Late adolescent animals are more susceptible to the somatic, HPA axis, and neuropeptide effects of chronic stress.
Abstract
Adolescent development is proposed to represent a time of increased susceptibility to stress. During adolescence, the brain demonstrates a high level of plasticity and can be positively or negatively affected by the environment. This study tests the hypothesis that adolescent development is a stage of enhanced vulnerability to chronic stress. Male Sprague-Dawley rats were exposed to our 14-d chronic variable stress (CVS) paradigm at three developmental stages: 1) early adolescence (35 d; age at initiation of CVS); 2) late adolescence (50 d); or 3) adulthood (80 d). We examined the effects of CVS on the following: 1) depression-like behavior; 2) somatic indices; 3) hypothalamic-pituitary-adrenal (HPA) axis activity; and 4) neuropeptide expression in the hypothalamus. Results show, regardless of age, CVS exposure: 1) decreased body weight; 2) increased adrenal size; 3) decreased fat weight; and 4) increased HPA response to stress. The somatic effects of CVS were exaggerated in late adolescent animals, and late adolescent animals were the only group where CVS decreased oxytocin expression and increased basal corticosterone. In response to CVS, adult animals increased immobility during the forced-swim test while early and late adolescent animals were resistant to the effects of chronic stress on depression-like behavior. Results show that adolescent animals were protected from the effect of chronic stress on depression-like behavior while late adolescent animals were more susceptible to the somatic, HPA axis, and neuropeptide effects of chronic stress. Thus, adolescent development is a unique window of vulnerabilities and protections to the effects of chronic stress.
Adolescence is a period of continued growth, behavioral and social development, and sexual maturation (1, 2). During this growth stage there is continued development of the neural pathways involved in the coordinated stress responses (3, 4). The biological and behavioral development that occurs during adolescence determines how the individual interacts with, and is affected by, their environment. Thus, adolescence is proposed to be a window of unique vulnerabilities to stress (3, 5).
Prepubertal animals respond to stress differently than adult animals. Although basal stress hormones do not change during puberty (6), the hypothalamic-pituitary-adrenal (HPA) axis response to acute stress is exaggerated in prepubertal animals, and there is a delayed return to basal values after stress exposure (7, 8). In response to chronic homotypic stress, prepubertal animals exposed to repeated restraint exhibit an exaggerated response to stress compared with adults (9). Thus, the developmental period between prepuberty and adulthood is a stage where the HPA axis response to stress is highly plastic.
The incidence of depression greatly increases during adolescence (10, 11), and this may be the consequence of genetic, maturational, and/or experiential factors (3, 12). Regional differences in the trajectory of developmental changes may increase the vulnerability of the adolescent brain to depression (12). During adolescence, there is an increase in glucocorticoid receptor expression (13), and an overproduction and pruning of synapses (3). Thus, the maturation of the adolescent brain may predispose individuals for stress susceptibility (12).
Despite the awareness of adolescence as a critical time for social and physical development, the stress susceptibility of adolescents is poorly understood. Indeed, in terms of age groups, the time period of adolescent development has received the least attention, with the bulk of research attention being directed to the study of stress in prenatal, early postnatal, and of course adult life. Therefore, the purpose of this study was to test the vulnerability of the adolescent animal to chronic stress. We hypothesized that adolescent development is a time period of increased susceptibility to the effects of chronic stress. To test this hypothesis, we measured the effects of chronic, unpredictable stress on behavior, HPA axis activity, body composition, and neuropeptide expression in adolescent and adult animals. Two age groups of adolescent animals were included to represent an early and late stage of adolescent development. Age-matched controls were included for all groups, and the relative effects of stress were compared between age groups.
