Abstract
Purpose: We studied the hypothesis that possibly via shared inflammatory mechanisms, psoriatic arthritis (PsA) is associated with increased prevalence of cardiovascular (CV) conditions.
Methods: Among a multiethnic population of 76,465 men and women with known demographics, we studied all persons (n = 99) with confirmed outpatient diagnoses of PsA. Associations of PsA with CV diagnoses were studied in the entire population by logistic regression with six covariates. With two matched control study subjects for each study subject with PsA, selected risk traits for CV conditions at time of PsA diagnosis were compared with findings on t-tests.
Results: Study subjects with PsA did not exhibit more atherothrombotic disease (coronary and cerebrovascular) or diabetes mellitus but had increased prevalence of systemic hypertension and heart failure compared with study control subjects. In the case-control analysis, study subjects with PsA had a lower mean blood cholesterol, a higher mean body mass index, and a higher mean blood pressure compared with study control subjects; mean blood glucose was similar in both groups.
Conclusions: In this analysis the associations of PsA with CV risk factors and CV conditions are mixed. Except for increased systemic hypertension, it is unclear whether PsA is related to higher prevalence of CV disease.
Introduction
Psoriasis is a common inflammatory skin condition with an extensive medical literature.1,2 Although less common, psoriatic arthritis (PsA) has also been studied substantially,1,3–6 but there are only limited data about PsA from population-based studies. Available reports suggest relations of both psoriasis7,8 and PsA9,10 to atherothrombotic cardiovascular diseases (CV), but some studies are limited by small numbers of participants, self-report of diagnoses, and homogeneity of study populations.
We present here the results of a study of PsA in a large, multiethnic, free-living population. We hypothesized that PsA and atherothrombotic vascular conditions might be associated because of shared underlying inflammatory mechanisms. Thus, we examined associated diagnoses made by physicians. A matched case-control analysis yielded data about selected traits usually considered risk factors for CV disease. Because study subjects with PsA were determined from among persons with known computer-stored previous data, in the case-control analyses it was possible to also study changes in the CV risk traits.
Materials and Methods
Study Population
The study protocols were approved by the institutional review board of the Kaiser Permanente Medical Care Program. The study population consisted of 76,425 persons who, on January 1, 1995, were members of a comprehensive prepaid Northern California Health Plan and whose demographic traits were known from previous health examinations11 for the period 1978–1985. These persons are thought to represent a cross-section of Health Plan members and were classified as follows: 43% men, 52% white, 30% African American, 11% Asian American, and 5% Hispanic. The Health Plan members reflect the full socioeconomic spectrum of the general population except for the extremes of income.
Study Subjects with Psoriatic Arthritis
The date for study population selection was based on the fact that outpatient diagnoses at visits to Health Plan facilities was first stored in computers in January 1995. We searched these computer files for diagnoses of PsA (International Classification of Diseases, 9th revision [ICD-9] code 696.0) through 2004, identifying 120 individuals (0.16%) with a diagnosis of PsA. A physician investigator performed a detailed record review of the 120 possible study subjects with PsA for confirmation of diagnosis. Strong consideration was given to acceptance of the PsA diagnosis if it was made by a rheumatologist, but inclusion ultimately depended on a compelling clinical picture, including radiologic evidence of erosive disease and negative findings on tests for rheumatoid factor. Uncertain diagnoses were resolved by consensus. We excluded 21 study subjects from analysis either because they did not fulfill PsA criteria established for this study or because other (than PsA) rheumatologic diagnoses were judged more likely. Thus, there were 99 confirmed study subjects with PsA in the analyses (0.13% of study subjects); of these, 50% were men, 70% were white, 8% were African American, 16% were Asian American, and 2% were Hispanic.
Analysis of Coexistent Outpatient Conditions
These associations were studied by logistic regression in all 76,425 persons. Included were the following CV conditions (ICD-9 codes): systemic hypertension (400–405), coronary heart disease (410–414), heart failure (428), cerebrovascular disease (430–438), and diabetes mellitus (250). Diabetes was included because from the risk viewpoint, it is widely considered a “coronary disease equivalent.” Age-adjusted and multivariate logistic models used covariate data obtained from the 1978–1985 health examinations, including self-classified ethnicity, education, marital status, body mass index (BMI), cigarette smoking status, and alcohol intake. These models yielded odds ratios (ORs) for relations of PsA to each coexistent condition, 95% confidence intervals (CIs), and P values. For this study, we defined “statistical significance” as p < 0.05.
Record Review and Case-Control Study Subjects
In addition to confirmation of PsA diagnosis, chart review included abstraction of data about weight, blood pressure, total cholesterol, and blood sugar at the time of diagnosis. For each confirmed study subject with PsA, two study control subjects were selected who were Health Plan patients at the time of first outpatient PsA diagnosis but who were free of diagnosed PsA, psoriasis, and other rheumatic inflammatory conditions (rheumatoid arthritis, lupus erythematosus, scleroderma, dermatomyositis, and polyarteritis nodosa). Each study control subject was matched to the corresponding study subject with PsA for age, sex, and ethnicity. Data similar to those for study subjects with PsA were abstracted for these study control subjects, using date for study control subjects closest to corresponding study subjects with PsA.
