Loss of canonical insulin signaling results in increased ERK-1/2 activation in response to physiological insulin that decreases p27Kip1 mRNA, demonstrating a potential mechanism where changes in insulin receptor signaling could lead to a decrease in p27Kip1 thereby accelerating VSMC proliferation and migration.
Abstract
Insulin resistance is associated with an accelerated rate of atherosclerosis. Vascular smooth muscle cell (VSMC) migration and proliferation are important components of atherosclerosis. To elucidate the effects of the loss of normal insulin receptor (IR) signaling on VSMC function, we compared the proliferation and migration of murine VSMCs lacking the IR (L2-VSMCs) with wild type (WT-VSMCs). We also examined changes in the response of L2-VSMCs to insulin stimulation and to inhibition of the mammalian target of rapamycin (mTOR), a kinase critical in VSMC proliferation and migration. The L2-VSMCs exhibit greater proliferation and migration rates compared with WT-VSMCs. L2-VSMCs also exhibit a resistance to the effects of rapamycin, an mTOR inhibitor, on proliferation, migration, and cell cycle progression. The resistance to mTOR inhibition is coupled with a loss of effect on the cyclin-dependent kinase inhibitor p27Kip1, an inhibitor of cell cycle progression and VSMC migration. In response to stimulation with physiological insulin, the L2-VSMCs exhibit a loss of Akt phosphorylation and a significantly increased activation of the ERK-1/2 compared with WT-VSMCs. Insulin stimulation also decreased p27Kip1 mRNA in L2-VSMCs but not in WT-VSMCs. The effect of insulin on p27Kip1 mRNA was blocked by pretreatment with an ERK-1/2 pathway inhibitor. We conclude that loss of canonical insulin signaling results in increased ERK-1/2 activation in response to physiological insulin that decreases p27Kip1 mRNA. These data demonstrate a potential mechanism where changes in IR signaling could lead to a decrease in p27Kip1, accelerating VSMC proliferation and migration.
Diabetes mellitus is a major risk factor for cardiovascular disease (CVD), and aspects of diabetes have been associated with increased vascular smooth muscle cell (VSMC) proliferation and migration. Initial studies into the interplay of diabetes and CVD have focused on the role of hyperglycemia (1–3), inflammatory mediators (1, 4), and reactive oxygen species (5–7) on the vasculature. Diabetes is complex, and the increase in inflammation and oxidative stress under diabetic conditions clearly promotes increased CVD in the diabetic population (8, 9). However, cellular and molecular changes in the response of VSMCs to mitogenic stimuli have not been fully addressed. Recent large clinical studies have demonstrated that intensive control of blood glucose does not by itself reduce CVD events in type 2 diabetics (10–14). This finding may depend on the strategy used to achieve glucose control, or it may suggest that targeting changes in the vasculature under diabetic conditions is necessary to reduce CVD events in this population.
A critical step in VSMC proliferation and migration is the down-regulation of the cyclin-dependent kinase inhibitor, p27Kip1. Elevated levels of this protein arrest VSMCs in the G1-phase, block proliferation, and inhibit cellular migration (15, 16). Down-regulation of p27Kip1 is tightly regulated, in part, by the mammalian target of rapamycin (mTOR). mTOR is a phosphoinositol kinase that regulates cellular responses to mitogens and nutrients. Inhibition of mTOR is effective at blocking VSMC proliferation and migration in response to vascular injury (17). It exists as two functionally distinct complexes: mTOR complex (mTORC)1, identified by the presence of the regulatory associated protein of mTOR (Raptor), regulates protein translation through control of phosphorylation of p70S6kinase and 4EBP1 (18); and mTORC2 is identified by the presence of the rapamycin-insensitive companion of mTOR (Rictor) and regulates Akt phosphorylation and the cytoskeleton (19).
Because insulin resistance is the result of the loss of normal insulin signaling, we hypothesized that VSMCs in which the normal (canonical) insulin signaling pathway has been disrupted would exhibit increased proliferation and migration. Additionally, we proposed that these VSMCs would exhibit a resistance to the effects of mTOR inhibition on proliferation and migration. We compared the response of VSMCs lacking the insulin receptor (IR) with wild-type (WT)-VSMCs. Our results demonstrate that the loss of canonical insulin signaling results in changes in the regulation of VSMC function that result in increased proliferation and migration.