Materials and Methods
Animals
Male Sprague-Dawley animals were used for all experiments. Time-pregnant Sprague-Dawley females were acquired from Charles River Laboratories and arrived at our animal facility approximately 1 week before giving birth. Pups remained with their mothers in their home cage until weaning at 25–28 d of age. At weaning, littermates were separated and animals were housed three per cage with animals from different litters. To avoid litter effects, animals from an individual litter were assigned to different experimental groups. Thus, each experimental group (n ≥ 9) had rats from at least seven different litters with no more than two animals from a given litter. Animals were divided into three age groups (early adolescent, 35–48 d; late adolescent, 50–64 d; adult, 80–93 d), and subdivided into 2 stress groups [chronic variable stress (CVS); controls; n = 9–12]. Control animals remained in their home cage and were only handled to collect body weight and to mark tails (permanent marker) for identification. Within each age group, a control group of animals was used for the forced swim test (FST) and a separate control group of animals was used for the collection of basal measures (somatic measures, basal corticosterone and neuropeptide mRNA). Animals were housed in temperature- and humidity-controlled rooms (lights on 0600–1800 h) with ad libitum access to food and water. All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Chronic variable stress
Animals in the CVS groups underwent our standard CVS regimen for 14 d (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The CVS regimen consisted of twice daily exposures to alternating stressors, once in the morning (0800–1100 h) and once in the afternoon (1300–1700). In addition, animals were exposed to overnight stressors [individually housed or social crowding (six rats per cage)] approximately every third night. The stressors used in the CVS paradigm included: 1) 1 h in plastic restraint tube; 2) 1 h shaker stress (100 rpm); 3) 30 min hypoxia (8% O2 and 92% N2); 4) 5 min open field; and 5) 1 h cold room (4 C). Adjustable tubes were used for restraint to ensure a similar degree of confinement for all groups (i.e., animals were unable to turn around in tubes).
FST
On the morning of d 14 of the CVS paradigm, animals went through the FST to assess depression-like behavior. Corticosterone responses to forced-swim stress were determined after cessation of the test. Animals were placed in a cylindrical container filled with 30 cm of 31 C water for 10 min. A video recording of the animal's behavior was scored every 5 sec. Scoring was done by an observer blinded to the experimental condition of each animal. The following behaviors, immobility, swimming, and climbing, were scored as previously described (14). Animals were removed from the swim cylinders and blood samples collected by tail clip in EDTA tubes at 15, 40, 70, and 130 min after stressor initiation. Corticosterone was measured by RIA as previously described (15).
Tissue collection
The morning after the completion of CVS (at least 16 h since last stress exposure), blood samples were collected by tail clip in EDTA tubes. Animals were given an overdose of sodium pentobarbital and subsequently perfused with 0.9% saline followed by 3.7% phosphate-buffered formaldehyde. Brains were collected and immersed in 3.7% phosphate-buffered formaldehyde for 24 h, then stored in 30% sucrose in diethylpyrocarbonate (DEPC)-treated water at 4 C. Thymus and adrenal glands were dissected and weighed. Rat bodies were individually stored in plastic bags and body composition measured by nuclear magnetic resonance (Echo NMR, Waco, TX).
mRNA analysis
In situ hybridization assays were used to quantify CRH, arginine vasopressin (AVP), and oxytocin mRNA expression in the paraventricular nucleus (PVN) and SON. Brains were sectioned and a one-in-12 series collected. Sections were mounted on slides, fixed in 4% phosphate-buffered formaldehyde for 10 min, rinsed twice in 5 mm potassium PBS (KPBS) for 5 min, twice in KPBS containing 0.2% glycine for 5 min, and twice in KPBS for 5 min. Sections were immersed in 0.25% acetic anhydride [suspended in 0.1 m triethanolamine (pH 8)] for 10 min, rinsed twice in 2× saline sodium citrate buffer (SSC) for 5 min, then dehydrated through graded alcohols.
Antisense cRNA probes complementary to CRH (765 bp) (16), AVP (161 bp) (17), and oxytocin (477 bp) were generated by in vitro transcription using 35S-UTP. The CRH and AVP probes were generated as previously described (15). The oxytocin fragment was cloned into a BSSK vector, linearized with HindIII, and transcribed with T7 RNA polymerase (Fisher Scientific Co., Pittsburgh, PA). Each transcription reaction (15 μl) consisted of 1× transcription buffer, 62.5 μCi 35S-UTP, 330 μm ATP, 330 μm GTP, 330 μm CTP, 10 μm cold UTP, 66.6 mm dithiothreitol, 40 U ribonuclease inhibitor, 20 U T7 RNA polymerase, and 2.5 μg linearized DNA. The transcription reaction was incubated at 37 C for 60 min and labeled probe separated from free nucleotide by ammonium acetate precipitation.
