NOX-4/p22PHOX-derived reactive oxygen species play an integral role in initiating the endometrial decidual response through regulation of CCAAT/enhancer binding protein-β.
Abstract
Differentiation of human endometrial stromal cells into specialized decidual cells is critical for embryo implantation and survival of the conceptus. Initiation of this differentiation process is strictly dependent on elevated cAMP levels, but the signal intermediates that control the expression of decidual marker genes, such as prolactin (PRL) and IGFBP1, remain poorly characterized. Here we show that cAMP-dependent decidualization can be attenuated or enhanced upon treatment of primary cultures with a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor (diphenylen iodonium) or activator (apocynin), respectively. Time-course analysis demonstrated that cAMP enhances endogenous reactive oxygen species production, apparent after 12 h of stimulation, which coincides with a dramatic increase in decidual PRL and IGFBP1 expression. Knockdown of the Rho GTPase RAC1, which disables activation of the NADPH oxidase homologs NADPH oxidase (NOX)-1, NOX-2, and NOX-3, had no effect on PRL or IGFBP1 expression. In contrast, silencing of NOX-4, or its cofactor p22PHOX, inhibited the expression of both decidual markers. Finally, we show that the NOX-4/p22PHOX complex regulates the DNA-binding activity of CCAAT/enhancer binding protein-β, a key regulator of human endometrial stromal cell differentiation. Thus, NOX-4 activation and reactive oxygen species signaling play an integral role in initiating the endometrial decidual response in preparation of pregnancy.
Decidualization denotes the differentiation of human endometrial stromal cells (HESCs) into specialized, epitheloid decidual cells that govern various aspects of embryo implantation and placenta formation. For example, differentiating HESCs are biosensors of embryo quality, capable of recognizing and responding selectively to developmentally compromised blastocysts (1, 2). Decidual cells also rapidly encapsulate the early conceptus (3), protect it from environmental stress signals (4, 5), control subsequent trophoblast invasion and placenta formation, and regulate the recruitment of specialized immune cells, such as uterine natural killer cells, to the fetomaternal interface (6). A defining feature of decidualizing HESCs is the acquisition of a secretory phenotype with two factors, prolactin (PRL) and IGF-binding protein 1 (IGFBP1), widely used as markers for this differentiation process (7–10).
In most mammals, decidualization is triggered by signals from the implanting embryo, but in human beings, and a handful of other species, this process is primarily under maternal control and initiated during the midsecretory phase of each cycle, irrespective of pregnancy (6). Contrary to what is often stated, progesterone is not the primary trigger of the decidual process, although it is essential to maintain and enhance the differentiated phenotype (8, 9, 11). Instead, there is overwhelming evidence that initiation of the decidual process is dependent on increased cellular cAMP levels and sustained activation of the protein kinase A (PKA) pathway, as reviewed in detail elsewhere (12–14). Major nuclear targets of PKA are the cAMP response element binding protein (CREB) and the related cAMP response element modulators (CREMs), transcription factors that control gene expression by binding to cAMP response elements (CREs) in target promoters. Due to alternative splicing, translation, and promoter usage, numerous CREM isoforms can be generated, often in a cell-specific manner, which serve either as transcriptional activators or repressors, depending on the presence or absence of a transactivation domain, respectively. Moreover, the CREM gene carries an internal highly sensitive, cAMP-inducible promoter, termed P2, which drives the expression of the inducible cAMP early repressor (ICER) (15). ICER is a potent repressor of cAMP-dependent promoters, including its own, and thus responsible for terminating the cAMP response (16–18).
However, cAMP signaling in differentiating HESCs does not follow this model insofar that induction of ICER is not transient, as predicted, but sustained. In other words, a negative feedback loop is not established on activation of the PKA pathway, and decidualizing HESCs remain permissive to continuous cAMP signaling (19). Furthermore, analysis of the decidual PRL promoter demonstrated that the single consensus CRE, located near the decidua-specific transcriptional start site, is important for the initial weak expression in response to cAMP signaling but dispensable for the much stronger induction, apparent after 12 h of stimulation (13). These observations suggest that initiation of the decidual process is a biphasic process, with the classic PKA/CREB/CREM pathway responsible for the rapid but very modest induction of marker genes. The cAMP-dependent signaling events responsible for the subsequent much stronger induction, apparent after 12 h of stimulation, remain ill defined (13).
Redox signaling is implicated in diverse cellular processes, including cell cycle progression, differentiation, apoptosis, and carcinogenesis (20, 21). Reactive oxygen species (ROS) are generated either as by-products of mitochondrial metabolism or as a consequence of different enzymatic reactions. The main nonmitochondrial sources of ROS are nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, enzyme complexes that transfers electron to molecular oxygen using NADPH as an electron donor to generate superoxide by one-electron reduction reaction (22). NADPH oxidase (NOX)-2, the prototypical NOX family member, is critical for the microbicidal activity of phagocytic cells (23). Upon stimulation, NOX-2, which makes up flavocytochrome b558 with its cofacor p22PHOX, assembles at the plasma membrane together with at least four cytosolic subunits (p67PHOX, p47PHOX, p40PHOX, and RAC1) to form the active complex capable of generating large amounts of ROS (24). Six homologs of NOX-2 have been identified in nonphagocytic cells, namely NOX-1, NOX-3, NOX-4, NOX-5, dual oxidase-1 (DUOX-1), and DUOX-2, which differ in not only their expression pattern but also their requirement for the cytosolic components to assemble an active NADPH oxidase complex (23). Moreover, more than one NOX isoform can be present in the same cell but often localized to different organelles, suggesting that they exert specific cellular functions (25).
Several recent studies reported that the transient activation of the NADPH oxidase complex and ROS production is an important cue for the differentiation of a number of very diverse cell types, including embryonic stem cells, megakaryocytes, vascular smooth muscle cells, and adipocytes (26–31), raising the possibility that a similar mechanism may be involved in initiating the decidual transformation of HESCs in response to cAMP signaling.
Materials and Methods
Primary cell culture
The Research Ethics Committee of the participating center approved the study: Hammersmith and Queen Charlotte's and Chelsea Research Ethics Committee (1997/5065). Written informed consent was obtained from all participating subjects before tissue collection. Endometrial samples were obtained from premenopausal women without uterine pathology, and HESCs were isolated and cultured and maintained as described previously (32). For decidual differentiation, cultures were seeded in six-well plates and treated with 0.5 mm 8-bromoadenosine-cAMP (8-br-cAMP) alone (Sigma, St Louis, MO) or in combination with 1 μm medroxyprogesterone acetate (MPA; Sigma). For activating and inhibiting the NADPH oxidase complex, cultures were treated with 1.5 mm apocynin (Sigma) or 10 μm diphenylen iodonium (DPI; Invitrogen, Carlsbad, CA), respectively. 4-(2-Amino-ethyl)benzenesulfonyl fluoride (AEBSF), allopurinol, and N-omega-nitro-l-arginine methyl ester (L-NAME; Sigma) were used at 250, 100, and 500 μm, respectively.