Case-Control Comparisons
The matched groups (study subjects with PsA vs study control subjects) were compared with student's t-test, with results expressed as χ-square and p values. In this manner, we examined traits at time of PsA diagnosis, data from the earlier examinations, and changes in the measurements between the earlier examinations and date of diagnosis.
Similar mediators are involved in the genesis of atherosclerosis, atherothrombotic diseases, the group of traits known as metabolic syndrome, and systemic hypertension.13,14
Results
Outpatient Diagnoses Associated with Psoriatic Arthritis
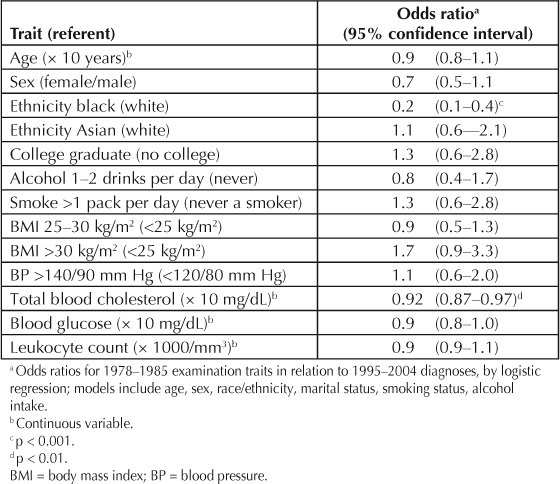
These associations are presented in Table 1. There was increased prevalence of systemic hypertension and of heart failure among the study subjects with PsA. In contrast, although the p values were >0.05 for each, the ORs for associated coronary disease, cerebrovascular disease, and diabetes mellitus were <1.0. Several of the relations of the covariates are presented in Table 2. Age and sex were unrelated to likelihood of the diagnosis of PsA. African Americans had a lower PsA prevalence, but there was no difference between Asian Americans, largely of Chinese or Filipino ethnicity, and whites. Total blood cholesterol level was inversely related to PsA. Unrelated traits included education level, obesity, heavy smoking, daily moderate alcohol drinking, BMI, blood pressure, blood glucose level, and leukocyte count.
Table 1.
Associations a of outpatient diagnoses b with psoriatic arthritis
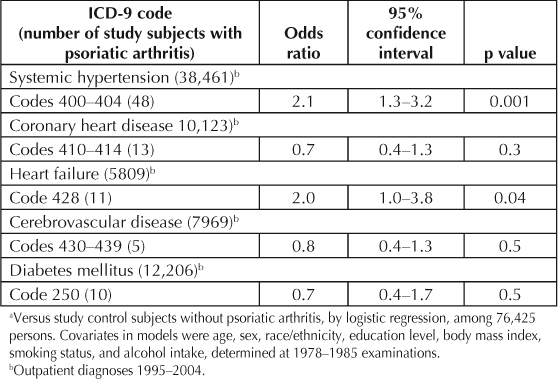
Table 2.
Relations of covariates and other traits a to psoriatic arthritis
Comparisons of Study Subjects with Psoriatic Arthritis and Matched Study Control Subjects
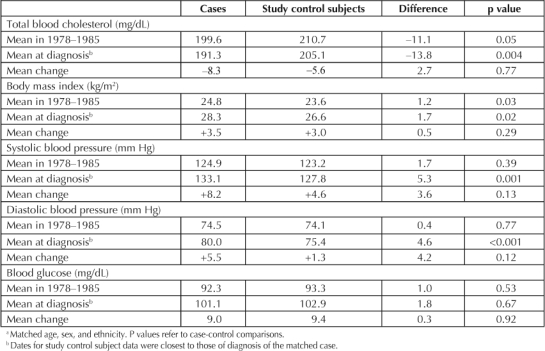
Case-control comparisons for selected CV risk traits are shown in Table 3. Mean cholesterol levels were lower in study subjects with PsA than in study control subjects both at the earlier examinations in 1978–1985 and at date of diagnosis. There were decreases in mean cholesterol levels from the earlier to the later measures, slightly greater in the study subjects with PsA. The study subjects with PsA had a higher mean BMI both in 1978–1985 and at diagnosis, but the increase in BMI over time was similar in both groups. Mean systolic and diastolic blood pressure was similar in 1978–1985 for study subjects with PsA and study control subjects but increased more in the study subjects with PsA, resulting in significant case-control differences at time of diagnosis. Blood glucose levels were similar in cases and study control subjects at both measurements, and they increased to a similar extent over time.
Table 3.