35S-probes were diluted in hybridization buffer [50% formamide, 20 mm Tris-HCl (pH 7.5), 1 mm EDTA, 335 mm NaCl, 1× Denhardt's solution, 200 μg/ml herring sperm DNA, 100 μg/ml yeast tRNA, 20 mm dithiothreitol, and 10% dextran sulfate] at an activity count of 1,000,000 cpm per 50 μl buffer. Fifty microliters of buffer was added to each slide. Slides were coverslipped then incubated overnight at 55 C in humidified chambers containing 50% formamide. The coverslips were removed in 2× SSC and the slides incubated in 100 μg/ml ribonuclease A for 30 min at 37 C. Slides were rinsed in 2× SSC, incubated in 0.2× SSC (65 C) for 1 h, then dehydrated through graded alcohols. Slides were exposed to Kodak Biomax MR-2 film (Eastman Kodak, Rochester, NY) for 7 d, 16 h, and 8 h for CRH, AVP, and oxytocin, respectively. Tissue sections from all age groups were processed in a single assay for each probe.
Scion Image 1.62 software (Scion, Frederick, MD) was used for semiquantitative analyses of autoradiographs. The anatomical regions of interest [CRH: PVN; AVP: parvocellular PVN (pPVN), magnocellular PVN (mPVN) and SON; oxytocin: dorsal pPVN, lateral pPVN, ventral zone of the medial pPVN, anterior mPVN, posterior mPVN and SON] were determined based on Paxinos and Watson's rat brain atlas (18). Supplemental Figure 1 outlines some of the subregions used for neuropeptide analysis. Gray level units were collected for specified brain regions and the background signal over a nonhybridized area within the same section subtracted to obtain corrected gray level units. Volume of hybridized area was also determined, and area was multiplied by the corrected gray level to calculate the integrated gray level. With each film, 14C radioactive standards (ARC) were included to assure that all signal intensities were within the linear range of detection. Analysis of autoradiographs was completed by a researcher blind to experimental groups.
Statistical analysis
Data are expressed as mean ± sem. Data [basal corticosterone, corticosterone area under the curve (AUC), neuropeptide mRNA, and immobility] were analyzed by two-way ANOVA with stress (control, CVS) and age (early adolescence, late adolescence and adulthood) as main factors. Planned comparisons were performed after the two-way ANOVA to assess the effect of stress within adolescent and adult animals (control vs. CVS within each age group). Because specific hypotheses were formed a priori on the effects of CVS within groups, the planned comparisons were performed regardless of whether a significant interaction was observed (19). Body weights, tissue weights, and body composition were analyzed by one-way ANOVA within each age group with stress (control, CVS) as the main factor. To compare the relative effect of CVS on somatic indices, data were expressed as percent change relative to age-matched control (to correct for differences in body size) and the effect of age within the CVS groups analyzed by one-way ANOVA. The corticosterone response to FST was analyzed by three-way repeated measures ANOVA, with stress (control, CVS), age (early adolescence, late adolescence and adulthood), and time (15, 40, 70, and 130 min after stressor initiation) as main factors. Outliers were detected using the Grubbs' test (GraphPad Software) and removed from analysis. Data were analyzed by SigmaStat 9.0 software with statistical significance set at P ≤ 0.05.
Results
The somatic effects of chronic stress on early adolescent, late adolescent, and adult animals are shown in Table 1. Chronic stress decreased body weight gain, increased adrenal size, and decreased body fat content in all three age groups (P < 0.05). Thymus size was decreased after chronic stress in both adolescent groups (P < 0.05), whereas thymus size was not altered in adult animals.