Transient transfection of primary cultures
Primary HESCs were transfected with DNA vectors or small interfering RNA (siRNA) oligonucleotides by the Ca3(PO4)2 coprecipitation method using the ProFection mammalian transfection kit (Promega, Madison, WI) according to the manufacturer's instructions. Reporter assays were done in 24-well plates. The concentration of reporter constructs and expression vectors was 500 and 125 ng/well, respectively. The control vector pCH110 (50 ng/well), which leads to constitutive β-galactosidase expression, was used to compare transfection efficiency. The CCAAT/enhancer-binding protein (C/EBP)-pGL4 reporter construct was a kind gift from Dr. P. Bennett (Imperial College London, London, UK). The catalase vector was a kind gift from Dr. Stefan Grimm (Imperial College London) Selective expression vector pSG-C/EBP have been previously described (33). HESCs were lysed in 50 μl of reporter lysis buffer in each well. The plate was then put on an orbital shaker for 15 min at 4 C and then frozen at −80 C. After thawing the lysate at room temperature, the firefly luciferase activity in the lysates was assayed by addition of 10 μl of the lysate to 50 μl luciferase assay reagent, containing substrate and assay buffer (Promega) and reading the luminescence on a Victor II plate reader (PerkinElmer, Boston, MA). For gene-silencing studies, HESCs were cultured in six-well plates until confluency and transiently transfected with 100 nm of the following siRNA reagents (Dharmacon, Lafayette, CO): siCONTROL nontargeting (NT) siRNA pool, RAC1 siGENOME SMARTpool siRNA, NOX4 siGENOME SMARTpool siRNA, and p22PHOX siGENOME SMARTpool siRNA.
Western blot analysis
Whole-cell protein extracts were obtained by direct lysis in Laemmli buffer heated to 100 C. Proteins resolved by SDS-PAGE were transferred to a polyvinyl difluoride membrane (GE Healthcare, Uppsala, Sweden) and probed with antibodies raised against Ras-related C3 botulinum toxin substrate (RAC)-1, 1:1000 [MAB3735 (Chemicon, Temecula, CA), Merck, KGaA (Darmstadt, Germany)]; NOX4, 1:1000 (kindly provided by J. D. Lambeth, Emory University, Atlanta, GA), p22phox (FL-195; Santa Cruz Biotechnology, Santa Cruz, CA); steroid receptor coactivator (SRC), 1:1,000 (L4A1; Cell Signaling, Beverly, MA); C/EBP-β, 1:400 (sc-150; Santa Cruz Biotechnology); Forkhead box O1 (FOXO1), 1:1,000 (C29H4; Cell Signaling); signal transducer and activator of transcription (STAT)-5, 1:1,000 [(Upstate, Lake Placid, NY) Merck, KGaA)]; β-actin, 1:100,000 (A1978; Sigma); α-tubulin, 1:1,000 (sc-8035; Santa Cruz Biotechnology); lamin B (N412; Calbiochem, San Diego, CA). After incubation with horseradish peroxidase-conjugated secondary antibodies, 1:2500, (Roche Diagnostics, Mannheim, Germany), chemiluminescence was visualized using the ECL+ chemoluminescent detection kit (Amersham, Little Chalfont, UK).
Real-time quantitative PCR
Total RNA was extracted from primary HESC cultures using STAT-60 reagent (AMS Biotechnology, Milton Park, Abingdon, UK). After treatment with amplification grade deoxyribonuclease I (Invitrogen), cDNA was generated using the SuperScript first-strand synthesis system for RT-PCR kit (Invitrogen). Template quantification was performed with an ABI Step One system (Applied Biosystem, Foster City, CA) using SYBR Green JumpStart Taq ReadyMix (Sigma) as dye layer and the relative standard curve calculation method. RNA input variances were normalized against the levels of the L19 housekeeping gene, which encodes a ribosomal protein. All measurements were performed in triplicate. Specific primer pairs were designed using Primer3 software (http://frodo.wi.mit.edu): L19 sense, 5′-GCG GAA GGG TAC AGC CAA T-3′, L19-R antisense, 5′-GCA GCC GGC GCA AA-3′; decidual PRL sense, 5′-AAG CTG TAG AGA TTG AGG AGC AAA C-3′, decidual PRL antisense, 5′-TCA GGA TGA ACC TGG CTG ACT A-3′; IGFBP1 sense, 5′-CGA AGG CTC TCC ATG TCA CCA-3′, IGFBP1 antisense, 5′-TGT CTC CTG TGC CTT GGC TAA AC-3′; Nox4 sense, 5′-AGC AAC ATT TGG GGT TCA TC-3′, Nox4 antisense, 5′-GGC AGA ATT TCG GAG TCT TG-3′; p22PHOX sense, 5′-ATG ACC GCC GTG GTG AAG-3′, p22PHOX antisense, 5′-GGC ACC GAG AGC AGG AGA-3′; RAC1 sense, 5′-TGC AGA CAC TTG CTC TCC TAT GTA G-3′, RAC1 antisense, 5′-GAG TTC AAT GGC AAC GCT TCA-3′.
Electrophoretic mobility shift assay
Five micrograms of nuclear protein extracts was incubated for 1 h with nonradiolabeled nonspecific [octamer transcription factor-1 (OCT1)] and specific oligonucleotide (C/EBP) in binding buffer [20% (vol/vol) glycerol; 5 mm MgCl2, 2 mm EDTA; 50 mm Tris-HCL, pH 7.5; 250 mm NaCl; and 2 mm dithiothreitol], followed by a 45-min incubation with 0.035 pmol [γ-32P]ATP ended-labeled oligonucleotide probes. DNA-protein complex was separated from unbound DNA probe on a 4% nondenaturing acrylamide gel in 0.25× running buffer of 9 mm Tris-borate and 0.2 mm EDTA (pH 8.0). Gels were vacuum dried and exposed to Amersham MP films at −80 C for 16–48 h. For supershift analysis, samples were incubated with 2 μg anti-C/EBPβ antibody (sc-150; Santa Cruz Biotechnology) for 1 h on ice before incubation with the probe. The consensus oligonucleotides for OCT1 were purchased from Promega Life Science (E 3241), and Santa Cruz Biotechnology for C/EBP (sc-2525).
