Prenatal androgenization of female mice causes enhanced firing activity of GnRH neurons and irregular estrous cycles that are reversible by metformin treatment in adulthood; central AMPK activation by metformin may play a role in these effects.
Abstract
Prenatal androgenization (PNA) of female mice with dihydrotestosterone programs reproductive dysfunction in adulthood, characterized by elevated luteinizing hormone levels, irregular estrous cycles, and central abnormalities. Here, we evaluated activity of GnRH neurons from PNA mice and the effects of in vivo treatment with metformin, an activator of AMP-activated protein kinase (AMPK) that is commonly used to treat the fertility disorder polycystic ovary syndrome. Estrous cycles were monitored in PNA and control mice before and after metformin administration. Before metformin, cycles were longer in PNA mice and percent time in estrus lower; metformin normalized cycles in PNA mice. Extracellular recordings were used to monitor GnRH neuron firing activity in brain slices from diestrous mice. Firing rate was higher and quiescence lower in GnRH neurons from PNA mice, demonstrating increased GnRH neuron activity. Metformin treatment of PNA mice restored firing activity and LH to control levels. To assess whether AMPK activation contributed to the metformin-induced reduction in GnRH neuron activity, the AMPK antagonist compound C was acutely applied to cells. Compound C stimulated cells from metformin-treated, but not untreated, mice, suggesting that AMPK was activated in GnRH neurons, or afferent neurons, in the former group. GnRH neurons from metformin-treated mice also showed a reduced inhibitory response to low glucose. These studies indicate that PNA causes enhanced firing activity of GnRH neurons and elevated LH that are reversible by metformin, raising the possibility that central AMPK activation by metformin may play a role in its restoration of reproductive cycles in polycystic ovary syndrome.
GnRH neurons form the final common pathway for the central regulation of fertility. Alterations in GnRH pulse frequency help drive the female reproductive cycle, with lower frequencies favoring FSH release and higher frequencies favoring LH (1). Improper patterning of GnRH release, such as that suggested by the persistent high frequency LH release in women with polycystic ovary syndrome (PCOS), is associated with infertility due to altered reproductive cyclicity, poor follicular development, and impaired ovulation (2–5).
Altered reproductive cyclicity can be programmed in several animal models, and in humans, by prenatal exposure to androgens (6). Developing young may be exposed to androgen via environmental sources, via elevated androgens in maternal circulation as occurs in late gestation in women with PCOS (7), or via endogenous production as in congenital adrenal hyperplasia. Disrupted reproductive cyclicity in these models is often accompanied by altered gonadotropin and ovarian steroid levels and/or metabolic phenotypes (8).
Prenatal androgenization (PNA) of female mice with dihydrotestosterone (DHT) causes irregular estrous cycles in adulthood (9, 10). PNA mice also have elevated LH levels and increased γ-aminobutyric acid (GABA)ergic neurotransmission to GnRH neurons (9), both of which are consistent with increased central reproductive drive (11). This is also consistent with studies in rats and sheep showing that androgen exposure in utero masculinizes activity of the GnRH pulse generator, generating a chronically high LH pulse frequency (12–14). In terms of metabolism, PNA mice exhibit impairments in glucose tolerance and pancreatic islet function but do not exhibit obesity or peripheral insulin resistance (10). Disrupted reproductive cyclicity in PNA mice is therefore likely driven in large part by developmental androgen programming of the central GnRH pulse generator.
Metformin is a biguanide antihyperglycemic agent frequently employed to treat women with PCOS, who often exhibit insulin resistance (15–17). In addition to its metabolic effects, metformin can improve menstrual cyclicity, reduce hyperandrogenemia, and increase the rate of ovulation in PCOS (18–22). The predominant line of thinking is that insulin sensitization, and a subsequent reduction in insulin levels, is the primary mechanism by which metformin exerts these reproductive system effects. However, recent studies have shown that metformin can improve PCOS symptoms in normoinsulinemic women (23–26), suggesting it may have additional reproductive effects that are independent of insulin sensitization. Recently, one mechanism of action of metformin was identified to be activation of AMP-activated protein kinase (AMPK) (27). AMPK is widely expressed and exhibits functional roles in metabolic tissues (muscle, liver, pancreas, and adipocytes) (28), as well as the ovary (29–32), pituitary (33), and hypothalamus (34–39), suggesting that diverse mechanisms may account for the reproductive effects of metformin. Because PNA mice exhibit altered reproductive biology but are not insulin resistant or hyperinsulinemic (10), they serve as a unique model to assess the reproductive effects of metformin that are independent of its insulin-lowering effects. Specifically, we can study the effects of metformin at the hypothalamic level by directly recording the activity of green fluorescent protein-identified GnRH neurons (40).
This study had three aims. First, we tested whether metformin could restore estrous cyclicity to PNA mice. Second, we assessed directly the native GnRH neuron activity in PNA mice, which has not been previously characterized in any PNA model, and whether it was altered by metformin in a manner consistent with changes in estrous cycles. Third, we tested whether GnRH neurons showed changes consistent with AMPK activation after systemic metformin administration.
Materials and Methods
Generation of PNA mice
Reagents were purchased from Sigma (St. Louis, MO) unless otherwise indicated. Adult (2–4 months) female GnRH-GFP transgenic mice were used to generate PNA mice. Mice were housed under a 14-h light, 10-h dark cycle with chow (2916; Harlan, Indianapolis, IN) and water available ad libitum. Females were paired with males and checked for copulatory plugs. The date of plug was considered d 1 of gestation. Pregnant mice were injected daily sc with 50 μl sesame oil containing 250 μg of DHT on d 16, 17, and 18 of gestation. Control (CON) mice were offspring of oil-injected dams or untreated mice; no differences were observed between these, and they were combined for analysis. All procedures were approved by the University of Virginia Animal Care and Use Committee and conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Estrous cycle monitoring
Beginning at 8 wk of age, estrous cycles were monitored by vaginal cytology for 16 d before metformin administration, for the first 16 d of administration, and during the 8th to 10th week of administration. Mean cycle length was the average estrus-to-estrus interval; for mice that did not enter estrus, 16 d was used as cycle length, although actual length may have been longer.
Metformin administration
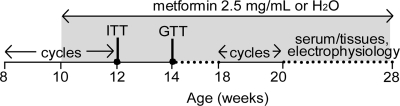
A schematic representation indicating the time of metformin administration relative to physiological assessments is shown in Fig. 1. Mice from each litter were randomized to receive either metformin or regular water. Metformin (1,1-dimethylbiguanide hydrochloride) was administered in drinking water at a dose of 2.5 mg/ml. Based on measured water consumption of approximately 4 ml/d by a 25-g mouse, this equates to approximately 400 mg/kg·d. This is approximately 10 times the maximum human dose (41). However, similar high doses have been used in mice and may be necessary to induce metabolic changes in this species (42–44). Indeed, this dose was the lowest effective in previous studies to reverse the effects of a high-fat diet on the onset of puberty in mice, without affecting growth or food and water intake (45). No differences in water consumption were measured between metformin-treated and untreated mice in this study (data not shown).