Table 1.
Somatic effects of chronic stress
| Early adolescent |
Late adolescent |
Adult |
||||
|---|---|---|---|---|---|---|
| Control | CVS | Control | CVS | Control | CVS | |
| Age | ||||||
| CVS | 35–48 d | 50–63 d | 80–93 d | |||
| Tissue collection | 49 d | 49 d | 64 d | 64 d | 94 d | 94 d |
| Number | 9 | 9 | 9 | 9 | 9 | 12 |
| Body weight, mean ± se | ||||||
| Initial, g | 148 ± 2.0 | 148 ± 3.3 | 251 ± 5.3 | 252 ± 6.2 | 360 ± 7.8 | 362 ± 6.4 |
| Final, g | 244 ± 3.8 | 223 ± 3.6a | 319 ± 8.4 | 291 ± 6.1a | 384 ± 8.9 | 361 ± 6.4a |
| Gain, g | 96.1 ± 2.2 | 74.4 ± 2.2a | 67.8 ± 3.6 | 39.0 ± 1.6a | 23.6 ± 1.8 | −1.3 ± 1.7a |
| Gain, % | 65.1 ± 1.0 | 50.4 ± 2.0a | 27.0 ± 1.1 | 15.5 ± 0.8a | 6.5 ± 0.4 | −0.4 ± 0.5a |
| Tissue weight, mean ± se | ||||||
| Adrenal, mg | 17.4 ± 0.6 | 19.9 ± 0.9a | 18.6 ± 0.9 | 20.3 ± 0.9 | 23.1 ± 0.6 | 27.2 ± 0.3a |
| mg/bw × 100 | 7.1 ± 0.2 | 8.9 ± 0.3a | 5.8 ± 0.2 | 6.9 ± 0.2a | 6.0 ± 0.2 | 7.6 ± 0.2a |
| Thymus, g | 570 ± 17 | 524 ± 15a | 516 ± 27 | 386 ± 16a | 410 ± 30 | 363 ± 16 |
| mg/bw × 10 | 23.4 ± 0.6 | 23.5 ± 0.7 | 16.2 ± 0.7 | 13.3 ± 0.5a | 10.7 ± 0.7 | 10.1 ± 0.5 |
| Body composition, mean ± se | ||||||
| Fat, g | 20.3 ± 0.7 | 17.3 ± 0.6a | 26.3 ± 1.3 | 19.8 ± 0.9a | 37.6 ± 2.6 | 31.7 ± 0.7a |
| Fat, % | 8.4 ± 0.2 | 7.8 ± 0.2a | 8.4 ± 0.4 | 6.8 ± 0.3a | 9.7 ± 0.5 | 8.8 ± 0.2 |
| Lean, g | 195 ± 1.0 | 186 ± 2.6a | 243 ± 4.2 | 234 ± 6.7 | 305 ± 7.2 | 285 ± 2.6a |
| Lean, % | 81.3 ± 1.0 | 82.6 ± 0.9 | 77.5 ± 1.4 | 80.3 ± 0.9 | 79.5 ± 0.9 | 81.1 ± 0.4 |
Effect of chronic stress on body weight, tissue weight and body composition in adolescent and adult animals. Results are presented as mean ± se.
P < 0.05 vs. control within age group.
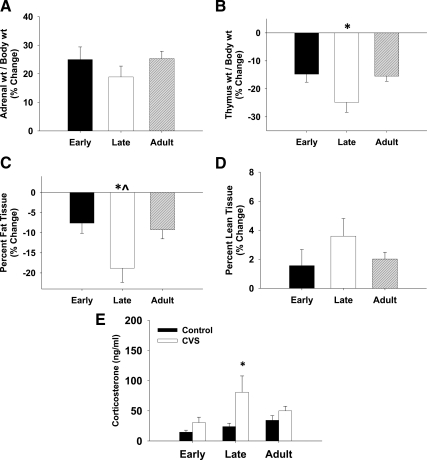
To determine whether the magnitude of the impact of chronic stress was age-dependent, data were normalized to age-matched control animals and the percent change in chronic stress animals was compared between age groups (Fig. 1, A–D). The impact of chronic stress on adrenal size (Fig. 1A) and percent lean tissue (Fig. 1D) did not differ between age groups. The impact of chronic stress was age-dependent for thymus size [H (2, 25) = 9.61, P = 0.008; Figure 1B] and percent body fat [F (2, 25) = 4.55, P = 0.02; Figure 1C], with the effect of chronic stress being exaggerated in late adolescent animals. There was no difference between early adolescent and adult animals in the impact of chronic stress on tissue weights and body composition.