Measurement of ROS in primary HESCs
Cultured HESCs were seeded in 96-well black plates with clear bases and maintained in 10% dextran-coated charcoal/DMEM until they become confluent. Cells in which either decidualized or left untreated. Thirty minutes before measuring the amount of ROS, cultured HESCs were loaded with 2′,7′-dichlorodihydrofluoresein diacetate (DCFH-DA) at a final concentration of 30 μm and incubated at 37 C in a cell culture incubator in a humid condition with 5% (vol/vol) CO2 for 30 min. The cells were then washed twice with PBS, and the appropriate media with or without the treatment were added to the cells. H2O2 was used as a positive control to ensure the validity of the DCFH-DA probe. Measurement was then taken using the fluorometer at an exitation and emission wavelengths of λ-485 and λ-535, respectively, every 2 min for a 30-min period. To control for the amount of cells in the wells, the media were aspirated and the cells were washed twice with PBS. Consequently the cells were stained with propodium iodide (PI) prepared in a 1% Nonidet P-40-PI 10 μg/ml solution. The plate was placed on an orbital shaker for 15 min at room temperature. Measurement was then taken using the fluorometer at an exitation wavelength λ-535, and emission wavelength λ-600. When 2′,7′-dichlorofluoresein (DCF) fluorescence was measured using flow cytometry, HESCs were cultured and treated in six-well plates. The cells were then stained with DCF diacetate/PI (30 μm/10 μg) in suspension for 30 min, washed, and resuspended in PBS. Subsequently DCF and PI fluorescence were measured using the following flow cytometer detectors, respectively, FL-H1 and FL-3H.
Statistical analysis
Data presented in this study are a representative of three or more biological replicates (i.e. primary cultures established from different biopsies). Statistical analysis was performed using a Student's t test after normalization of the data. The level of significance was defined as P < 0.05.
Results
NADPH oxidase-dependent ROS signaling in differentiating HESCs
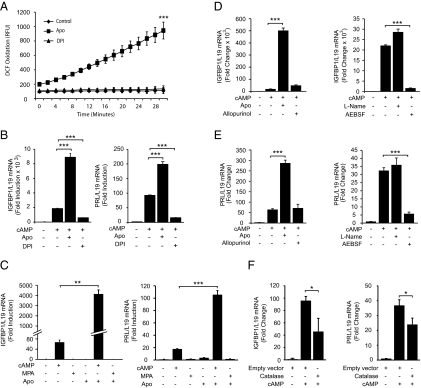
DPI and apocynin are chemicals widely used to inhibit or activate the NADPH oxidase complex, respectively (23). However, the effects of apocynin are cell type specific. For example, it acts as an inhibitor of the NADPH oxidase complex in phagocytes but stimulates ROS production in other cells, including vascular fibroblasts (23, 34, 35). In undifferentiated HESCs, apocynin strongly induced intracellular ROS production, as measured by the oxidation of 2′,7′-(DCFH-DA to a fluorescent DCF, whereas DPI had no discernible effect (Fig. 1A). These observations suggest that HESCs contain functional NADPH oxidase complexes, yet basal ROS production appears low.
Fig. 1.
NADPH-dependent ROS production upon initiation of the decidual process. A, Undifferentiated primary HESCs were loaded with DCFH-DA for 30 min and then pulsed with apocynin (Apo) or DPI. DCF fluorescence was measured every 2 min over a 30-min period. The data represent the mean DCF oxidation level (±sem) at each interval. B, HESCs were maintained untreated or treated with 8-br-cAMP in the presence or absence of Apo or DPI for 24 h. Quantitative RT-PCR (RTQ-PCR) analysis was carried out for PRL and IGFBP1 mRNA levels normalized to L19 mRNA. Data are depicted as fold induction relative to transcript levels of untreated samples and represent means (±sd) of triplicate determinations. C, HESCs were either maintained untreated or treated with 8-br-cAMP or MPA in the presence or absence of Apo or with Apo alone for 24 h. Total RNA was harvested and RTQ-PCR analysis was carried out for IGFBP1 and PRL, normalized to L19 transcript levels. Data are depicted as fold induction relative to transcript levels of untreated samples and represent means (±sd) of triplicate determinations. D and E, Primary HESCs were maintained untreated or treated with 8-br-cAMP in the presence or absence of Apo, allopurinol, L-NAME, or AEBSF. RTQ-PCR analysis was carried out for PRL and IGFBP1 mRNA levels normalized to L19 mRNA. Data are depicted as fold induction relative to transcript levels of undifferentiated samples and represent means (±sd) of triplicate determinations. F, Primary cultures were transfected with a catalase expression vector or empty vector. The cells were treated 2 d later with 8-br-cAMP for 24 h. Total mRNA was harvested and PRL and IGFBP1 transcript levels were measured using RTQ-PCR analysis. Data are depicted as fold induction relative to transcript levels of undifferentiated samples and represent means (±sd) of triplicate determinations. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether NADPH oxidase activation plays a role in HESC differentiation, primary cultures were decidualized with 8-br-cAMP, in the presence or absence of apocynin or DPI, and the transcript levels of two decidual marker genes, IGFBP1 and PRL, measured after 24 h. As shown in Fig. 1B, apocynin markedly increased the expression of both decidual markers in 8-br-cAMP-treated cells, whereas DPI exerted an inhibitory effect. Notably, treatment with apocynin, alone or in combination with the progestin MPA, was insufficient to induce either IGFBP1 or PRL expression (Fig. 1C), indicating that the effect of endogenous ROS signaling on HESC differentiation is dependent on simultaneous activation of the cAMP pathway.
Although DPI is a widely used inhibitor of the NADPH oxidase complex, it can also antagonize ROS production from other sources, such as the xanthine oxidase and nitric oxide synthase. To determine the mechanism of the inhibitory effect of DPI on the decidual phenotype, HESCs were treated with 8-br-cAMP for 24 h in the presence or absence of a xanthine oxidase inhibitor (allopurinol) or the nitric oxide synthase inhibitor L-NAME. Both chemicals failed to antagonize IGFBP1 and PRL expression, whereas treatment with AEBSF, another NOX inhibitor, mimicked the effects of DPI (Fig. 1, D and E).