Fig. 1.
Timeline of metformin administration and physiological assessments. cycles, Estrous cycle monitoring.
Insulin tolerance tests (ITTs)
ITTs were performed 2 wk after the start of metformin administration. Studies were performed 10 h after lights-on in singly-housed fed mice. Although mice had free access to food before testing, their active (feeding) period normally ends at the time of lights-on. The tail was anesthetized with the skin refrigerant ethyl chloride (Gebauer, Cleveland, OH) and the tip removed with a sterile scalpel blade. Tail blood (∼1 μl/sample) was collected for glucose measurement with a OneTouch Ultra glucometer (Lifescan, Milpitas, CA). After an initial glucose measurement, mice were injected ip with a bolus of 0.75 U/kg of insulin (Novolin R, Novo Nordisk, Denmark) in sterile 0.9% NaCl. Blood glucose was determined at 10, 20, 30, 45, 60, and 75 min after injection, and percent change from baseline was calculated for each time point.
Glucose tolerance tests (GTTs)
GTTs were performed after a 2-wk recovery period from ITTs (4 wk after initiation of metformin). Mice were singly housed on Sani-Chips (Harlan) and fasted overnight for 16 h (1600 to 0800 h) before the test. After a fasting glucose measurement, mice were injected ip with a bolus of 1 g/kg glucose in 0.9% NaCl. Blood glucose was assessed at 10, 20, 30, 45, 60, 75, 90, and 120 min thereafter.
Brain slice preparation
Mice were killed on diestrus between 0900 and 1100 h (5–7 h after the time of lights-on). Blood glucose, body mass, parametrial fat mass (mass of left fat pad), and uterine mass were recorded for all mice. Brain slices were prepared with slight modifications of previously described methods (46, 47). Briefly, the brain was rapidly removed and placed in ice-cold high-sucrose saline solution containing (in mm): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.3 Na2HPO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal (300 μm) slices were cut with a Vibratome 3000 (Ted Pella, Inc., Redding, CA). Slices were incubated for 30 min at 30–32 C in 50% high-sucrose saline and 50% artificial cerebrospinal fluid (ACSF) containing (in mm): 135 NaCl, 3.5 KCl, 26 NaHCO3, 0 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 2.5 CaCl2 (pH 7.4). Slices were then transferred to 100% artificial cerebrospinal fluid solution (glucose adjusted to 5 mm) at room temperature (∼21–23 C) for 0.5–5 h.
Electrophysiological recordings
Targeted single-unit extracellular recordings (loose patch) were used in this study, because this configuration minimally affects the intrinsic properties of the cell, including glucose metabolism, during long-term recordings (48). Recording pipettes (1–3 mΩ) were filled with normal HEPES-buffered solution, and low-resistance (<50 mΩ) seals were formed between the pipette and the GnRH neuron. Recordings were made in voltage-clamp mode with the pipette holding potential at 0 mV, and signals were filtered at 10 kHz. Experiments were performed using an EPC 10 amplifier and Patchmaster software (HEKA Electronics, Lambrecht/Pfalz, Germany). Cells targeted for recording were located in preoptic and septal areas of hypothalamus. In some cells, the AMPK antagonist compound C (CC) (40 μm) was acutely applied for 10 min after a 45- to 60-min baseline recording. In another subset of cells, the effect of a 10-min application of 0.2 mm glucose was tested after a 10-min CON recording period.
Hormone measurements
All hormones were measured in serum from random-fed mice exhibiting diestrous vaginal cytology on the day of killing. LH was measured using a two-site sandwich immunoassay with a sensitivity of 0.07 ng/ml (49–51). Testosterone was measured in serum using a RIA kit according to the manufacturer's instructions (catalog no. TKTT2; Siemens Medical Solutions, Los Angeles, CA). Sensitivity was 7.5 ng/dl. Serum androstenedione was measured using a RIA kit (catalog no. DSL 3800; Beckman Coulter, Fullerton, CA) with a sensitivity of 0.12 ng/ml. An adipokine panel was used to assess insulin, leptin, IL-6, TNF-α, plasminogen activator inhibitor-1 (PAI-1), and resistin (Millipore mouse serum adipokine kit, catalog no. MADPK-71K-07; Millipore, Billerica, MA). Sensitivity was 12.2 pg/ml for resistin, 195 pg/ml for insulin, 48.8 pg/ml for leptin and PAI-1, 2 pg/ml for TNF-α, and 5 pg/ml for IL-6. Adiponectin was measured via RIA (catalog no. MADP-60K; Millipore); assay sensitivity was 1.3 ng/ml. Inter and intraassay coefficients of variation were less than 10% for all analytes.
Analysis and statistics
PNA and CON mice with body mass more than 2 sds from the mean (>29 g) were excluded from analysis of electrophysiology data to avoid possible confounding effects of excess adiposity on the function of GnRH neurons. Mean firing rate of GnRH neurons in CON mice less than 29 g: 0.11 ± 0.04 Hz, n = 17; in CON mice more than 29 g: 0.22 ± 0.09 Hz, n = 9; P < 0.08; in PNA mice less than 29 g: 0.32 ± 0.09 Hz, n = 17; in PNA mice more than 29 g: 0.60 ± 0.59 Hz, n = 3. The percent change in response to CC was calculated and compared among groups using Fisher's exact test and two-way ANOVA. For effects of low glucose, one-way repeated measures ANOVA with Dunnett's multiple comparison test was used to compare treatment vs. CON frequencies. ANOVA with Bonferroni or Duncan's post hoc testing was used for all other comparisons. Data are reported as mean ± sem. Significance was set at P < 0.05.