Materials and Methods
Cell culture
Murine VSMCs were isolated from the abdominal aortae of L2 mice and C57Bl/6 WT mice according to the protocol of Sakata et al. (20). Aortic tissue was obtained from Domenico Accili's laboratory in accordance with the Columbia University Institutional Animal Care and Utilization Committee. Greater than 90% purity of the VSMC cultures was confirmed by positive staining for α-smooth muscle actin. VSMCs were subcultured in DMEM (Invitrogen, Inc. Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS) (Invitrogen, Inc.) at 37 C in a humidified 5% CO2 atmosphere with the media being replaced approximately every 48 h.
Cell proliferation
Cell proliferation was measured using two methods, cell counts and bromodeoxyuridine (BrdU) incorporation. Equal numbers of WT-VSMCs and VSMCs lacking the IR (L2-VSMCs) were seeded at approximately 50% confluence and incubated in DMEM supplemented with 0.5% FBS for 24 h followed by incubation in DMEM supplemented with 20% FBS. For cell counts, VSMCs were trypsinized and counted at the indicated time using an Auto T4 Cellometer (Nexcelom Bioscience LLC, Lawrence, MA). For BrdU incorporation, VSMCs were incubated for 48 h in DMEM supplemented with 20% FBS and treated with 10 μm BrdU for the final 4 h. BrdU incorporation was measured using the fluorescein isothiocyanate BrdU Flow kit (BD Biosciences, San Jose, CA) according to manufacturer's instructions and analyzed using a FACSCalibur Flow Cytometry System (BD Biosciences).
Cell migration
Cell migration was measured as previously described (21). VSMCs at less than 50% confluence were pretreated with rapamycin in normal growth media for up to 48 h before seeding onto the cell inserts. VSMCs (2 × 105) were seeded into the cell inserts and incubated for 4 h over DMEM containing chemoattractant [platelet-derived growth factor (PDGF)] (10 ng/ml) or 0.1% BSA. For comparison of migration rates, data are expressed as the mean of four high power fields of triplicate samples normalized to the number of cells migrating toward media without chemoattractant. For studies of the effects of rapamycin and PD98059, data are expressed as the average of four high power fields of triplicate samples after subtraction of the average of cells migrating toward media without chemoattractant.
Cell cycle analysis
VSMCs were serum starved for 48 h, exposed to growth medium for 48 h, and harvested via trypsinization. Pelleted cells were resuspended in 100 mm sodium acetate, 5.4 mm EDTA (pH 5.2), 700 U/ml ribonuclease, 0.2% Triton X-100, and 1 μg/ml propidium iodide. DNA content was measured using a FACSCalibur, and the percentages of cells in G0/G1-phase, S-phase, and G2/M phase were determined using Flowing Software (Cell Imaging Core, Turku Centre for Biotechnology, Turku, Finland).
Western blotting and ERK-1/2 kinase assay
Western blottings were prepared as previously described (16) and probed with antibodies purchased from BD Biosciences (p27Kip1), Cell Signaling Technology (Danvers, MA) [β-actin, p-ERK-1/2 (Thr202/Thr204), total ERK-1/2, p-Akt (Ser473), total Akt, and p-p70S6kinase (Thr389)], and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) (total p70S6kinase). All primary antibodies were used at a 1:1000 dilution except for the β-actin, which was used at 1:2000. Blots were incubated with primary antibody overnight at 4 C with the exception of the p27Kip1 and p70S6kinase antibodies, which were incubated for 1 h at 25 C. Blots were incubated with secondary antibodies (Vector Laboratories, Inc., Burlingame, CA) for 1 h at 25 C. ERK-1/2 kinase activity was determined by measuring the ability of cell lysates to phosphorylate a fusion protein of glutathione S-transferase and residues 307–428 of the Ets like-1 protein (Elk-1) using the p42/44 MAPK Activity Assay kit (Cell Signaling Technology).