Fig. 1.
Impact of chronic stress on tissue weights, body composition, and basal corticosterone. The relative impact of chronic stress on thymus size (B) and percent fat tissue (C) was exaggerated in late adolescent animals. The relative impact of chronic stress on the adrenal size (A) and percent lean tissue (D) did not differ between age groups. Data are presented as the percent change from age-matched control animals (A–D; *, P < 0.05 vs. early adolescent; ^, P < 0.05 vs. adult). E, The impact of chronic stress on basal corticosterone in adolescent and adult animals. There was a main effect of chronic stress, and of age, on basal corticosterone. *, P < 0.05 vs. control within age group.
There was a main effect of chronic stress [F (1, 49) = 8.18, P = 0.006], and age [F (2, 49) = 12.11, P = 0.047] on basal corticosterone (Fig. 1E). There was no interaction between chronic stress and age on basal corticosterone [F (2, 49) = 0.77, P = 0.5]. Planned comparisons revealed an enhanced effect of chronic stress on basal corticosterone only in late adolescent animals (P = 0.011).
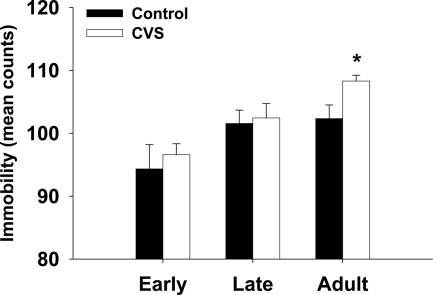
FST was used to test the effect of chronic stress on depression-like behavior in adolescent animals. Chronic stress increased immobility in adult animals (P < 0.05; Figure 2). Of interest, early and late adolescent animals that underwent CVS did not show an increase in immobility during the FST but demonstrated a resistance to the effect of chronic stress on depression-like behavior.
Fig. 2.
Behavioral response to FST. Animals were exposed to the FST for 10 min, and behavior was scored every 5 sec for activity for early adolescent (A), late adolescent (B), and adult (C) animals. Data displayed as mean counts of immobility (total counts for 10 min equals 120). Chronic stress increased immobility in adult animals but did not affect behavior in adolescent animals. *, P < 0.05 vs. age-matched control.
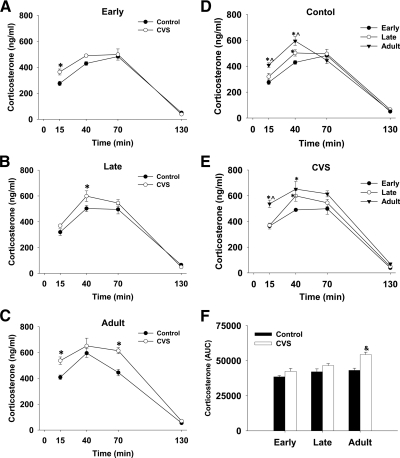
In addition to behavioral scoring, blood samples were collected at 5, 30, 60, and 120 min after stressor cessation to test the effect of chronic stress on the HPA response to a novel stressor. The corticosterone response to FST data were analyzed by three-way repeated measures ANOVA. For clarity of data presentation, the corticosterone response to FST is presented in two ways in Fig. 3. Figure 3, A–C shows the effect of chronic stress within an age group, while panels D and E highlight the difference in the corticosterone response to FST between age groups. After FST, mean corticosterone levels increased in all groups [main effect of time; F (3, 201) = 452.8, P < 0.001] and returned close to baseline levels by 130 min (2 h after stressor cessation; Figure 3).