Results
Metformin has minimal effects on metabolic parameters in PNA mice
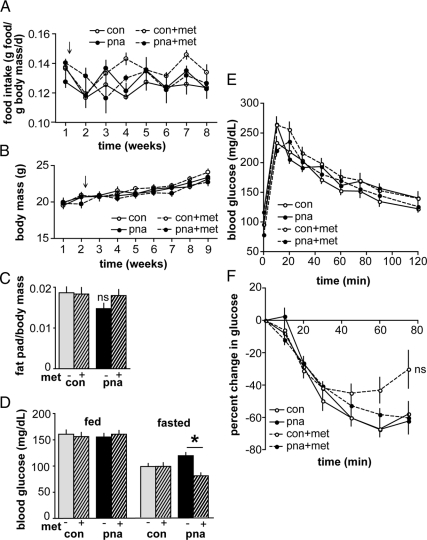
Because metformin's primary application is as an antihyperglycemic in diabetic patients, we first assessed metabolic parameters in metformin-treated mice. Food intake and body mass were not different between CON and PNA mice and were unaffected by metformin (n = 11–12 per group) (Fig. 2, A and B). Parametrial fat mass/body mass ratio (determined at euthanasia) was similar in CON and PNA mice and unchanged by metformin (CON, n = 24; PNA, n = 24; CON metformin, n = 21; and PNA metformin, n = 21) (Fig. 2C). Random-fed blood glucose levels did not differ among groups, but fasting glucose was reduced by metformin in PNA mice (P < 0.05) (Fig. 2D). This is consistent with the drug's primary mechanism of action to inhibit hepatic gluconeogenesis (52) and indicates that an effective dose was used. ITT and GTT were performed after 2 and 4 wk, respectively, of metformin administration. Metformin did not significantly alter either glucose disposal or insulin sensitivity in PNA mice (n = 10 mice per group, P > 0.05) (Fig. 2, E and F). Further, an adipokine panel in serum collected from random-fed mice at the time of killing showed no differences in adipokine or insulin levels (n = 11–12 mice per group) (Table 1). The data used to generate ITT curves for untreated CON and PNA mice were reported previously (10), as were adiponectin values from untreated CON and PNA mice (10).
Fig. 2.
Metformin has minimal effects on metabolic parameters in PNA mice. A, Average daily food intake per unit body mass was similar in PNA and CON mice and unaffected by metformin (met) (n = 11–12 per group). Arrow indicates start of metformin administration to treated groups. B, Body mass was unaffected by metformin (n = 11–12 per group). Arrow indicates start of treatment. C, Ratio of parametrial fat pad mass to body mass was not different among groups (n = 21–24 per group). D, Blood glucose levels in random-fed (n = 21–24 per group) and fasted (n = 10 per group) mice. Metformin significantly decreased fasting blood glucose in PNA mice. E, GTT indicated no difference in glucose tolerance among groups (n = 10 per group). F, ITT showed no differences in insulin sensitivity (n = 10 per group). *, P < 0.05. ns, Not significant. ITT curves from untreated CON and PNA mice were generated from data reported previously (10).
Table 1.
Mean ± sem serum insulin and adipokine levels in random-fed diestrous mice
| CON | CON+met | PNA | PNA+met | |
|---|---|---|---|---|
| Adiponectin (ng/ml) | 16.2 ± 0.7 | 14.3 ± 1.2 | 14.0 ± 1.0 | 14.8 ± 0.2 |
| Insulin (ng/ml) | 1.3 ± 0.2 | 1.9 ± 0.4 | 1.7 ± 0.4 | 1.1 ± 0.2 |
| Leptin (ng/ml) | 2.5 ± 0.4 | 3.2 ± 0.6 | 1.7 ± 0.6 | 2.2 ± 0.5 |
| TNF-α (pg/ml) | 5.2 ± 0.4 | 5.3 ± 0.4 | 5.3 ± 0.7 | 4.1 ± 0.2 |
| IL-6 (pg/ml) | 16.8 ± 2.3 | 21.0 ± 3.0 | 28.2 ± 7.1 | 17 ± 2.7 |
| Resistin (ng/ml) | 2.0 ± 0.2 | 2.3 ± 0.2 | 1.7 ± 0.2 | 2.0 ± 0.1 |
| PAI-1 (ng/ml) | 10.2 ± 0.9 | 9.3 ± 0.8 | 8.7 ± 1.5 | 9.2 ± 0.9 |
Adiponectin levels in untreated CON and PNA mice were reported previously (10). met, Metformin.
Metformin restores estrous cyclicity to PNA mice
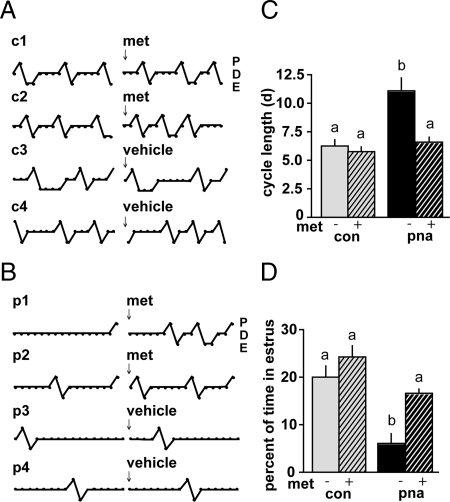
Estrous cycles were monitored before and after metformin administration in individual mice and quantified over a 16-d period (Fig. 3). In accordance with previous observations (9, 10), PNA mice exhibited abnormal estrous cycles before metformin administration. Cycles were lengthened, with PNA mice rarely entering estrus (Fig. 3, B and C). After 10 wk of metformin administration, cycles in PNA mice were normalized, with mean cycle length and percent of time in estrus no longer different from CON (n = 10 mice per group, P < 0.05) (Fig. 3, B and C). In a subsequent study, cycles were monitored before and during metformin administration without interruption by metabolic testing. Significant differences in cycle duration and time in estrus were apparent by 4 wk of treatment (n = 11–12 mice per group, data not shown). Cycle parameters did not change over time in mice not treated with metformin (data not shown).
Fig. 3.
Metformin improves estrous cycles in PNA mice. A and B, Representative estrous cycle plots from metformin (met)- and vehicle-treated CON (A) and PNA (B) mice. c1–c4 and p1–p4 refer to individual CON and PNA mouse numbers. Daily cycle stage designated as diestrus (D), proestrus (P), or estrus (E). C, Estrous cycle length was significantly longer in PNA mice before metformin but restored to CON levels after treatment (n = 10 per group). D, Percent of time in estrous was significantly lower in PNA than CON mice but no longer different after metformin. Different lowercase letters indicate groups with significantly different means. P < 0.05.