Real-time PCR
Total RNA was isolated from the VSMCs using the RNeasy Plus Mini kit (QIAGEN, Inc., Valencia, CA); 10 ng of total RNA from each sample were analyzed to determine the relative amounts of mRNA encoding p27Kip1 and β-actin using QuantiTect SYBR Green One-Step RT-PCR (QIAGEN, Inc.) and QuantiTect primer assays for mouse p27 (QT01058708) and mouse actin (QT01136772) (QIAGEN, Inc.). The expression of p27Kip1 was normalized to the expression of β-actin for each sample, and the fold difference between samples was calculated using the 2−ΔΔCt method. For measurement of the RNA half-life, VSMCs were treated with actinomycin D (5 ng/ml), insulin (10 nm), and either vehicle or PD98059 (20 μm). The levels of p27Kip1 and β-actin were determined as described above, and the half-life was calculated as t1/2 = ln(2)/k, where k is the first order rate of decay constant.
Statistics
All data are expressed as the mean ± sem. For comparisons across time or increasing doses of rapamycin or insulin, analysis of covariance (ANCOVA) was used to test for statistical differences with time or dose treated as a covariate. Student's t test was used to compare the means of two samples. Statistical analysis between multiple groups was performed using one-way ANOVA, and Tukey's honestly significant difference test was used to compare the individual mean values. A P value of less than 0.05 was considered significant.
Results
VSMCs lacking the IR exhibit increased proliferation and migration
To test whether loss of canonical insulin signaling affects VSMC proliferation and migration, we isolated VSMCs (L2-VSMCs) from the abdominal aortae of an Insr knockout mouse expressing the human IR under the control of the transthyretin promoter limiting expression to the liver and the pancreatic β-cells (Fig. 1A) (22). VSMCs isolated from the abdominal aortae of these mice allow examination of VSMC proliferation and migration in the absence of canonical insulin signaling.
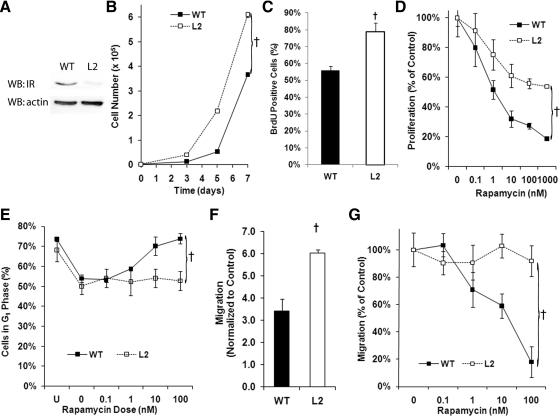
Fig. 1.
Proliferation and migration are increased in VSMCs lacking the IR. A, Representative Western blot (WB) analysis demonstrating down-regulation of the IR in VSMCs from the L2 mouse. B, Cell counts of serum-stimulated L2- and WT-VSMCs incubated for the indicated times demonstrating increased proliferation of L2-VSMCs (F = 46.28; †, P < 0.001, ANCOVA). C, BrdU incorporation by WT- and L2-VSMCs after 48 h of serum stimulation demonstrating increased proliferation of L2-VSMCs (t = 3.88; †, P < 0.05). D, Comparison of the proliferation of serum-stimulated L2- and WT-VSMCs incubated in the presence of the indicated doses of rapamycin for 72 h (F = 7.35; †, P < 0.01, ANCOVA). E, Comparison of the percentage of L2- and WT-VSMCs in the G1-phase after serum stimulation in the presence of the indicated doses of rapamycin for 24 h (F = 14.44; †, P < 0.001, ANCOVA). F, Migration of L2- and WT-VSMCs toward the chemoattractant PDGF (10 ng/ml) measured using a transwell assay (t = 6.24; †, P < 0.05). G, Comparison of L2- and WT-VSMC migration toward PDGF after pretreatment with the indicated doses of rapamycin for 48 h (F = 14.09; †, P < 0.001, ANCOVA).