Although the activated NADPH oxidase generates superoxide from molecular oxygen, this can be rapidly dismutated to hydrogen peroxide, and both radicals have been implicated in signal transduction. Overexpression of a vector encoding catalase, a highly efficient enzyme that converts hydrogen peroxide to water and oxygen, also attenuated the subsequent induction of PRL and IGFBP1 transcripts in 8-br-cAMP-treated HESCs (Fig. 1F), which provides further evidence that endogenous ROS signaling plays an integral role in initiating the decidual response. Catalase expression was validated by Western blot analysis (data not shown).
Kinetics of cAMP-dependent NADPH oxidase activation
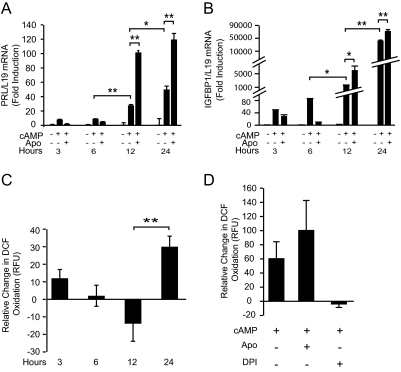
Based on the kinetics of cAMP-dependent induction of the decidual PRL promoter activity in reporter assays (14), initiation of the HESC differentiation has been described as a biphasic process, characterized by an initial rapid but modest response, followed by a sharp rise in promoter activity after 12 h of stimulation. To provide insight into the role of endogenous ROS signaling, we repeated these time-course studies in primary cultures treated with 8-br-cAMP, alone or in combination with apocynin, but measured endogenous PRL and IGFBP1 expression. As shown in Fig. 2, A and B, PRL and IGBP1 transcript levels rose by 8- and 48-fold, respectively, after 3 h of 8-br-cAMP treatment, and the levels remained relatively unchanged at 6 h, before rising sharply after 12 h of treatment. If anything, cotreatment with apocynin attenuated the early response somewhat before enhancing PRL and IGFBP1 expression from 12 h onward.
Fig. 2.
Kinetics of cAMP-dependent ROS generation in differentiating HESCs. A and B, Cultured HESCs were treated or untreated with 8-br-cAMP in the presence or absence of apocynin (Apo) for 3, 6, 12, and 24 h. PRL (A) and IGFBP1 (B) transcript levels were normalized to L19 mRNA. Data are depicted as fold induction relative to transcript levels of untreated samples and represent means (±sd) of triplicate determinations. C, HESCs were treated or untreated with 8-br-cAMP for 3, 6, 12, and 24 h. Cultures were loaded with DCFH-DA (30 μm) 25 min before terminating the treatments. DCF fluorescence measurement was taken every 2 min over a 30-min period using a fluorometer. Data are depicted as the difference in ROS production between 8-br-cAMP treated and untreated cultures and represent means (±sem) of sextuplicate determinations. D, Primary cultures were untreated or treated with 8-br-cAMP for 24 h and then pulsed with Apo or DPI for 30 min. Subsequently the cells were loaded with DCF-DA for 25 min and DCF fluorescence was measured using flow cytometry. *, P < 0.01; **, P < 0.001.
Next we set out to determine the kinetics of changes in cellular ROS production on initiation of the decidual process. To do this, primary cultures, treated with 8-br-cAMP for various time points, were loaded with the oxidant-sensing fluorescent DCFH-DA probe and the level of fluorescence normalized to that of parallel untreated cultures. Surprisingly, a triphasic response was observed in all cultures tested (n = 4), characterized by a modest increase in DCF fluorescence on 3 h of 8-br-cAMP treatment, followed by a gradual decline, with levels falling below those of control cultures by 12 h, and finally a sharp rise between 12 and 24 h (Fig. 2C). Thus, cAMP signaling triggers rapid, complex, but coordinated changes in DCF fluorescence, characterized most prominently by a burst in prooxidant activity between 12 and 24 h, which in turn coincided with the marked induction of the decidual marker genes and their responsiveness to apocynin. Flow cytometry confirmed that 8-br-cAMP enhances cellular oxidation by 24 h, and this response was abolished on cotreatment with DPI but enhanced by apocynin (Fig. 2D).
Decidualization requires NOX-4 activation
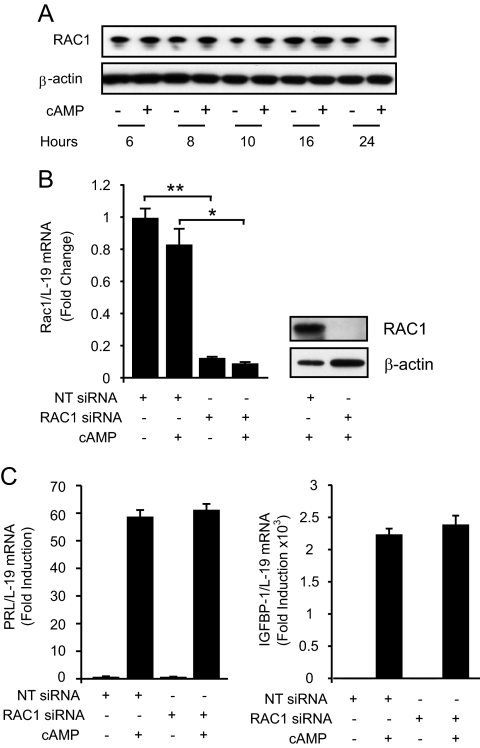
We set out to characterize the NOX homolog(s) responsible for ROS signaling in differentiating HESCs. RAC GTPases are master activators of the catalytic subunits NOX-1, NOX-2, and to a lesser extent NOX-3 (23). A previous study demonstrated that HESCs selectively express RAC1 and that this Rho family member critically regulates embryo implantation (36). To determine whether the induction of the decidual phenotype is RAC1 dependent, we first treated primary cultures with 8-br-cAMP for 24 h and harvested total protein lysates for Western blot analysis. RAC1 levels were comparable in undifferentiating and decidualizing cells, at least when differentiated for 24 h (Fig. 3A). Next, we transfected cultures with NT or RAC1 siRNA and 2 d later treated the cells with 8-br-cAMP for 24 h. Although RAC1 silencing was highly efficient, both at mRNA and protein level (Fig. 3B), it had no effect on the induction of PRL or IGFBP1 transcripts in differentiating cells (Fig. 3C). Thus, initiation of the decidual process is RAC1 independent, rendering it unlikely that NOX-1, NOX-2, or NOX-3 is the primary source of ROS generation and signaling in differentiating HESCs.
Fig. 3.