PNA increases firing activity of GnRH neurons
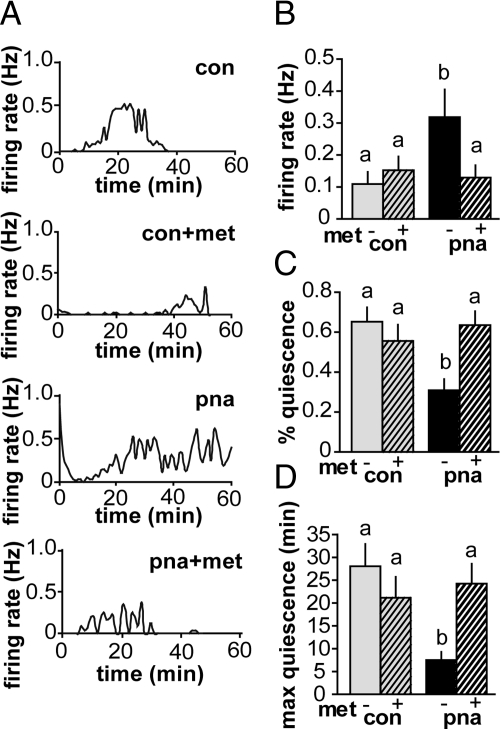
In contrast to most mature neurons, in which GABAA receptor activation is inhibitory, due to the high intracellular chloride maintained by GnRH neurons GABAA receptor activation can excite these cells (11). Because GABAergic transmission to GnRH neurons and LH levels were previously shown to be increased in PNA mice (9), we hypothesized that firing activity of GnRH neurons from PNA mice would be elevated. Using extracellular recordings, we assessed firing activity of GnRH neurons from PNA mice. Mean firing rate was increased, and percent and maximum duration of quiescence were decreased in GnRH neurons from PNA mice (CON, n = 17 cells from eight mice; PNA, n = 17 cells from 10 mice; P < 0.05 frequency and P < 0.01 percent and max duration quiescence) (Fig. 4). Firing activity is associated with hormone secretion in other neuroendocrine systems (53), suggesting that GnRH release may be elevated or altered in pattern in PNA mice.
Fig. 4.
GnRH neurons from PNA mice have increased firing activity that is reversed by metformin. A, Representative graphs of firing rate over time in GnRH neurons from mice from each group. Events are binned in 60-sec intervals. B, Firing rate of GnRH neurons was higher in PNA mice (n = 17 cells from 10 mice) compared with CON (n = 17 cells from eight mice). Firing rate was lower in GnRH neurons from PNA mice treated with metformin (met) (n = 14 cells from seven mice) but was not different in cells from CON mice on metformin (n = 19 cells from eight mice). Percent quiescence (C) and maximum duration of quiescence (D) were lower in GnRH neurons from PNA mice not treated with metformin. Metformin restored quiescence measures of GnRH neurons from PNA mice but had no effect on CON mice. Quiescence is defined as 0–1 events/min. Different lowercase letters indicate groups with significantly different means. P < 0.05.
Metformin reverses the increase in firing activity of GnRH neurons from PNA mice
To test whether the normalization of estrous cyclicity in PNA mice by metformin could be attributed to changes in GnRH neuron activity, we examined GnRH neuron firing rate in animals treated with metformin. Metformin treatment normalized firing activity and quiescence measures in GnRH neurons from PNA mice, without altering firing parameters of GnRH neurons from CON mice (CON+met, n = 19 cells from eight mice; PNA+met, n = 14 cells from seven mice; P < 0.05 frequency and P < 0.01 quiescence measures vs. PNA) (Fig. 4). This suggested that a normalization of GnRH neuron activity by metformin may have contributed to the restoration of estrous cyclicity in PNA mice.
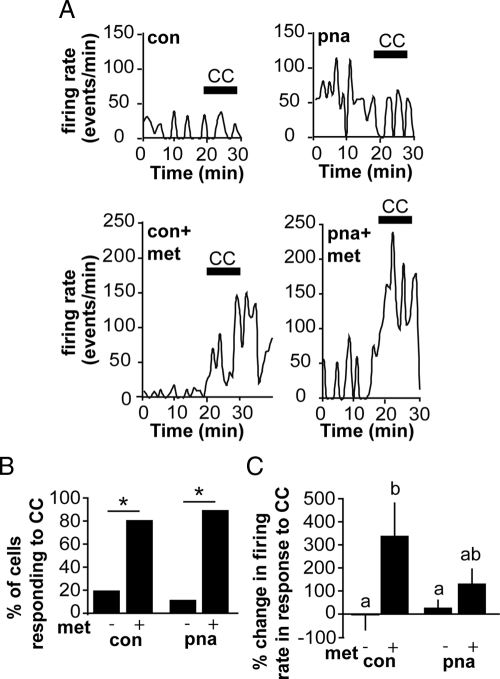
GnRH neurons from metformin-treated, but not untreated, mice respond to an AMPK antagonist
To test whether AMPK was activated in GnRH neurons from metformin-treated mice, we used the specific AMPK antagonist CC. Bath application of CC caused an increase in firing in the majority of GnRH neurons from metformin-treated, but not untreated, CON and PNA mice (Fig. 5). The percent of cells that responded with a more than 20% increase in firing was higher in metformin-treated vs. untreated CONs (CON, 2/10 or 20% vs. CON metformin, 8/10 or 80%; P = 0.023) (Fig. 5B) and similarly greater in metformin-treated vs. untreated PNA mice (PNA, 1/8 or 12.5% vs. PNA metformin, 10/11 or 90.9%; P = 0.001). There was a main effect of metformin to increase the percent change in firing in response to CC (P < 0.05, two-way ANOVA) (Fig. 5C); post hoc testing revealed a greater percent change in metformin-treated compared with untreated CONs but not between metformin-treated PNA and either metformin-treated CON or untreated PNA. Overall, these data suggest that metformin renders GnRH neurons responsive to CC, consistent with AMPK activation in GnRH neurons or their afferents as a result of metformin administration.
Fig. 5.
An AMPK antagonist stimulates firing in metformin-treated but not untreated mice. A, Representative plots of firing rate over time from each group. Black bar indicates time of CC application. CC induces an acute increase in firing in GnRH neurons from metformin (met)-treated mice. B, Percent of cells responding with a greater than 20% increase in firing rate in response to CC application. Significantly more cells from metformin-treated compared with untreated mice responded to the antagonist (P < 0.05). C, Mean percent change in firing rate in response to CC application (CON, n = 10 cells from five mice; PNA, n = 8 cells from three mice; CON metformin, n = 10 cells from four mice; and PNA metformin, n = 11 cells from five mice). Different lowercase letters indicate groups with significantly different means. P < 0.05.