The L2-VSMCs exhibited increased cell proliferation and BrdU incorporation compared with WT (Fig. 1, B and C). Given the importance of the mTOR pathway in VSMC proliferation and migration, we compared the ability of the mTOR inhibitor, rapamycin, to inhibit L2-VSMCs proliferation in response to serum stimulation. The L2-VSMCs exhibited a relative resistance to rapamycin's antiproliferative effects with an IC50 approximately 10-fold greater than the WT-VSMCs and a reduced maximal effect (Fig. 1D). To better understand the mechanisms underlying this resistance to rapamycin's effects on cell proliferation, we measured the ability of rapamycin to arrest WT- and L2-VSMCs in G1-phase. Figure 1E shows that although rapamycin led to arrest of WT-VSMCs in G1-phase, the L2-VSMCs exhibited no response.
L2-VSMCs also exhibited increased migration toward the chemoattractant PDGF (Fig. 1F). Pretreatment of VSMCs for 24–48 h with rapamycin is effective in inhibiting VSMC migration (23). In our studies, the WT-VSMCs exhibited the expected response to rapamycin pretreatment, but the L2-VSMCs exhibited a complete resistance to rapamycin's effects (Fig. 1G). These data suggest an attenuation of the role of the mTOR pathway in VSMC function when normal insulin signaling is lost.
Regulation of p27Kip1 by mTOR is lost in the L2-VSMCs
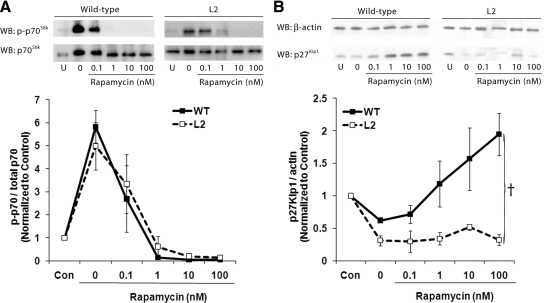
Having demonstrated a change in the cellular response to mTOR inhibition, we next measured the ability of rapamycin to inhibit the downstream effectors of mTOR. Figure 2A demonstrates that rapamycin was effective in blocking phosphorylation of p70S6kinase in both the WT- and L2-VMSCs. This suggests that the resistance to rapamycin the L2-VSMCs exhibit is not due to a loss of the ability of rapamycin to bind to its intercellular receptor, FK506 binding protein 12, and inhibit mTORC1 activity. Figure 2B demonstrates that although treatment with rapamycin induces an increase in p27Kip1 in WT-VMSCs, this increase is not observed in the L2-VSMCs. This loss of the effect may explain the loss of G1 arrest in the rapamycin-treated L2-VSMCs.
Fig. 2.
Inhibition of mTOR does not increase p27Kip1 in insulin resistant VSMCs. Representative Western blottings (WB) and densitometric analysis measuring levels of p70S6kinase phosphorylation (A) and p27Kip1 protein levels (B) in lysates obtained from WT- and L2-VSMCs after serum stimulation for 1 h (p70S6kinase) or 24 h (p27Kip1) in the presence of the indicated doses of rapamycin (F = 14.49; †, P < 0.001, ANCOVA).
Insulin stimulation of ERK-1/2 is increased in VSMCs lacking the IR
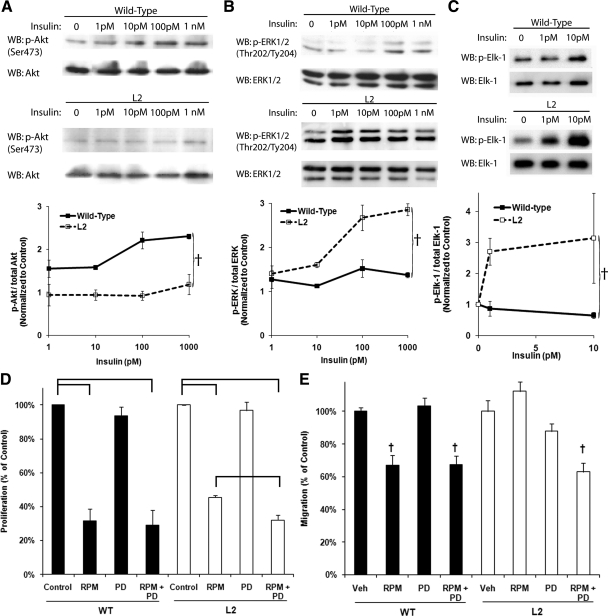
Because regulation of p27Kip1 protein levels appears shifted to a pathway other than mTOR in the L2-VSMCs, we examined how loss of the IR altered Akt and ERK-1/2 phosphorylation in response to stimulation with physiological insulin concentrations. As expected in cells lacking the IR, there was a complete loss of Akt phosphorylation in response to insulin stimulation, except at superphysiological doses (Fig. 3A). In contrast, phosphorylation of ERK-1/2 occurred at lower concentrations of insulin in the L2-VSMCs compared with the WT (Fig. 3B). We confirmed this increase in ERK-1/2 activation by measuring the ability of ERK-1/2 immunoprecipitated from lysates obtained from insulin-stimulated L2- and WT-VSMCs to phosphorylate Elk-1 in vitro (Fig. 3C).