RAC1 is not required for the induction of the decidual markers. A, Cultured HESCs were untreated or treated with 8-br-cAMP for 24 h. Whole-cell lysates were extracted and blotted for RAC1 expression. β-Actin served as a loading control. B, The left panel shows quantitative RT-PCR (RTQ-PCR) analysis of RAC1 transcript levels, normalized to L19 mRNA, in cells transfected with NT or RAC1 siRNAs and treated 2 d later with 8-br-cAMP for 24 h. Data are depicted as fold change relative to transcript levels of undifferentiated samples and represent means (±sd) of triplicate determinations. The right panel shows Western blot analysis of RAC1 expression in protein lysates from parallel cultures. β-Actin served as a loading control. C, RTQ-PCR analysis for PRL and IGFBP1 levels, normalized to L19 mRNA, upon RAC1 silencing. B, The results are fold change relative to transcript levels of undifferentiated samples transfected with NT siRNA and represent means (±sd) of triplicate determinations. *, P < 0.01; **, P < 0.001.
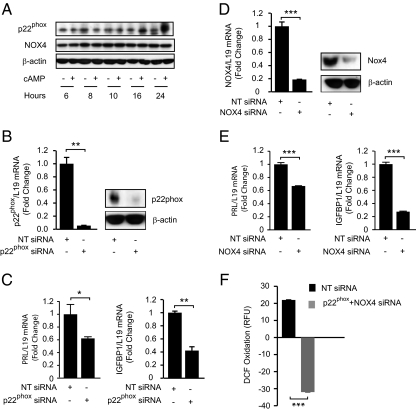
We next focused on p22PHOX, the subunit essential for the catalytic activities of NOX-1 to -4 but not for NOX-5 or DUOX-1/2 (22, 23). Interestingly, p22PHOX expression at protein level increased in response to 8-br-cAMP when the cells were treatment for 16 h or longer (Fig. 4A). In short-term cultures, siRNA-mediated knockdown of p22PHOX was sufficient to reduce cAMP-depedendent induction of PRL and IGFBP1 transcripts by 38 and 62%, respectively (Fig. 4, B and C). The observation that induction of decidual marker genes in response to cAMP signaling is p22PHOX dependent, yet RAC1 independent, suggested that NOX4 could be a major source of ROS production in differentiating HESCs. Like p22PHOX, NOX-4 expression levels increase on decidualization, but this was strictly a delayed response apparent after 2 or more days of differentiation (Fig. 4A and data not shown). As expected, NOX-4 knockdown was as effective as p22PHOX silencing in dampening the induction of decidual PRL and IGFBP1 in cultures treated with 8-br-cAMP for 24 h (Fig. 4, D and E).
Fig. 4.
p22PHOX and NOX4 knockdown perturbs cAMP-dependent HESC decidualization. A, Cultured HESCs were untreated or treated with 8-br-cAMP for 6, 8, 10, 16, and 24 h. Whole-cell lysates were extracted and blotted for p22PHOX and NOX4 expression. β-Actin served as a loading control. B, Validation of p22PHOX knockdown at mRNA (left) and protein level (right) in HESCs transfected with NT or p22PHOX siRNAs. C, Quantitative (RTQ-PCR) analysis for both PRL and IGFBP1 levels, normalized to L19 mRNA upon p22PHOX silencing. D, Validation of NOX4 knockdown at mRNA (left) and protein level (right) in HESCs transfected with NT or NOX4 siRNAs. E, RTQ-PCR analysis for both PRL and IGFBP1 levels, normalized to L19 mRNA upon NOX4 silencing. Data in C and E are depicted as fold change relative to transcript levels of samples transfected with NT siRNA and represent means (±sd) of triplicate determinations. F, Primary cultures were transfected with NT siRNA or siRNA targeting p22PHOX and NOX4 and treated 2 d later with 8-br-cAMP for 24 h. The cells were then loaded with DCF-DA for 30 min and DCF fluorescence was measured over a 30-min period. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next set out to determine whether p22PHOX/NOX-4 activation underpins the changes in cellular redox on HESC differentiation. Although NOX-4 knockdown had little effect on the changes in DCF fluorescence in HESCs treated with 8-br-cAMP for 12 h or less (data not shown), it abolished the rise in cellular oxidation above that of control cells by 24 h of differentiation (Fig. 4F).
NOX-4-dependent ROS signaling enhances C/EBPβ activity
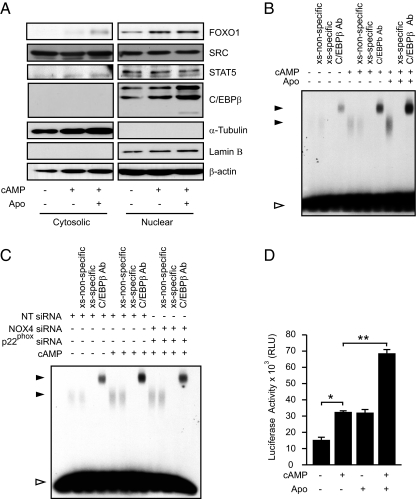
Decidualization of HESCs is associated with the induction or activation of a number of redox-sensitive transcription factors and kinases, most prominently FOXO1, STAT5, and SRC kinase (32, 37–40). However, activation of these signal intermediates tends to be a delayed response, apparent after 2 or more days of differentiation (12). A notable exception is C/EBPβ, which is induced in HESCs between 8 and 24 h of 8-br-cAMP treatment (33). In agreement, Western blot analysis of cytosolic and nuclear protein extract demonstrated enrichment of nuclear C/EBPβ in HESCs treated with 8-br-cAMP for 24 h, a response further enhanced on cotreatment with apocynin (Fig. 5A). Conversely, apocynin had no effect on 8-br-cAMP-dependent subcellular distribution of FOXO1, STAT5, or SRC, at least not within the first 24 h of the differentiation process (Fig. 5A).
Fig. 5.