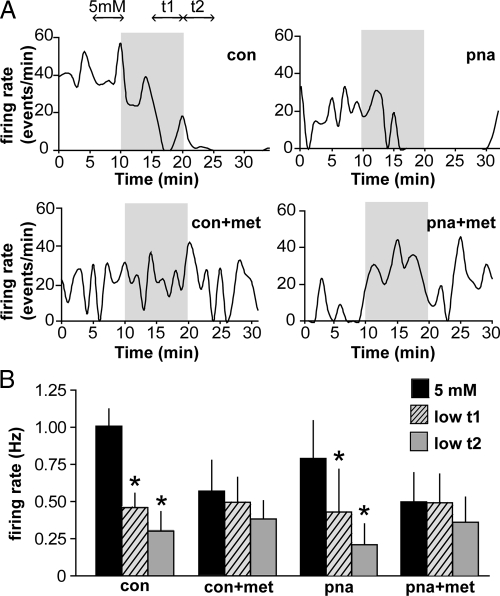
Glucosensing is attenuated in GnRH neurons from metformin-treated mice
Recent work in our lab has demonstrated a critical role for AMPK in glucosensing by GnRH neurons (54). AMPK is activated by low glucose, inhibiting firing activity. We hypothesized that in metformin-treated mice, AMPK is already activated; thus, the inhibitory response to low glucose may be diminished. To test this hypothesis, firing activity was recorded for a CON period in 5 mm glucose and after a switch to 0.2 mm glucose for 10 min. All cells from untreated PNA and CON mice were markedly inhibited by low glucose (CON, n = 8 cells from five mice, P = 0.003; PNA, n = 8 cells from four mice, P = 0.0001) (Fig. 6). However, cells from metformin-treated PNA and CON mice were less sensitive to low glucose, with the majority failing to show inhibition (metformin CON, n = 6 cells from three mice, P = 0.4; metformin PNA, n = 8 cells from three mice, P = 0.4) (Fig. 6). Basal firing rate in 5 mm glucose did not differ among groups in this experiment, although there was a trend for metformin to reduce firing rate (two-way ANOVA, P = 0.08; main effect of metformin). It is important to point out that firing rates in this experiment differ from those in longer-term recordings (Fig. 4), because here, only cells that were actively firing at the onset of recording were used to enable us to see a potential suppression; previously, all cells were included (if verified to be viable) to record the native firing activity. In conjunction with the above data using the AMPK antagonist, these experiments support the hypothesis that AMPK in GnRH neurons (or in the network controlling their activity) is activated by peripheral metformin administration.
Fig. 6.
Glucosensing is attenuated in GnRH neurons from metformin (met)-treated compared with untreated mice. A, Representative graphs of firing rate over time. Shaded region indicates period of low (0.2 mm) glucose application. Double-headed arrows in first graph indicate periods during which frequency was averaged for analysis. B, Firing rates in 5 mm glucose and during time intervals t1 and t2 after low-glucose application. Firing rate was significantly reduced by low glucose in GnRH neurons from untreated CON and PNA mice but not those treated with metformin (CON, n = 8 cells from five mice; PNA, n = 8 cells from four mice; CON metformin, n = 6 cells from three mice; and PNA metformin, n = 8 cells from three mice). *, P < 0.05 vs. 5 mm.
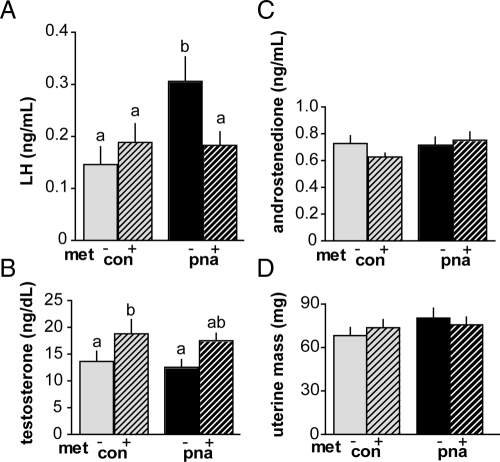
Metformin reduces LH in PNA mice but does not alter androgen levels
Hormones were measured in serum samples taken at the time of killing on the day of recording from mice exhibiting diestrous vaginal cytology. Consistent with previous observations (9), LH was elevated in PNA mice (Fig. 7A, n = 9–14 per group, P < 0.05). Neither testosterone nor androstenedione were elevated, however (Fig. 7, B and C). Metformin restored LH levels to normal in PNA mice (PNA vs. PNA metformin, P < 0.05). Metformin increased testosterone levels (n = 9–14 per group; P < 0.05 main effect of metformin) (Fig. 7B). However, post hoc testing only found this increase significant in CONs (CON vs. CON metformin, P < 0.05). Metformin had no effect on androstenedione levels (P > 0.05) (Fig. 7C). Uterine mass, a bioassay for estradiol levels (55), was similar among groups (n = 19–23 per group, P > 0.5) (Fig. 7D), suggesting neither PNA nor metformin markedly altered estradiol levels.
Fig. 7.
Serum reproductive hormone levels and uterine mass. A, LH levels were elevated in PNA mice. Metformin (met) restored LH in PNA mice to the level of CON mice (n = 9–14 per group). B and C, Testosterone and androstenedione levels were not different between PNA and CON mice; metformin increased testosterone but had no effect on androstenedione levels (n = 9–14 per group). D, Uterine mass was not different among groups (n = 19–23 per group). Different lowercase letters indicate groups with significantly different means. P < 0.05.
Discussion
GnRH neuronal function is critical for fertility. Here, we demonstrate for the first time that GnRH neuronal activity is elevated in mice prenatally androgenized with DHT; this elevated activity may be causal to disrupted estrous cyclicity in these mice. Based on these findings, we tested the hypothesis that metformin can affect the reproductive system by modulating the activity of central AMPK, which is emerging as an important regulator of GnRH neuronal function. Alterations in both estrous cyclicity and GnRH neuron activity were restored to CON values by treatment with metformin. Metabolic parameters were largely unchanged by metformin, and serum androgen levels were not reduced, suggesting that metformin's effectiveness to improve reproductive cyclicity in this model was not dependent on reduction of circulating glucose, insulin, or androgens. GnRH neurons from metformin-treated mice showed changes in activity consistent with activation of AMPK, supporting a central site of metformin action. These data indicate that prenatal exposure to androgen programs altered GnRH neuron function, and suggest that one mechanism of metformin action to ameliorate infertility may be through central effects to either directly or indirectly regulate GnRH neurons.
The demonstration that GnRH neuron firing activity is abnormally high in PNA mice supports and extends our previous finding of increased excitatory GABAergic transmission to GnRH neurons and elevated LH levels in these mice (9). Firing rate has been correlated with hormone release in neuroendocrine systems (53), suggesting that GnRH release may be elevated in PNA mice. Metformin restored GnRH neuron activity in PNA mice to CON levels. The reduction in firing activity by metformin is consistent with central AMPK activation, which has been shown to inhibit GnRH neurons in brain slices (54) and in GT1-7 immortalized GnRH neurons (56). AMPK activation may signal low energy availability, which suppresses fertility. Interestingly, measures of firing activity of GnRH neurons did not differ between untreated and metformin-treated CON mice. This was surprising, because metformin would be expected to suppress firing in CONs if it indeed activates AMPK. We surmise that chronic metformin administration may lead to compensatory mechanisms that maintain a basal level of firing because AMPK can influence cellular metabolic processes such as glucose uptake (57) that may affect excitability long term. It is also possible that assessment of firing patterns over a period longer than 1 h might reveal differences between untreated and metformin-treated CONs.