Fig. 3.
The effect of chronic stress and age on the corticosterone response to a novel stressor. Animals completed the 10 min FST and blood samples were collected at 15, 40, 70, and 130 min after the initiation of the stressor. Within early adolescent (A), late adolescent (B), and adult (C) animals there was an increase in the corticosterone response to stress (*, P < 0.05 vs. control within time point). The corticosterone response to swimming at 15 min and 40 min was higher in adult animals in both the control (D) and chronic stress (E) groups. At 40 min, the corticosterone response was higher in the late adolescent group compared with the early adolescent group within control (D) and chronic stress (E) animals. Area under the curve data (F) shows a main effect of stress and that this effect was significant within the adult animals. *, P < 0.05 vs. early adolescent; ^, P < 0.05 vs. late adolescent; &, P < 0.05 vs. control within age group.
There was a main effect of chronic stress [F (1, 201) = 30.7, P < 0.001] and an interaction between chronic stress and time [F (3, 201) = 4.02, P = 0.008], demonstrating that chronic stress leads to a facilitation of the corticosterone response to a novel stressor (Fig. 3). There was also a main effect of age [F (2, 201) = 26.4, P < 0.001] and an interaction between age and time [F (6, 201) = 5.21, P < 0.001], indicating that the corticosterone response to novel stress is age-dependent (Fig. 3). There was a trend for an interaction between age and chronic stress [F (2, 201) = 2.61, P = 0.076], suggesting that the relative effect of chronic stress on the corticosterone response to a novel stressor may be age dependent. There was not an interaction between all three factors [age × time × chronic stress; F (6, 201) = 1.34, P = 0.24].
The total corticosterone response to FST, as show by the corticosterone AUC data, is presented in Figure 3E. There was a main effect of chronic stress [F (1, 48) = 21.84, P < 0.001] and age [F (2, 48) = 12.11, P < 0.001] on the AUC for corticosterone (Fig. 3E). Similar to the effect of chronic stress on immobility, planned comparisons show that the effect of chronic stress on corticosterone AUC was only significant in adult animals (P < 0.05). The interaction between chronic stress and age on corticosterone AUC [F (2, 48) = 3.04, P = 0.057] did not reach statistical significance.
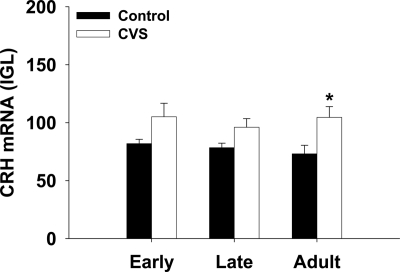
To assess the impact of chronic stress on neuropeptide expression in the adolescent brain, CRH (Fig. 4), oxytocin (Fig. 5), and vasopressin (Fig. 6) mRNA were measured by in situ hybridization. There was a main effect of chronic stress on CRH expression in the hypothalamus [F (1, 49) = 11.77, P = 0.001]. Planned comparisons show a significant difference in the adult group (P = 0.006), and no statistically significant effect of CVS on CRH expression within the early adolescent (P = 0.10) or late adolescent (P = 0.15) groups.
Fig. 4.
The effect of chronic stress on CRH mRNA in adolescent and adult animals. CVS increased CRH mRNA in adult animals. *, P < 0.05 vs. control within age group.
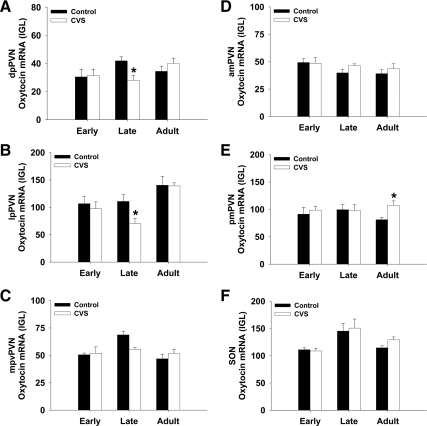
Fig. 5.
