome Validation
Complications, revisions, and reoperations are identified by TJRR operative and postoperative forms and KP administrative data sources using International Classification of Diseases, Ninth Revision, Clinical Modification codes (ICD 9-CM) as a screening mechanism. Registry clinical staff validate identified complications, revisions, and re-operations through chart review to ensure Centers for Disease Control and Prevention guidelines5 and Agency for Healthcare Research and Quality Patient Safety Indicators6 are met.
Results
Current Registry Population
As of March 31, 2007, the TJRR recorded 16,945 primary total hip arthroplasties (THAs) and 2144 revision THAs for a THA revision burden (number of revision THA procedures per number of primary and revision THA combined) of 11.2%. During the same time period, the registry contained over 30,815 total knee arthroplasty (TKA) cases and 1794 revision TKAs resulting in a TKA revision burden of 5.5%.
Revision, complication, and failure rates documented in our database represent the first direct measurement of these outcomes in a community hospital setting in the US.
Results indicate that revision THA patients are older than primary THA patients (mean age [SD]: 67 [13] vs 66 [12] years, p < 0.001). The percentage of female patients was slightly higher in the primary compared to revision THA group but the difference was not statistically significant (57.4% vs 55.8%, p = 0.067) (Table 1). Revision THA patients have higher American Society of Anesthesiology (ASA) risk scores than primary THA patients (p < 0.001). Osteoarthritis is the most common diagnosis among primary THAs and aseptic loosening and instability are the most common among revision THAs (Figure 2).
Table 1.
Total hip arthroplasty demographics (04/2001 to 03/2007)
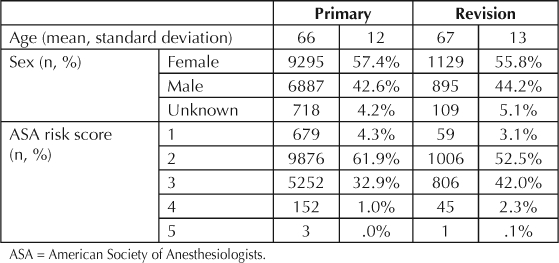
Figure 2.
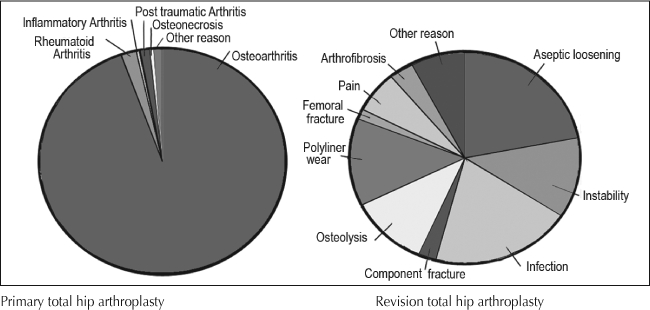
Diagnosis for primary total hip arthroplasty and revision total hip arthroplasty.
Two thirds of the primary TKAs were performed in female patients but only 51.3% of the revision procedures were performed in female patients (p < 0.001) (Table 2). Mean age is similar between the primary and revision TKA groups. Osteoarthritis is the most common diagnosis in the primary TKA group and infection, aseptic loosening, and instability are the most common reasons for revisions (Figure 3).
Table 2.
Total knee arthroplasty demographics (04/2001 to 03/2007)
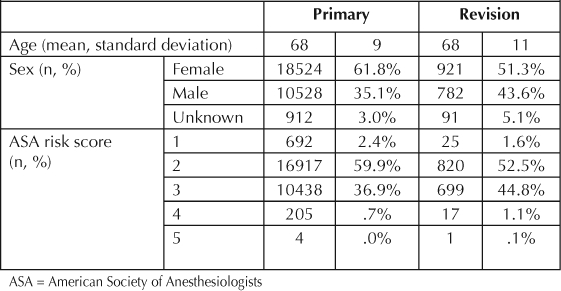
Figure 3.
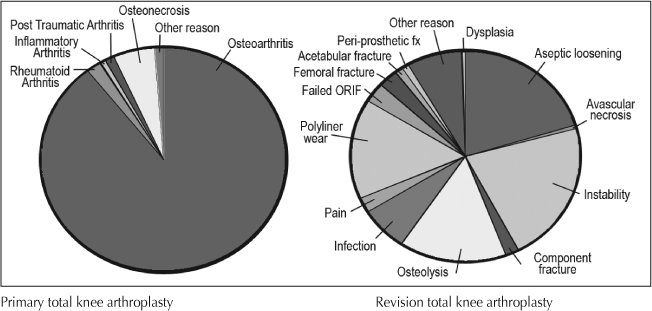
Diagnosis for primary total knee arthroplasty and revision total knee arthroplasty.
Changes in Practice
A critical component of the TJRR has been the implementation of a dynamic feedback mechanism. Clinical best practices are identified and shared nationally through chiefs and administrator meetings, the TJRR Web site, site visits, newsletters, e-mails, annual reports, quarterly reports, and national conference presentations to improve the quality of care for patients.