AMPK activation is typically assessed using Western blot analysis to assay for phosphorylation. Multiple studies have demonstrated AMPK phosphorylation in the brain after peripheral metformin administration (8, 36); however, this could not be assessed directly in GnRH neurons due to their scattered localization. Instead, we used both a pharmacological and physiological assay for AMPK activation. Acute application of the AMPK antagonist CC increased firing only in GnRH neurons from metformin-treated mice, suggesting that AMPK is activated in these neurons or their afferents remaining within the brain slice. This robust response to CC did not appear to be simply a relief of suppression but instead an activation (i.e. GnRH neurons do not typically reach a firing rate of 4 Hz when averaged over several minutes, which some did after CC). This could represent an unmasking of compensatory mechanisms induced by long-term metformin administration. Another possibility is that AMPK phosphorylates multiple intracellular targets, and by acute antagonism, we are able to block only those with short-term effects, whereas other sustained excitatory effects remain. It is also possible that the observed effect of CC is nonspecific (non-AMPK mediated), but this would still be of interest, because the effect of CC is only observed in cells from mice treated with metformin.
Because the pharmacologic specificity of CC may not be limited to AMPK, we performed a second experiment to assess AMPK activation. Because AMPK plays a role in glucosensing in GnRH neurons (54), we tested the response to low glucose of GnRH neurons from untreated and metformin-treated mice. Consistent with enhanced basal AMPK activation, GnRH neurons from metformin-treated mice were less sensitive to the inhibitory effects of low glucose. Notably, mean basal firing rate in all groups in the glucosensing study (Fig. 6) differed from that for long-term recordings of firing activity (Fig. 4). This is explained by the fact that basal firing rate in Fig. 6 represents cells that were selected on the basis of being active at the time when they were patched. Quiescent cells, which occur more frequently in the CON group, were excluded from these averages; the absence of basal firing precluded testing the effects of low glucose, which suppresses firing (54).
Because these studies were performed in whole-animal models, we cannot rule out the possibility that observed central changes were secondary to peripheral changes. Studies have shown that metformin can have direct effects in the ovary to alter morphology (58, 59), vascularity (60), stromal blood flow (59), and steroidogenic function (23, 29, 30, 61, 62). With regard to the latter, metformin has been shown to directly inhibit androgen production by theca cells in vitro (62). A reduction in androgens would be expected to reduce GnRH neuron activity (63). In this study, metformin increased rather than decreased testosterone levels. This contradicts a direct effect of metformin to reduce ovarian androgen production and suggests that lowered androgens result from reduced insulin levels in women with PCOS. Metformin may have altered levels of estradiol, progesterone, or other secreted ovarian factors (29, 30, 61). These were not evaluated due to limitations in blood volume in mice, but the lack of a difference in uterine mass among groups suggested that estradiol levels were similar. Adipokines were evaluated because metformin affects adipocyte function (64, 65) and has been shown to alter secretion of adiponectin in vivo (66) and various adipokines in vitro (67–71). Similar to steroids, circulating adipokines can cross the blood brain barrier and influence neuronal function (72, 73). Of interest, adiponectin has been shown to activate AMPK in immortalized GnRH neurons (74), as well as in other cell types (33, 75–77). We found no changes in the circulating adipokines measured, suggesting that changes in levels of these hormones did not contribute to the observed changes in GnRH neuron activity.
Although the small blood volume of mice hinders the assessment of LH pulses, we did assess LH in single-point samples and repeated our previous finding of increased LH in PNA mice. This increase was reversed by metformin, consistent with the reduction in firing activity of GnRH neurons. Reduced LH could reflect reduced LH pulse frequency, amplitude, or both. Two studies in normal-weight women with PCOS demonstrated a decrease in LH pulse amplitude but no change in frequency after metformin administration (78, 79); another study in lean women with PCOS showed a decrease in overall serum LH levels after metformin (80). LH pulse amplitude is determined by the magnitude of the GnRH stimulus as well as the response of the pituitary to GnRH, either of which could be altered by metformin or PNA. Thus, the observed postmetformin decrease in LH in this study could be due to the observed decrease in activity of GnRH neurons and subsequent reduction in GnRH release, diminished pituitary responsiveness mediated by direct effects of metformin on the pituitary (33), or both. A recent study of women with PCOS demonstrating a reduction in LH by metformin in vivo found no effect of metformin on LH release in LβT2 gonadotroph cells in vitro (78), suggesting that the effects in vivo may have been centrally mediated.
Other animal models of PNA have examined the effects of insulin-sensitizing agents on reproductive parameters. In PNA rhesus monkeys, which are reported to exhibit numerous PCOS-like characteristics (6), the peroxisome proliferator-activated receptor-γ agonist pioglitazone normalized menstrual cycles in conjunction with a reduction in basal plasma insulin levels (81). Pioglitazone had no effect on basal or human chorionic gonadotropin-stimulated testosterone production, and it increased androstenedione levels, suggesting that a reduction in androgens did not underlie improved cyclicity in this model, similar to our findings. Pioglitazone increased the basal LH/FSH ratio, suggesting a paradoxical increase in GnRH pulse frequency in this model. This suggests possible differences in the reproductive effects of various insulin-sensitizing agents. In PNA sheep, which similarly are reported to mimic some aspects of PCOS, the peroxisome proliferator-activated receptor-γ agonist rosiglitazone halted the deterioration of estrous cycles that typically occurs in the second breeding season and improved insulin sensitivity (82). LH levels, pulsatility, or androgen levels were not reported in the sheep study, making it difficult to ascertain the contribution of central and/or pituitary changes to the improvements in reproductive function.
The antihyperglycemic drug metformin has been used clinically for decades and has demonstrated benefits in women with PCOS, but the mechanisms by which metformin improves reproductive cyclicity in this disorder are unclear. Although no animal model can perfectly replicate a human disease, the similarity of some phenotypes of PNA animals with women with PCOS suggests common pathophysiological aspects. The findings presented in this study raise a new possibility that in addition to its metabolic effects, metformin may alter the function of the reproductive axis through central mechanisms to alter GnRH release.
Acknowledgments
We thank Debra Fisher and Laura Burger for excellent technical assistance; Dr. Farah Morgan, Dr. Christopher McCartney, and Dr. Justyna Pielecka for editorial comments; and Kimberly Cox for assistance with statistical analyses. Portions of this work were presented in abstract form at the 92nd meeting of The Endocrine Society.