C/EBPβ is a downstream mediator of NADPH oxidase produced ROS signaling in HESCs. A, Cultured HESCs were untreated or treated for 24 h with 8-br-cAMP in the presence or absence of apocynin (Apo) and/or DPI. Cytosolic/nuclear extracts were prepared and blotted for SRC, STAT5, FOXO1, and C/EBPβ. The expression of lamin B and α-tubulin was assessed to confirm the integrity of the cell fractionation. β-Actin was used as a loading control. B, HESCs were treated for 24 h with 8-br-cAMP in the presence or absence of Apo, and DNA binding of CEBPβ contained in the nuclear extracts was analyzed by EMSA. Nuclear lysates were incubated with excess of [32P]-labeled C/EBP, OCT1 (xs nonspecific) and unlabeled C/EBP (xs specific) oligonucleotides. Both OCT1 and unlabeled C/EBP oligonucleotides served as DNA binding competitors to verify binding specificity. For supershift analysis, nuclear extracts were incubated with an antibody against C/EBPβ before addition of the hot probe. Solid arrowhead indicate position of specific complexes; open arrowhead marks the position of uncomplexed DNA probe. C, Undifferentiated HESCs were transfected with NT siRNA or siRNA targeting p22PHOX and NOX4 and treated 2 d later with 8-br-cAMP. DNA binding of C/EBPβ contained in the nuclear extracts was analyzed by EMSA. To verify DNA binding, binding specificity and for supershift analysis, nuclear lysates were incubated with excess of [32P]-labeled C/EBP, OCT1, and unlabeled C/EBP oligonucleotides. For supershift analysis, nuclear extracts were incubated with an antibody against C/EBPβ before addition of the hot probe. Solid arrowhead indicate position of specific complexes; open arrowhead marks the position of uncomplexed DNA probe. D, Primary cultures were transfected with C/EBP-pGL4, pSG5-C/EBPβ (encoding for full length C/EBPβ), and pCH110 (encoding for β-galactosidase). The cells were left untreated or treated with 8-br-cAMP, Apo, or the combination of 8-br-cAMP and Apo for 24 h. Subsequently firefly luciferase activity was measured. *, P < 0.01; **, P < 0.001.
Next, we used EMSAs to substantiate the notion that endogenous ROS signaling on HESC differentiation impacts on C/EBPβ activity. As shown in Fig. 5B, 8-br-cAMP stimulated endogenous C/EBPβ DNA-binding activity in HESCs, and again this response was enhanced on cotreatment with apocynin. Conversely, siRNA-mediated NOX-4/p22PHOX knockdown before 8-br-cAMP treatment attenuated the induction of C/EBPβ DNA-binding activity. Transfection of HESCs with a luciferase reporter under the control of two cognate response elements revealed that apocynin enhances C/EBP-dependent transcription to the same degree as 8-br-cAMP (Fig. 5D). Moreover, combined treatment of apocynin and 8-br-cAMP in HESCs resulted in an additive response.
Discussion
Decidualization of the endometrial stromal compartment is an obligatory event in all species in which implantation involves breaching of the luminal endometrial epithelium by the embryo (41, 42). Depending on severity, failure to express an adequate decidual phenotype results either in implantation failure, early pregnancy loss, or predisposes to obstetrical complications associated with impaired placental function, such as preeclampsia, fetal growth restriction, and preterm birth (42). The onset of the decidual process is exquisitely regulated, temporally as well as spatially, starting in the stromal cells underlying the luminal epithelium approximately 9 d after the postovulatory rise in circulating progesterone levels. Initiation of this process in vivo coincides with elevated endometrial cAMP production, which is accounted for by increased expression of local factors that activate adenylate cyclase in stromal cells (e.g. relaxin, CRH, and prostaglandin E2) and by the down-regulation of phosphodiesterase-4, a phosphodiesterase family member that converts cAMP back to AMP (43–47). Upon continuous cAMP stimulation, HESCs become first responsive and then dependent on progesterone signaling for maintaining the decidual phenotype (48).
PRL in the endometrium is transcribed from an alternative promoter upstream of a noncoding exon, located approximately 6 kb upstream of the pituitary-specific transcriptional start site (49). Its expression is under control of a transposable element, termed MER20, which emerged in evolution on divergence of eutherian (placental) from nonplacental mammals (10, 50). MER20 is located at −395/−148 relative to the decidual PRL transcription start site and contains response elements for many transcription factors indispensable for decidualization and pregnancy, including the forkhead protein FOXO1, the homeobox protein HOXA11, v-ets erythroblastosis virus E26 oncogene homolog 1, C/EBPβ, and progesterone receptor (51). In fact, MER20 enhancer regions are found in the vicinity of many decidua-specific genes. Although our knowledge is still fragmented, it is increasingly apparent that decidual PRL expression involves sequential and highly coordinated actions of specific transcription factors. As mentioned, the initial weak response to cAMP stimulation reflects binding of CREB to a nonpalindromic CRE at position −12 of the decidual PRL promoter (14), thus outside the MER20 enhancer region. Binding of C/EBPβ to a core sequence within MER20 then triggers a more pronounced response, apparent after 12 h of cAMP stimulation. C/EBPβ in turn has been shown to bind FOXO1 (37) and recruitment of FOXO1 to MER20 converts HOXA11 from a repressor to a strong activator of the promoter (51). It is now widely accepted that expression of decidual genes depends on the formation of distinct multimeric transcriptional complexes, which in turn are regulated by upstream signaling pathways and in response to distinct posttranslational modifications, such as sumoylation (11).
Here we present several lines of evidence that implicate redox signaling in the initiation of the decidual process. First, we demonstrated that apocynin, a formidable inducer of endogenous ROS production in HESCs, strongly enhances cAMP-dependent decidual PRL and IGFBP1 expression in primary cultures, whereas DPI, AEBSF, and catalase had the opposite effect. Notably, the effect of apocynin was delayed and only apparent after 12 h of cAMP stimulation, which pointed toward possible redox regulation of C/EBPβ activity. Furthermore, apocynin alone did not induce the expression of these decidual markers, nor did it sensitize the cells to progestins like MPA and thus reinforces the indispensable role of cAMP signaling in the decidual process. Second, cAMP signaling triggered rapid but complex changes in DCF fluorescence. The results, however, should be interpreted with some caution. For example, DPI had no discernible effect on DCF oxidation in undifferentiated cells, indicating that basal ROS production is low, yet treatment with 8-br-cAMP triggered first a reduction in DCF fluorescence before a marked increase between 12 and 24 h. It is possible that rapid cAMP effects on the cytoskeleton and secretome of HESCs interfere with the uptake or retention of the probe, thereby accounting for the initial rise and decline in DCF fluorescence. On the other hand, the subsequent increase in cellular oxidation fits well with the up-regulation of p22PHOX. Furthermore, NOX-4 knockdown had no effect on the initial transient fluctuations in DCF fluorescence but abolished the rise in cellular oxidation by 24 h of differentiation. Next, we showed that either p22PHOX or NOX4 knockdown is sufficient to impair PRL and IGFBP1 expression in differentiating HESCs. In contrast, RAC1 silencing had no discernible effect, indicating that NOX-1, NOX-2, and NOX-3 are not major sources of cAMP-dependent ROS production in differentiating HESCs. However, we cannot exclude a role for these homologs in regulating subsequent aspects of the decidual process. Finally, we demonstrated that C/EBPβ is a major downstream target of the activated p22PHOX/NOX-4 complex. C/EBPβ is not only essential for reproduction in all eutherian mammals tested to date (32, 52–54), but it is also a key regulator of differentiation in numerous other cell types, perhaps most prominently so in adipocytes (55). Interestingly, a recent study demonstrated that low levels of ROS trigger phosphorylation and activation of C/EBPβ in 3T3-L1 preadipocytes, which in turn promotes mitotic clonal expansion and terminal adipocyte differentiation (55). As reported for an increasing number of cell types, NOX-4 was subsequently identified as a major source of ROS production during adipocyte differentiation (26). Combined with our findings, these observations suggest NOX-4-dependent redox regulation of C/EBPβ activity may be a highly conserved mechanism that controls differentiation of very diverse cell types.