Case-control comparisons for study subjects with psoriatic arthritis a
Discussion
Although the etiology of most rheumatic conditions is not well established, it is accepted that autoimmune phenomena and chronic tissue inflammation are involved and possibly causal.9,12 The same is true for psoriasis, a T-helper-cell type 1 immunologic inflammatory skin disease7 and, presumably, for PsA, now classified as one of the spondyloarthritides.6 In general, T-cell activation induces proinflammatory cytokines and chemokines that are noxious to a variety of tissues. Similar mediators are involved in the genesis of atherosclerosis, atherothrombotic diseases, the group of traits known as metabolic syndrome, and systemic hypertension.13,14 Epidemiologic studies show that atherosclerosis has a number of causal risk factors, several of which (cigarette smoking, atherogenic lipids, hypertension, and hyperglycemia) involve cytokines, other bioactive substances, and cells characteristic of the inflammatory process. In fact, endovascular inflammation is now considered involved in all stages of atherosclerosis development and may well be causal.13,14 Despite the apparent similarities in hypothetical noxious biologic pathways related to inflammation, the link between rheumatic conditions and atherosclerosis remains a statistical association without established causal mechanisms.
We found inconsistent positive associations of CV conditions with PsA. Although hypertension and heart failure were associated, atherothrombotic disease was not. Even though this could be interpreted as indicating a lesser role for common systemic inflammatory mechanisms than we expected, inflammation is not the only possible mechanism for a link among atherosclerosis, PsA, and rheumatic conditions.8 Shared CV risk factors would be another possible basis for a link. Our data show inconsistent evidence about shared risk traits also, but we did not have data about several important potential CV risk traits. Several of these, such as amount physical activity and diet, might be modulated by the presence of PsA. Increased CV disease as a consequence of treatment is another interesting possibility. For example, use of nonsteroidal anti-inflammatory medications might increase risk of hypertension or of heart failure, the specific conditions we found related to PsA. Finally, shared genetic predilections are hypothetically possible, but our data cannot evaluate this aspect.
In the case-control comparison, weight gain was similar for study subjects with PsA and study control subjects. The greater increase in blood pressure in study subjects with PsA could be substantially responsible for the heart failure disparity, especially because coronary disease, another major risk factor for heart failure, was not more prevalent in the study subjects with PsA. Although this was not a strictly prospective analysis, it is noteworthy that there were no convincing relations to the 1978–1985 examination data about blood pressure, blood glucose levels, or leukocyte count. The unexplained inverse relation of total blood cholesterol in 1978–1985 to PsA might partially account for the absence of a relation of PsA to atherothrombotic disease. Leukocyte count, a marker of inflammation, was unrelated to PsA but has previously been reported to be related to acute myocardial infarction in this population.15
Not surprisingly, our data do show that the known relatively low prevalence of psoriasis in African Americans2 extends to PsA. Also, the data show that Asian Americans have rates of PsA similar to those of whites.
Limitations of our study start with the fact that the small number of study subjects with PsA precluded the statistical power to demonstrate modest relations to the condition. Another limitation was our inability to study temporal relations and thus incident disease. We emphasize that this was not a prospective analysis. This should not affect the data about relations of lifelong demographic traits (age, sex, and ethnicity) to PsA. However, the data about education, BMI, smoking and alcohol-drinking habits, and blood tests in Table 2 should not be over-interpreted as necessarily indicating predictive traits. Strengths of the study include the large, multiethnic, relatively stable cohort and the fact that all diagnoses were made by physicians.
We conclude that PsA is associated with increased prevalence of hypertension and heart failure. However, evidence about other independent associations between PsA and vascular conditions is unclear.
… the link between rheumatic conditions and atherosclerosis remains a statistical association without established causal mechanisms.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
This study was supported by a grant from the Kaiser Foundation Research Institute, Oakland, CA. Data collection from 1978 to 1985 was supported by a grant from the Alcoholic Beverage Medical Research Foundation, Baltimore, MD.



Acknowledgments
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Myers WA, Gottlieb AB, Mease P. Psoriasis and psoriatic arthritis: clinical features and disease mechanisms. Clin Dermatol. 2006 Sep–Oct;24(5):438–47. doi: 10.1016/j.clindermatol.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005 May 5;352(18):1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000 May;27(5):1247–50. [PubMed] [Google Scholar]
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005 Oct;53(4):573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Fitzgerald O, Dougados M. Psoriatic arthritis: one or more diseases? Best Pract Res Clin Rheumatol. 2006 Jun;20(3):435–50. doi: 10.1016/j.berh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Mease P. Psoriatic arthritis update. Bull NYU Hosp Jt Dis. 2006;64(1–2):25–31. [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006 Oct 11;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- Kremers HM, McEvoy MT, Dann FJ, Gabriel SEJ. Heart disease in psoriasis. Am Acad Dermatol. 2007 Aug;57(2):347–54. doi: 10.1016/j.jaad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006 Nov;33(11):2167–72. [PubMed] [Google Scholar]
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007 Nov–Dec;25(6):529–34. doi: 10.1016/j.clindermatol.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Collen MF, Davis LF. The multitest laboratory in health care. J Occup Med. 1969 Jul;11(7):355–60. [PubMed] [Google Scholar]
- Farzaneh-Far A, Roman MJ. Accelerated atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Pract. 2005 Jul;59(7):823–4. doi: 10.1111/j.1742-1241.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control & Prevention; American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974 Jun 6;290(23):1275–8. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]