This work was supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development U54 HD 28934 Project III and the Ligand Assay and Analysis Core, and by the National Institute of Neurological Disorders and Stroke Grant F31 NS 062646 (to A.V.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMPK
- AMP-activated protein kinase
- CC
- compound C
- CON
- control
- DHT
- dihydrotestosterone
- GABA
- γ-aminobutyric acid
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- PAI-1
- plasminogen activator inhibitor-1
- PCOS
- polycystic ovary syndrome
- PNA
- prenatal androgenization.
References
- 1. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 2. Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, Hall JE. 1997. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:2248–2256 [DOI] [PubMed] [Google Scholar]
- 3. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr 1988. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocri Metab 66:165–172 [DOI] [PubMed] [Google Scholar]
- 4. Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. 1976. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 57:1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blank SK, McCartney CR, Marshall JC. 2006. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 12:351–361 [DOI] [PubMed] [Google Scholar]
- 6. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. 2005. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11:357–374 [DOI] [PubMed] [Google Scholar]
- 7. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. 2002. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579 [DOI] [PubMed] [Google Scholar]
- 8. Dumesic DA, Abbott DH, Padmanabhan V. 2007. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan SD, Moenter SM. 2004. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA 101:7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roland AV, Nunemaker CS, Keller SR, Moenter SM. 2010. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol 207:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeFazio RA, Heger S, Ojeda SR, Moenter SM. 2002. Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- 12. Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. 2005. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology 146:4281–4291 [DOI] [PubMed] [Google Scholar]
- 13. Masek KS, Wood RI, Foster DL. 1999. Prenatal dihydrotestosterone differentially masculinizes tonic and surge modes of luteinizing hormone secretion in sheep. Endocrinology 140:3459–3466 [DOI] [PubMed] [Google Scholar]
- 14. Foecking EM, Szabo M, Schwartz NB, Levine JE. 2005. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod 72:1475–1483 [DOI] [PubMed] [Google Scholar]
- 15. DeUgarte CM, Bartolucci AA, Azziz R. 2005. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 83:1454–1460 [DOI] [PubMed] [Google Scholar]
- 16. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. 1992. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41:1257–1266 [DOI] [PubMed] [Google Scholar]
- 17. Dunaif A. 1997. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18:774–800 [DOI] [PubMed] [Google Scholar]
- 18. Diamanti-Kandarakis E, Kouli C, Tsianateli T, Bergiele A. 1998. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol 138:269–274 [DOI] [PubMed] [Google Scholar]
- 19. Palomba S, Falbo A, Zullo F, Orio F., Jr 2009. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 30:1–50 [DOI] [PubMed] [Google Scholar]
- 20. Essah PA, Apridonidze T, Iuorno MJ, Nestler JE. 2006. Effects of short-term and long-term metformin treatment on menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril 86:230–232 [DOI] [PubMed] [Google Scholar]
- 21. Palomba S, Orio F, Jr, Falbo A, Manguso F, Russo T, Cascella T, Tolino A, Carmina E, Colao A, Zullo F. 2005. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4068- 4074 [DOI] [PubMed] [Google Scholar]
- 22. Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. 1994. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 43:647–654 [DOI] [PubMed] [Google Scholar]
- 23. Romualdi D, Giuliani M, Cristello F, Fulghesu AM, Selvaggi L, Lanzone A, Guido M. 2010. Metformin effects on ovarian ultrasound appearance and steroidogenic function in normal-weight normoinsulinemic women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Fertil Steril 93:2303–2310 [DOI] [PubMed] [Google Scholar]
- 24. Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, Schmidt M, Elsenbruch S, Kimmig R, Mann K, Janssen OE. 2007. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol 157:669–676 [DOI] [PubMed] [Google Scholar]
- 25. Romualdi D, Costantini B, Selvaggi L, Giuliani M, Cristello F, Macrì F, Bompiani A, Lanzone A, Guido M. 2008. Metformin improves endothelial function in normoinsulinemic PCOS patients: a new prospective. Hum Reprod 23:2127–2133 [DOI] [PubMed] [Google Scholar]
- 26. Genazzani AD, Lanzoni C, Ricchieri F, Baraldi E, Casarosa E, Jasonni VM. 2007. Metformin administration is more effective when non-obese patients with polycystic ovary syndrome show both hyperandrogenism and hyperinsulinemia. Gynecol Endocrinol 23:146–152 [DOI] [PubMed] [Google Scholar]
- 27. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winder WW, Hardie DG. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277:E1–E10 [DOI] [PubMed] [Google Scholar]
- 29. Tosca L, Solnais P, Ferré P, Foufelle F, Dupont J. 2006. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol Reprod 75:342–351 [DOI] [PubMed] [Google Scholar]
- 30. Tosca L, Chabrolle C, Uzbekova S, Dupont J. 2007. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK). Biol Reprod 76:368–378 [DOI] [PubMed] [Google Scholar]
- 31. Richardson MC, Ingamells S, Simonis CD, Cameron IT, Sreekumar R, Vijendren A, Sellahewa L, Coakley S, Byrne CD. 2009. Stimulation of lactate production in human granulosa cells by metformin and potential involvement of adenosine 5′ monophosphate-activated protein kinase. J Clin Endocrinol Metab 94:670–677 [DOI] [PubMed] [Google Scholar]
- 32. Kayampilly PP, Menon KM. 2009. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology 150:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. 2008. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LβT2 gonadotropes. Mol Endocrinol 22:760–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramamurthy S, Ronnett GV. 2006. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol 574:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. 2009. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 109(Suppl 1):17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ropelle ER, Pauli JR, Zecchin KG, Ueno M, de Souza CT, Morari J, Faria MC, Velloso LA, Saad MJ, Carvalheira JB. 2007. A central role for neuronal adenosine 5′-monophosphate-activated protein kinase in cancer-induced anorexia. Endocrinology 148:5220–5229 [DOI] [PubMed] [Google Scholar]
- 37. Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. 2004. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 279:19970–19976 [DOI] [PubMed] [Google Scholar]
- 38. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574 [DOI] [PubMed] [Google Scholar]
- 39. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. 2004. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 40. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. 2000. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- 41. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. 2008. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol 199:596–609 [DOI] [PubMed] [Google Scholar]