Our study also raises a number of important but as-yet-unanswered questions. For example, whereas the p22PHOX/NOX-4 complex has been described to be constitutively active (56) and p22PHOX is modestly induced in response to cAMP treatment, it appears likely that other factors contribute to the activation of this complex in decidualizing HESCs. Furthermore, p22PHOX as well as NOX-4 is strongly induced on prolonged decidualization with 8-br-cAMP and MPA (data not shown), suggesting that continuous endogenous ROS production and redox signaling are important for the maintenance of the decidual phenotype, a hypothesis currently under investigation. It is also important to note that apocynin strongly activated a luciferase reporter driven by consensus C/EBP response elements in undifferentiated HESCs, yet it was insufficient to elicit PRL or IGFBP1 expression. Thus, cAMP signaling must exert additional effects that couple ROS-dependent C/EBPβ activation to the expression of decidual genes.
In summary, we have shown that redox signaling plays an integral role in initiating the endometrial differentiation program in preparation of pregnancy. We identified the p22PHOX/NOX-4 complex as the major source of ROS in decidualizing HESCs and C/EBPβ as an important downstream effector molecule. Interestingly, NOX4, which encodes for the catalytic subunit of the NADPH oxidase complex, was recently identified as a gene perturbed in eutopic endometrial cells from women with endometriosis (57), suggesting that perturbed redox signaling may clinically contribute to impaired decidualization and subsequent reproductive failure.
Acknowledgments
The authors are grateful to Drs. J. D. Lambeth (Emory University Medical School, Atlanta, GA), P. Bennett (Imperial College London, London, UK), and S. Grimm (Imperial College London, London, UK) for providing us with reagents. We are also indebted to all the patients who participated in this study.
M.A.-S. was supported by the Crown Prince International Scholarship Program, Kingdom of Bahrain, and the Overseas Research Student Fund, Imperial College London. J.J.B. received support from National Institute for Health Research Biomedical Research Centre funding scheme.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AEBSF
- 4-(2-Amino-ethyl)benzenesulfonyl fluoride
- 8-br-cAMP
- 8-bromoadenosine-cAMP
- C/EBP
- CCAAT/enhancer-binding protein
- CRE
- cAMP response element
- CREB
- cAMP response element binding protein
- CREM
- cAMP response element modulator
- DCF
- 2′,7′-dichlorofluoresein
- DCFH-DA
- 2′,7′-dichlorodihydrofluoresein diacetate
- DPI
- diphenylen iodonium
- DUOX-1
- dual oxidase-1
- FOXO1
- Forkhead box O1
- HESC
- human endometrial stromal cell
- ICER
- inducible cAMP early repressor
- IGFBP1
- IGF-binding protein 1
- L-NAME
- N-omega-nitro-l-arginine methyl ester
- MPA
- medroxyprogesterone acetate
- NADPH
- nicotinamide adenine dinucleotide phosphate
- NOX
- NADPH oxidase
- NT
- nontargeting
- OCT1
- octamer transcription factor-1
- PI
- propodium iodide
- PKA
- protein kinase A
- PRL
- prolactin
- RAC
- Ras-related C3 botulinum toxin substrate
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- SRC
- steroid receptor coactivator
- STAT
- signal transducer and activator of transcription.
References
- 1. Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ. 2010. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One 5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS. 2010. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One 5:e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. 2010. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 25:862–873 [DOI] [PubMed] [Google Scholar]
- 4. Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham JM, Lam EW, Brosens JJ. 2006. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol 20:2444–2455 [DOI] [PubMed] [Google Scholar]
- 5. Leitao B, Jones MC, Fusi L, Higham J, Lee Y, Takano M, Goto T, Christian M, Lam EW, Brosens JJ. 2010. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J 24:1541–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 7. Hochner-Celnikier D, Ron M, Eldor A, Segal S, Palti Z, Fuks Z, Vlodavsky I. 1984. Growth characteristics of human first trimester decidual cells cultured in serum-free medium: production of prolactin, prostaglandins and fibronectin. Biol Reprod 31:827–836 [DOI] [PubMed] [Google Scholar]
- 8. Brosens JJ, Hayashi N, White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 9. Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkilä M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. 2008. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149:4462–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch VJ, Tanzer A, Wang Y, Leung FC, Gellersen B, Emera D, Wagner GP. 2008. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc Natl Acad Sci USA 105:14928–14933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. 2006. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA 103:16272–16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gellersen B, Brosens J. 2003. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 [DOI] [PubMed] [Google Scholar]
- 13. Telgmann R, Gellersen B. 1998. Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 4:472–479 [DOI] [PubMed] [Google Scholar]
- 14. Telgmann R, Maronde E, Taskén K, Gellersen B. 1997. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937 [DOI] [PubMed] [Google Scholar]
- 15. Servillo G, Della Fazia MA, Sassone-Corsi P. 2002. Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res 275:143–154 [DOI] [PubMed] [Google Scholar]
- 16. Lamas M, Molina C, Foulkes NS, Jansen E, Sassone-Corsi P. 1997. Ectopic ICER expression in pituitary corticotroph AtT20 cells: effects on morphology, cell cycle, and hormonal production. Mol Endocrinol 11:1425–1434 [DOI] [PubMed] [Google Scholar]
- 17. Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. 1993. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell 75:875–886 [DOI] [PubMed] [Google Scholar]
- 18. Stehle JH, Foulkes NS, Molina CA, Simonneaux V, Pévet P, Sassone-Corsi P. 1993. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature 365:314–320 [DOI] [PubMed] [Google Scholar]
- 19. Gellersen B, Kempf R, Telgmann R. 1997. Human endometrial stromal cells express novel isoforms of the transcriptional modulator CREM and up-regulate ICER in the course of decidualization. Mol Endocrinol 11:97–113 [DOI] [PubMed] [Google Scholar]
- 20. Abid MR, Kachra Z, Spokes KC, Aird WC. 2000. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486:252–256 [DOI] [PubMed] [Google Scholar]