Fig. 3.
ERK-1/2 phosphorylation and activity are increased in insulin resistant VSMCs. Representative Western blottings (WB) and densitometric analysis measuring phosphorylation of Akt (A) and ERK-1/2 (B) in lysates obtained from WT- and L2-VSMCs after stimulation with the indicated dose of insulin for 15 min (A: F = 13.35; †, P < 0.01, ANCOVA; and B: F = 16.73; †, P < 0.001, ANCOVA). C, Representative Western blottings and densitometric analysis measuring in vitro phosphorylation of Elk-1 by ERK-1/2 immunoprecipitated from lysates obtained from WT- and L2-VSMCs after stimulation with the indicated dose of insulin for 15 min (F = 9.22; †, P < 0.01, ANCOVA). D, Comparison of the proliferation of WT- and L2-VSMCs incubated in DMEM supplemented with 20% FBS and vehicle (Control), rapamycin (RPM) (10 nm), PD98059 (PD) (5 μm), or rapamycin and PD98059 (RPM+PD) for 72 h. Brackets indicate significant differences (WT: F = 37.94, ANOVA, P < 0.001; and L2: F = 144.08, ANOVA, P < 0.001). E, Comparison of the migration of WT- and L2-VSMCs pretreated with vehicle (Control), rapamycin (10 nm), PD98059 (5 μm), or rapamycin and PD98059 for 48 h (WT: F = 23.00; †, P < 0.01, ANOVA; and L2: F = 10.616; †, P < 0.01, ANOVA).
To test the importance of the increased ERK-1/2 activity in the increased proliferation and migration of the L2-VSMCs, we measured the ability of a median concentration of the ERK-1/2 pathway inhibitor, PD98059 (5 μm), to restore sensitivity to rapamycin. Although PD98059 had minimal effects on VSMC proliferation alone, in the L2-VSMCs, a synergistic response to the combination of rapamycin and PD98059 on proliferation was observed (Fig. 3D). Treatment of L2-VSMCs with PD98059 also restored the effects of rapamycin on migration (Fig. 3E). These data suggest that the increased ERK-1/2 activity in the L2-VSMCs attenuates the role of mTOR in VSMC proliferation and migration.