To date the TJRR has resulted in significant improvement in quality and cost savings, including: 1) successful identification, monitoring, and notification of a hip implant recall and a knee implant advisory; 2) identification of a 10% difference in revision rates of partial and total knees—surgeons used this information to reduce partial knee volumes and prevent 16 revisions with cost savings of more than $550,000 (Figure 4); 3) identification of an uncemented total knee technique that was found to be associated with higher revision rates, reducing the usage of this technique (Figure 5); 4) reduced volume of minimally invasive hip and knee procedures, which effectively reduced pain and demonstrated an improvement in subjective patient outcomes (Figures 6 and 7); 5) evaluation of new implant technology that is more costly, with no difference in short-term outcome—this feedback changed practice with respect to implant selection; 6) identification of patient risk factors for postoperative infections, hospital readmissions, deep venous thrombosis, pulmonary embolism, and other complications, leading to significant changes in surgical indications and preoperative care.
Figure 4.
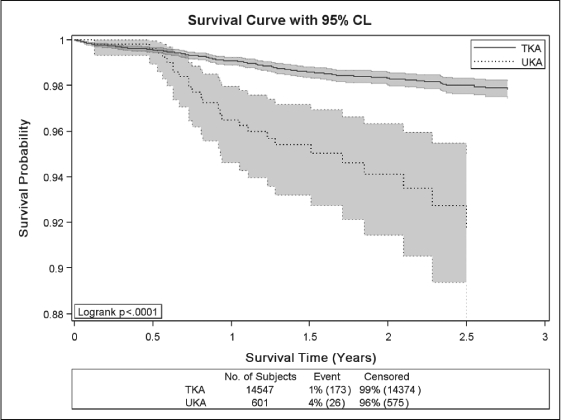
Kaplan-Meier survival curves with 95% confidence intervals for patients undergone total knee arthroplasty (TKA) vs unicompartmental knee arthroplasty (UKA).
X-axis shows postoperative survival time (time to revision) in years, Y-axis shows survival probability. P-value for Logrank test (p < 0.001) indicates statistically significant difference in survival experience between TKA and UKA patients. TKA patients have higher survival probability compared with UKA.
Figure 5.
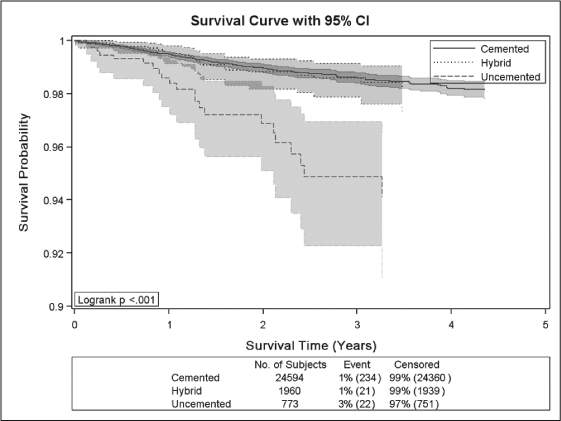
Kaplan-Meier survival curves with 95% confidence intervals for cemented, hybrid and uncemented total knee arthroplasty.
X-axis shows postoperative survival time (time to revision) in years, Y-axis shows survival probability. P-value for Logrank test (p < 0.001) indicates statistically significant difference in survival experience between implants. Uncemented implants have lower survival probability compared with hybrid and cemented.
Figure 6.
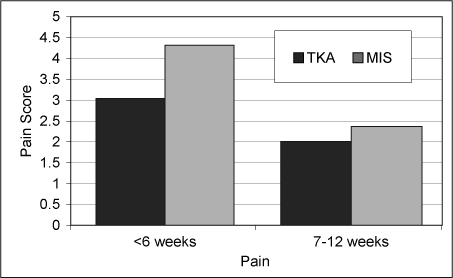
Significantly higher pain reported in minimally invasive total knee arthroplasty.
TKA = total knee arthroplasty; MIS = minimally invasive.
Figure 7.
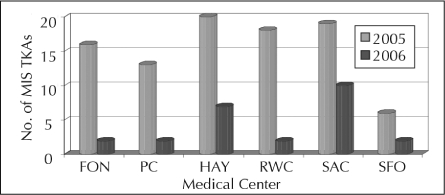
Significant decrease in minimally invasive total knee arthroplasty after providing feedback to surgeons.
TKA = total knee arthroplasty; MIS = minimally invasive.
Discussion
Using the Swedish Hip registry7 as a model, we implemented a national KP TJRR to reduce revision rates by identifying the best implants and surgical techniques for the population we serve. The TJRR's automated postmarket surveillance system tracks more than 68,000 TJAs implanted in Kaiser Foundation Health Plan, Inc (KFHP) patients. The ultimate purpose of the registry is to glean the highest value from total knee and hip replacements in terms of their effects on the health and productivity of KFHP patients. The TJRR represents a unique and unprecedented partnership between the surgeons who use the implants and the HP that pays for them. The program is entirely voluntary, yet participation rates exceed 95% among more than 350 surgeons in over 50 hospital areas.