- 21. Ushio-Fukai M, Alexander RW. 2004. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264:85–97 [DOI] [PubMed] [Google Scholar]
- 22. Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189 [DOI] [PubMed] [Google Scholar]
- 23. Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- 24. Babior BM, Lambeth JD, Nauseef W. 2002. The neutrophil NADPH oxidase. Arch Biochem Biophys 397:342–344 [DOI] [PubMed] [Google Scholar]
- 25. Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. 2008. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 28:1347–1354 [DOI] [PubMed] [Google Scholar]
- 26. Schröder K, Wandzioch K, Helmcke I, Brandes RP. 2009. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29:239–245 [DOI] [PubMed] [Google Scholar]
- 27. Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassègue B, Griendling KK. 2007. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, Iuchi Y, Fujii J, Vené R, Rubartelli A, Rhee SG, Sitia R. 2010. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal 13:1133–1144 [DOI] [PubMed] [Google Scholar]
- 29. Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Rokutan K. 2009. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest 56:33–41 [DOI] [PubMed] [Google Scholar]
- 30. Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, Sauer H. 2007. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci 120:885–894 [DOI] [PubMed] [Google Scholar]
- 31. Sardina JL, Lopez-Ruano G, Sanchez-Abarca LI, Perez-Simon JA, Gaztelumendi A, Trigueros C, Llanillo M, Sanchez-Yague J, Hernandez-Hernandez A. p22(phox)-dependent NADPH oxidase activity is required for megakaryocytic differentiation. Cell Death Differ 12:1842–1854 [DOI] [PubMed] [Google Scholar]
- 32. Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ. 2002. Functional association of PR and CCAAT/enhancer-binding protein β isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol 16:141–154 [DOI] [PubMed] [Google Scholar]
- 33. Pohnke Y, Kempf R, Gellersen B. 1999. CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem 274:24808–24818 [DOI] [PubMed] [Google Scholar]
- 34. Stefanska J, Pawliczak R. 2008. Apocynin: molecular aptitudes. Mediators Inflamm 2008:106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vejrazka M, Mícek R, Stipek S. 2005. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722:143–147 [DOI] [PubMed] [Google Scholar]
- 36. Grewal S, Carver JG, Ridley AJ, Mardon HJ. 2008. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc Natl Acad Sci USA 105:16189–16194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. 2002. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem 277:20825–20832 [DOI] [PubMed] [Google Scholar]
- 38. Mak IY, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, White JO. 2002. Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 87:2581–2588 [DOI] [PubMed] [Google Scholar]
- 39. Nagashima T, Maruyama T, Uchida H, Kajitani T, Arase T, Ono M, Oda H, Kagami M, Masuda H, Nishikawa S, Asada H, Yoshimura Y. 2008. Activation of SRC kinase and phosphorylation of signal transducer and activator of transcription-5 are required for decidual transformation of human endometrial stromal cells. Endocrinology 149:1227–1234 [DOI] [PubMed] [Google Scholar]
- 40. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 41. Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. 2009. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol 200:615.e1–615.e6 [DOI] [PubMed] [Google Scholar]
- 42. Brosens JJ, Pijnenborg R, Brosens IA. 2002. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187:1416–1423 [DOI] [PubMed] [Google Scholar]
- 43. Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. 2002. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod 66:1743–1748 [DOI] [PubMed] [Google Scholar]
- 44. Ivell R, Balvers M, Pohnke Y, Telgmann R, Bartsch O, Milde- Langosch K, Bamberger AM, Einspanier A. 2003. Immunoexpression of the relaxin receptor LGR7 in breast and uterine tissues of humans and primates. Reprod Biol Endocrinol 1:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gravanis A, Stournaras C, Margioris AN. 1999. Paracrinology of endometrial neuropeptides: corticotropin-releasing hormone and opioids. Semin Reprod Endocrinol 17:29–38 [DOI] [PubMed] [Google Scholar]
- 46. Milne SA, Perchick GB, Boddy SC, Jabbour HN. 2001. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab 86:4453–4459 [DOI] [PubMed] [Google Scholar]
- 47. Kasahara K, Takakura K, Takebayashi K, Kimura F, Nakanishi K, Noda Y. 2001. The role of human chorionic gonadotropin on decidualization of endometrial stromal cells in vitro. J Clin Endocrinol Metab 86:1281–1286 [DOI] [PubMed] [Google Scholar]
- 48. Brosens JJ, Gellersen B. 2006. Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol 36:389–398 [DOI] [PubMed] [Google Scholar]
- 49. DiMattia GE, Gellersen B, Duckworth ML, Friesen HG. 1990. Human prolactin gene expression. The use of an alternative noncoding exon in decidua and the IM-9-P3 lymphoblast cell line. J Biol Chem 265:16412–16421 [PubMed] [Google Scholar]
- 50. Wagner GP, Lynch VJ. 2010. Evolutionary novelties. Curr Biol 20:R48–R52 [DOI] [PubMed] [Google Scholar]
- 51. Lynch VJ, Brayer K, Gellersen B, Wagner GP. 2009. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One 4:e6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang W, Li Q, Bagchi IC, Bagchi MK. 2010. The CCAAT/enhancer binding protein β is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology 151:3929–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kannan A, Fazleabas AT, Bagchi IC, Bagchi MK. 2010. The transcription factor C/EBPβ is a marker of uterine receptivity and expressed at the implantation site in the primate. Reprod Sci 17:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schneider-Merck T, Pohnke Y, Kempf R, Christian M, Brosens JJ, Gellersen B. 2006. Physical interaction and mutual transrepression between CCAAT/enhancer-binding protein β and the p53 tumor suppressor. J Biol Chem 281:269–278 [DOI] [PubMed] [Google Scholar]
- 55. Lee H, Lee YJ, Choi H, Ko EH, Kim JW. 2009. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284:10601–10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. 2006. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18:69–82 [DOI] [PubMed] [Google Scholar]
- 57. Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, Giudice LC. 2010. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology 151:1341–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]