p27Kip1 mRNA is decreased in L2-VSMCs after insulin stimulation
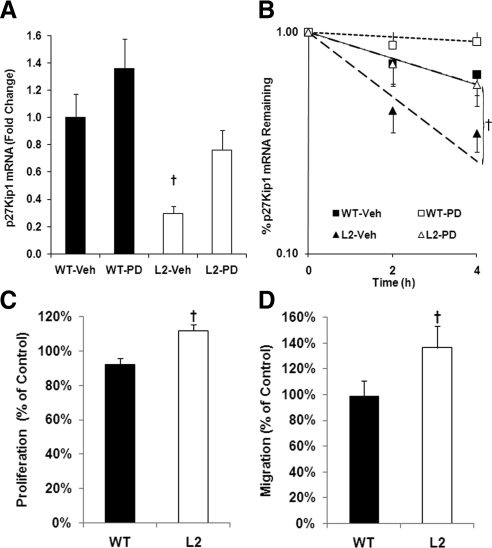
A link between ERK-1/2 activation and degradation of p27Kip1 mRNA has been previously reported in VSMCs (24). We measured the levels of p27Kip1 mRNA in L2- and WT-VSMCs stimulated for 1 h with 10 nm insulin in the presence and absence of PD98059. We found that p27Kip1 mRNA was significantly lower in the L2-VSMCs. Furthermore, inhibition of ERK-1/2 activation had little effect on p27Kip1 mRNA in the WT-VSMCs but restored normal levels of p27Kip1 in the L2-VSMCs (Fig. 4A). The half-life of p27Kip1 mRNA in insulin-stimulated L2-VSMCs is significantly reduced, and treatment with an ERK-1/2 inhibitor restored the half-life to levels similar to that of the WT-VSMCs (Fig. 4B). Our data suggest that in the absence of canonical IR signaling, insulin stimulation will increase the cellular response to mitogenic stimulation. Treatment of serum-stimulated L2-VSMCs with 1 nm of insulin resulted in a small, but significant, increase in VSMC proliferation compared with WT-VSMCs (Fig. 4C). Similarly, pretreatment with insulin (1 nm) increased L2-VSMC, but not WT-VSMC, migration toward PDGF (Fig. 4D). Thus, increased ERK-1/2 activity in the L2-VSMCs may be decreasing p27Kip1 mRNA levels and enhancing VSMC proliferation and migration. Regulation of p27Kip1 at the mRNA level would occur before the posttranslational regulation by the mTOR pathway, diminishing the role of mTOR in p27Kip1 protein regulation and VSMC function.
Fig. 4.
In L2-VSMCs, insulin stimulation reduces p27Kip1 mRNA in an ERK-dependent manner and increases proliferation and migration. A, Comparison of p27Kip1 mRNA levels in WT- and L2-VSMCs stimulated with 10 nm insulin for 1 h in the presence (PD) and absence (Veh) of 20 μm PD98059 (F = 5.06; †, P < 0.05, ANOVA). B, Comparison of the half-life of p27Kip1 mRNA in WT- and L2-VSMCs stimulated with 10 nm insulin in the presence (PD) and absence (Veh) of 20 μm PD98059. Note: The WT-Veh and L2-PD curves overlap (F = 161.03; †, P < 0.001, ANCOVA). C, Proliferation of WT- and L2-VSMCs incubated in DMEM containing 5% FBS and 1 nm insulin for 72 h. Data are normalized to WT- and L2-VSMCs incubated in DMEM containing 5% serum alone (t = 4.03; †, P < 0.05). D, Migration of WT- and L2-VSMCs toward PDGF after pretreatment with DMEM containing 0.5% FBS and 1 nm insulin for 48 h. Data are normalized to WT- and L2-VSMCs pretreated with DMEM containing 0.5% FBS (t = 3.19; †, P < 0.05).
Discussion
Development of vascular disease in diabetic patients is significantly greater than in nondiabetics. Furthermore, insulin resistance correlates with an increase in CVD, even in the absence of type 2 diabetes (25–27). This study was undertaken to determine whether a loss of canonical insulin signaling in VSMCs would lead to changes in proliferation and migration conducive to vascular disease. These data demonstrate that loss of the IR produced increases in VSMC proliferation and migration, two critical components of vascular disease progression. This increase in proliferation and migration was coupled with a relative resistance to the effects of mTOR inhibition on these processes.