- 42. Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. 2006. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology 130:2099–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heishi M, Ichihara J, Teramoto R, Itakura Y, Hayashi K, Ishikawa H, Gomi H, Sakai J, Kanaoka M, Taiji M, Kimura T. 2006. Global gene expression analysis in liver of obese diabetic db/db mice treated with metformin. Diabetologia 49:1647–1655 [DOI] [PubMed] [Google Scholar]
- 44. Hou M, Venier N, Sugar L, Musquera M, Pollak M, Kiss A, Fleshner N, Klotz L, Venkateswaran V. 2010. Protective effect of metformin in CD1 mice placed on a high carbohydrate-high fat diet. Biochem Biophys Res Commun 397:537–542 [DOI] [PubMed] [Google Scholar]
- 45. Brill DS, Moenter SM. 2009. Androgen receptor antagonism and an insulin sensitizer block the advancement of vaginal opening by high-fat diet in mice. Biol Reprod 81:1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nunemaker CS, DeFazio RA, Moenter SM. 2002. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- 47. Chu Z, Moenter SM. 2005. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nunemaker CS, DeFazio RA, Moenter SM. 2003. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. 1987. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol 4:157–165 [DOI] [PubMed] [Google Scholar]
- 50. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. 1993. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132:1687–1691 [DOI] [PubMed] [Google Scholar]
- 51. Fallest PC, Trader GL, Darrow JM, Shupnik MA. 1995. Regulation of rat luteinizing hormone β gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod 53:103–109 [DOI] [PubMed] [Google Scholar]
- 52. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. 1995. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 333:550–554 [DOI] [PubMed] [Google Scholar]
- 53. Dutton A, Dyball RE. 1979. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roland AV, Moenter SM. 2010. Role of AMP-activated protein kinase (AMPK) in glucosensing by gonadotropin-releasing hormone (GnRH) neurons. 40th Annual Meeting of The Society for Neuroscience, San Diego, CA, 2010 (Abstract 6326) [Google Scholar]
- 55. Shim WS, Conaway M, Masamura S, Yue W, Wang JP, Kmar R, Santen RJ. 2000. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology 141:396–405 [DOI] [PubMed] [Google Scholar]
- 56. Coyral-Castel S, Tosca L, Ferreira G, Jeanpierre E, Rame C, Lomet D, Caraty A, Monget P, Chabrolle C, Dupont J. 2008. The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1–7 cells and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol 20:335–346 [DOI] [PubMed] [Google Scholar]
- 57. Fujii N, Jessen N, Goodyear LJ. 2006. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291:E867–E877 [DOI] [PubMed] [Google Scholar]
- 58. Falbo A, Orio F, Venturella R, Rania E, Materazzo C, Tolino A, Zullo F, Palomba S. 2009. Does metformin affect ovarian morphology in patients with polycystic ovary syndrome? A retrospective cross-sectional preliminary analysis. J Ovarian Res 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ozcimen EE, Uckuyu A, Ciftci FC, Zeyneloglu HB. 2009. The effect of metformin treatment on ovarian stromal blood flow in women with polycystic ovary syndrome. Arch Gynecol Obstet 280:263–269 [DOI] [PubMed] [Google Scholar]
- 60. Palomba S, Orio F, Jr, Falbo A, Russo T, Tolino A, Zullo F. 2006. Effects of metformin and clomiphene citrate on ovarian vascularity in patients with polycystic ovary syndrome. Fertil Steril 86:1694–1701 [DOI] [PubMed] [Google Scholar]
- 61. Mansfield R, Galea R, Brincat M, Hole D, Mason H. 2003. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril 79:956–962 [DOI] [PubMed] [Google Scholar]
- 62. Attia GR, Rainey WE, Carr BR. 2001. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril 76:517–524 [DOI] [PubMed] [Google Scholar]
- 63. Pielecka J, Quaynor SD, Moenter SM. 2006. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147:1474–1479 [DOI] [PubMed] [Google Scholar]
- 64. Matthaei S, Reibold JP, Hamann A, Benecke H, Häring HU, Greten H, Klein HH. 1993. In vivo metformin treatment ameliorates insulin resistance: evidence for potentiation of insulin-induced translocation and increased functional activity of glucose transporters in obese (fa/fa) Zucker rat adipocytes. Endocrinology 133:304–311 [DOI] [PubMed] [Google Scholar]
- 65. Bourron O, Daval M, Hainault I, Hajduch E, Servant JM, Gautier JF, Ferré P, Foufelle F. 2010. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia 53:768–778 [DOI] [PubMed] [Google Scholar]
- 66. Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, Demissie M. 2008. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecol Endocrinol 24:378–384 [DOI] [PubMed] [Google Scholar]
- 67. Huypens P, Quartier E, Pipeleers D, Van de Casteele M. 2005. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur J Pharmacol 518:90–95 [DOI] [PubMed] [Google Scholar]
- 68. Rea R, Donnelly R. 2006. Effects of metformin and oleic acid on adipocyte expression of resistin. Diabetes Obes Metab 8:105–109 [DOI] [PubMed] [Google Scholar]
- 69. Mueller WM, Stanhope KL, Gregoire F, Evans JL, Havel PJ. 2000. Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes Res 8:530–539 [DOI] [PubMed] [Google Scholar]
- 70. Klein J, Westphal S, Kraus D, Meier B, Perwitz N, Ott V, Fasshauer M, Klein HH. 2004. Metformin inhibits leptin secretion via a mitogen-activated protein kinase signalling pathway in brown adipocytes. J Endocrinol 183:299–307 [DOI] [PubMed] [Google Scholar]
- 71. He G, Pedersen SB, Bruun JM, Lihn AS, Richelsen B. 2003. Metformin, but not thiazolidinediones, inhibits plasminogen activator inhibitor-1 production in human adipose tissue in vitro. Horm Metab Res 35:18–23 [DOI] [PubMed] [Google Scholar]
- 72. Pan W, Kastin AJ. 2007. Adipokines and the blood-brain barrier. Peptides 28:1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ahima RS. 2005. Central actions of adipocyte hormones. Trends Endocrinol Metab 16:307–313 [DOI] [PubMed] [Google Scholar]
- 74. Wen JP, Lv WS, Yang J, Nie AF, Cheng XB, Yang Y, Ge Y, Li XY, Ning G. 2008. Globular adiponectin inhibits GnRH secretion from GT1–7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem Biophys Res Commun 371:756–761 [DOI] [PubMed] [Google Scholar]
- 75. Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. 2002. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 77. Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. 2009. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem 284:22426–22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oride A, Kanasaki H, Purwana IN, Miyazaki K. 2010. Effects of metformin administration on plasma gonadotropin levels in women with infertility, with an in vitro study of the direct effects on the pituitary gonadotrophs. Pituitary 13:236–241 [DOI] [PubMed] [Google Scholar]
- 79. Genazzani AD, Battaglia C, Malavasi B, Strucchi C, Tortolani F, Gamba O. 2004. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril 81:114–119 [DOI] [PubMed] [Google Scholar]
- 80. Sahin Y, Unluhizarci K, Yilmazsoy A, Yikilmaz A, Aygen E, AQKelestimur F. 2007. The effects of metformin on metabolic and cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Clin Endocrinol 67:904–908 [DOI] [PubMed] [Google Scholar]
- 81. Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. 2007. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol 23:438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Veiga-Lopez A, Lee JS, Padmanabhan V. 2010. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology 151:4007–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]