Postmarket surveillance systems such as registries provide a mechanism to assess comparative safety and clinical effectiveness. Registries can assist in identifying clinical best practices, optimal implants, and patients at risk for failures and revisions. Identification of these underlying factors can reduce revision surgery. The effectiveness of registries in reducing TJA revision surgery has been demonstrated by the National Swedish Hip registry, which reduced their revision rate 50% by providing feedback to surgeons on clinical best practices.8
[Evaluation] feedback changed practice with respect to: implant selection, minimally invasive procedures, uncemented knees, and surgical indications and preoperative care.
The TJRR has provided a mechanism for recalls, identified clinical best practices and changed clinical practices through ongoing feedback to physicians. The TJRR has been successful in immediate identification and monitoring of several recalls. It has also allowed savings from decrease in certain higher risk procedures, techniques, and use of new implants that are more costly but offer no difference in short-term outcome. In addition, the TJRR has provided a mechanism to identify patients at high risk for postoperative infections, revisions, hospital readmissions, DVT, and other complications. Our clinicians share this information with their patients to set expectations and to identify an optimal treatment plan on the basis of patient risk factors.
Revision, complication, and failure rates documented in our database represent the first direct measurement of these outcomes in a community hospital setting in the US. These indicators are benchmarked across medical centers, regions, and program as well as compared to published rates within the literature. The Swedish National Hip Registry demonstrated a 50% reduction in revision rates nationwide following identification of best practices among Swedish hip replacement surgeons. The revision rate in the US, comparing Centers for Medicare and Medicaid Services data to the Swedish data, appears to be approximately twice as high as the rate in Sweden.2 As our length of follow-up has increased, we have begun the process of comparing our revision rates to those published in other population-based Joint Registries.9–13 These comparisons allow us to assess the effects of our registry on revision rates among KFHP members, with the goal of achieving similar improvements in quality and outcomes.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
We would like to thank the KP surgeons, clinical staff, administrators, and the Surgical Outcomes & Analysis unit of Clinical Analysis for contributing to the success of the TJRR.
References
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007 Apr;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- Kurtz Sm, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007 Oct;89(Suppl 3):144–51. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- Khatod M, Bini S, Namba R, Inacio M, Paxton L, Fithian D. 17,080 knee replacements from 1995 to 2004: epidemiology, outcomes, and trends. Acta Orthop Scand. 2008 doi: 10.1080/17453670810016902. In press. [DOI] [PubMed] [Google Scholar]
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990–2000. Arthritis Rheum. 2006 Aug 15;55(4):591–7. doi: 10.1002/art.22102. [DOI] [PubMed] [Google Scholar]
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999 Apr;20(4):250–78. doi: 10.1086/501620. quiz 279–80. [DOI] [PubMed] [Google Scholar]
- AHRQ quality indicators: guide to patient safety indicators Version 3.1 [monograph on the Internet] Department of Health and Human Services Agency Agency for Healthcare Research and Quality. 2003 Mar [cited 2008 Apr 7]. Available from: www.qualityindicators.ahrq.gov/psi_download.htm, click on: Guide to Patient Safety Indicators, Ver. 3.1, March 2007.
- Malchau H, Herberts P, Eisler T, Garellick G, Söderman P. The Swedish total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. Erratum in: J Bone Joint Surg Am 2004 Feb; 86-A(2):363. [DOI] [PubMed] [Google Scholar]
- Herberts P, Malchau H. How outcome studies have changed total hip arthroplasty practices in Sweden. Clin Orthop Relat Res. 1997 Nov;(344):44–60. [PubMed] [Google Scholar]
- Malchau H, Garellick G, Eisler T, Kärrholm J, Herberts P. Presidential guest address: the Swedish Hip Registry: Increasing the sensitivity by patient outcome data. Clin Orthop Relat Res. 2005 Dec;441:19–29. doi: 10.1097/01.blo.0000193517.19556.e4. [DOI] [PubMed] [Google Scholar]
- Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Early failures among 7174 primary total knee replacements: a follow-up study from the Norwegian Arthroplasty Register 1994–2000. Acta Orthop Scand. 2002 Apr;73(2):117–29. doi: 10.1080/000164702753671678. [DOI] [PubMed] [Google Scholar]
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000 Aug;71(40):337–53. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- Gioe TJ, Killeen KK, Mehle S, Grimm K. Implementation and application of a community total joint registry: a twelve-year history. J Bone Joint Surg Am. 2006 Jun;88(6):1399–404. doi: 10.2106/JBJS.E.01198. [DOI] [PubMed] [Google Scholar]
- Robertsson O, Dunbar MJ, Knutson K, Lewold S, Lidgren L. The Swedish Knee Arthroplasty Register, 25 years experience. Bull Hosp Jt Dis. 1999;58(3):133–8. [PubMed] [Google Scholar]