Activation of mTOR promotes cell proliferation and migration through activation of protein synthesis and down-regulation of p27Kip1. Rapamycin treatment of both VSMCs led to a reduction in p70S6kinase phosphorylation, suggesting that protein synthesis is inhibited after rapamycin treatment. Furthermore, that the phosphorylation dropped below basal levels supports a role for phosphatases, such as has been proposed for protein phosphatase 2A (28–30), in the regulation of p70S6kinase by mTOR. Although the effects of mTOR inhibition on protein synthesis remain intact in the L2-VSMCs, there is a loss of effect on p27Kip1. Elevated levels of p27Kip1 in the nucleus of VSMCs block cell cycle progression and proliferation through binding and inhibition of cyclin-dependent kinase 2 complexes (31, 32). In the cytoplasm, p27Kip1 inhibits activation of members of the Ras homolog gene family and the formation of actin stress fibers (21, 33). Previous studies have shown that myogenic cell lines made rapamycin resistant through prolonged incubation in high doses of rapamycin exhibit an inhibition of p70S6kinase in response to rapamycin but maintain constitutively lower levels of p27Kip1 that were not responsive to mTOR inhibition (32). Similarly, T lymphocytes and fibroblasts isolated from p27Kip1 null mice exhibit an attenuated response to rapamycin's effects on proliferation (32). Regulation of cellular migration of both VSMCs and vascular endothelial cells has also been linked to mTOR regulation of p27Kip1 (16, 21). Because p27Kip1 acts as a check on both proliferation and migration, constitutively low levels of p27Kip1 will promote these processes independent of mTOR activity.
Previous reports have suggested that increased ERK-1/2 activity accompanies insulin resistance in vascular cells (34–36). Similarly, we observed an increase in ERK-1/2 activation in the L2-VSMCs in response to stimulation with physiological insulin. Because periods of hyperinsulinemia often accompany type 2 diabetes, this represents a potential mechanism that could promote this increase in ERK-1/2 activation, even in the absence of the mitogenic stimuli associated with vascular disease. Activation of ERK-1/2 has been linked to down-regulation of p27Kip1 mRNA in VSMCs through multiple potential mechanisms (24, 37, 38). The L2-VSMCs used in this study exhibited a similar decrease in p27Kip1 mRNA, which was blocked by treatment with an ERK-1/2 pathway inhibitor. Because mTOR regulates p27Kip1 protein levels through posttranslational effects, down-regulation of p27Kip1 mRNA through activation of ERK-1/2 would reduce the role of mTOR mediated down-regulation of p27Kip1 in VSMC proliferation and migration.
Although this model may not represent a physiological insulin resistance, given the complete absence of the IR, it does provide insight into how loss or decreases in IR holoreceptors may promote VSMC proliferation and migration. Subunits of the IR bind to IGF-I receptor (IGF-IR) subunits to form hybrid receptors that function similarly to IGF-IRs. In the db/db mouse model of type 2 diabetes, an increase in IGF-IR expression has been observed (39). Increased IGF-IR levels in type 2 diabetes may reduce the number of IR holoreceptors and produce a situation similar to that observed in the L2-VSMCs. Overexpression of IGF-IR in VSMCs attenuates insulin-stimulated Akt phosphorylation (39) similar to that observed in the L2-VMSCs.
In summary, VSMCs lacking the IR exhibited increased proliferation and migration as well as a relative resistance to mTOR inhibition. Although maintaining normal responses to mTOR inhibition with regard to p70S6kinase, the effects on p27Kip1 were lost. This loss of effect may be mediated through an increase in ERK-1/2 activation in response to insulin stimulation that reduces p27Kip1 mRNA. Taken together, these data describe how a loss of the canonical insulin signaling pathway results in increased VSMC proliferation and migration, an important component of vascular disease.
Acknowledgments
We thank Domenico Accili for the gift of the tissue from which the VSMCs isolated.
This work was supported by the Greater Southeast Affiliate of the American Heart Association Grant 0665320B and by the National Center for Research Resources at the National Institutes of Health Award P20RR018766.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANCOVA
- Analysis of covariance
- BrdU
- bromodeoxyuridine
- CVD
- cardiovascular disease
- FBS
- fetal bovine serum
- IGF-IR
- IGF-I receptor
- IR
- insulin receptor
- L2-VSMC
- VSMC lacking the IR
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR complex
- PDGF
- platelet-derived growth factor
- VSMC
- vascular smooth muscle cell
- WT
- wild type.
References
- 1. Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. 2002. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep 4:228–237 [DOI] [PubMed] [Google Scholar]
- 2. Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. 2007. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology 148:2435–2443 [DOI] [PubMed] [Google Scholar]
- 3. Jones JI, Prevette T, Gockerman A, Clemmons DR. 1996. Ligand occupancy of the α-V-β3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci USA 93:2482–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. 1997. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100:2158–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. 2000. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945 [DOI] [PubMed] [Google Scholar]
- 6. Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. 2005. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol 25:1142–1147 [DOI] [PubMed] [Google Scholar]
- 7. Nakanishi H, Brewer KA, Exton JH. 1993. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 268:13–16 [PubMed] [Google Scholar]
- 8. Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. 2010. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 3:142–148 [DOI] [PubMed] [Google Scholar]
- 9. Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB, Sr, Savage PJ, Levy D, Fox CS. 2009. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham heart study, 1950 to 2005. Circulation 119:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. 2008. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 11. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. 2008. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. 2005. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. UK Prospective Diabetes Study Group 1998. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865 [PubMed] [Google Scholar]
- 14. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. 2009. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139 [DOI] [PubMed] [Google Scholar]
- 15. Tanner FC, Yang ZY, Duckers E, Gordon D, Nabel GJ, Nabel EG. 1998. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res 82:396–403 [DOI] [PubMed] [Google Scholar]
- 16. Sun J, Marx SO, Chen HJ, Poon M, Marks AR, Rabbani LE. 2001. Role for p27(Kip1) in vascular smooth muscle cell migration. Circulation 103:2967–2972 [DOI] [PubMed] [Google Scholar]
- 17. Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. 1999. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 99:2164–2170 [DOI] [PubMed] [Google Scholar]
- 18. Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. 2008. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 30:701–711 [DOI] [PubMed] [Google Scholar]
- 19. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14:1296–1302 [DOI] [PubMed] [Google Scholar]
- 20. Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. 2004. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol 24:2069–2074 [DOI] [PubMed] [Google Scholar]
- 21. Moss SC, Lightell DJ, Jr, Marx SO, Marks AR, Woods TC. 2010. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor, p27Kip1. J Biol Chem 285:11991–11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okamoto H, Obici S, Accili D, Rossetti L. 2005. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest 115:1314–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. 1996. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest 98:2277–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakakibara K, Kubota K, Worku B, Ryer EJ, Miller JP, Koff A, Kent KC, Liu B. 2005. PDGF-BB regulates p27 expression through ERK-dependent RNA turn-over in vascular smooth muscle cells. J Biol Chem 280:25470–25477 [DOI] [PubMed] [Google Scholar]
- 25. Zethelius B, Lithell H, Hales CN, Berne C. 2005. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. A population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia 48:862–867 [DOI] [PubMed] [Google Scholar]
- 26. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW. 2005. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham offspring study. Diabetes 54:3252–3257 [DOI] [PubMed] [Google Scholar]
- 27. Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. 2002. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med 19:470–475 [DOI] [PubMed] [Google Scholar]
- 28. Peterson RT, Beal PA, Comb MJ, Schreiber SL. 2000. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275:7416–7423 [DOI] [PubMed] [Google Scholar]
- 29. Peterson RT, Desai BN, Hardwick JS, Schreiber SL. 1999. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci USA 96:4438–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterson RT, Schreiber SL. 1998. Translation control: connecting mitogens and the ribosome. Curr Biol 8:R248–50 [DOI] [PubMed] [Google Scholar]
- 31. Marx SO, Jayaraman T, Go LO, Marks AR. 1995. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res 76:412–417 [DOI] [PubMed] [Google Scholar]
- 32. Luo Y, Marx SO, Kiyokawa H, Koff A, Massagué J, Marks AR. 1996. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol 16:6744–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. 2004. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev 18:862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gogg S, Smith U, Jansson PA. 2009. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes 58:2238–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. 1999. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. 2005. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res 97:725–733 [DOI] [PubMed] [Google Scholar]
- 37. Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. 2009. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 104:476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. 2009. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284:3728–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Engberding N, San Martín A, Martin-Garrido A, Koga M, Pounkova L, Lyons E, Lassègue B, Griendling KK. 2009. Insulin-like growth factor-1 receptor expression masks the antiinflammatory and glucose uptake capacity of insulin in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 29:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]