The expression of oxytocin mRNA after CVS in adolescent and adults animals. dpPVN, dorsal pPVN; lpPVN, lateral pPVN; mpvPVN, medial parvocellular ventral PVN; amPVN, anterior mPVN; pmPVN, posterior mPVN. *, P < 0.05 vs. control within age group.
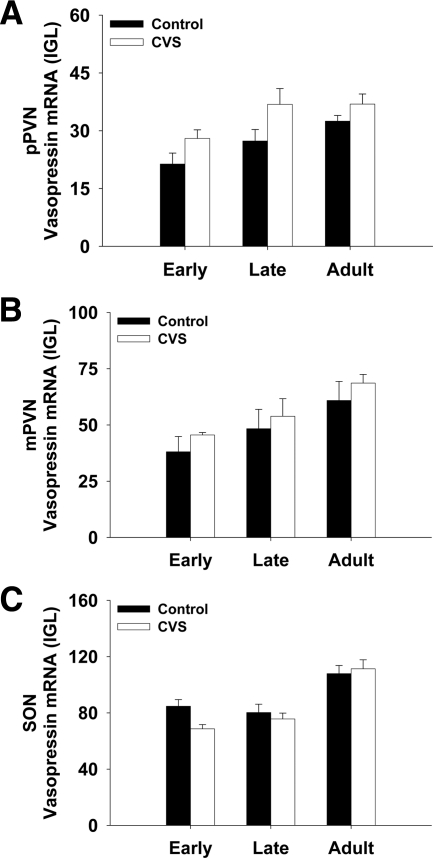
Fig. 6.
The impact of CVS on vasopressin expression in the PVN and SON.
The analysis of oxytocin mRNA was measured in the SON and in subdivisions of the PVN (Fig. 5). Oxytocin was measured in both parvocellular (A–C) and magnocellular (D and E) divisions. In late adolescent animals, chronic stress significantly decreased oxytocin expression in the dorsal pPVN (Fig. 5A) and the lateral pPVN (Fig. 5B). This effect was not observed in he early adolescent or adult animals. In adult animals, oxytocin expression in the pmPVN was increased (Fig. 5E) after chronic stress. There was no effect of chronic stress on oxytocin expression within early adolescent animals. In addition, there was also a main effect of age on oxytocin expression (Supplemental Table 2), demonstrating that oxytocin expression in the hypothalamus is dependent on the developmental stage.
The expression of vasopressin in the hypothalamus was both age- and stress-dependent (Supplemental Table 2). There was a main effect of stress to increase vasopressin expression in the pPVN (Supplemental Table 2), although planned comparisons did not reveal any within age-group differences (Fig. 6A).
Table 2 summarizes the results included in this manuscript and highlights the developmental age where the effects of chronic stress were exacerbated. There was not a single measure where the effect of CVS was exaggerated in early adolescent animals. In addition, there was not a single neuropeptide measure that was affected by CVS in the early adolescent age group. In contrast, late adolescent animals had exaggerated somatic effects and were the only group to show a decrease in oxytocin expression in the pPVN. Adult animals were the only group susceptible to the behavioral effects of CVS and were more susceptible than adolescent animals to the effect of CVS on increasing the corticosterone response to a novel stressor. Thus, the effect of CVS differs between adolescent and adult animals.
Table 2.
Summary of the effects of chronic stress
| Early | Late | Adult | ||
|---|---|---|---|---|
| Behavior | Immobility | — | — | ↑aE,L |
| HPA axis | CORT (basal) | — | ↑ | — |
| CORT (stress-induced) | — | — | ↑aE,L | |
| Body weight and tissue weights | Body weight | ↓ | ↓ | ↓ |
| Body weight gain | ↓ | ↓aE,A | ↓aE,L | |
| Adrenal size | ↑ | — | ↑ | |
| Adrenal ratio | ↑ | ↑ | ↑ | |
| Thymus size | ↓ | ↓aE,A | — | |
| Thymus ratio | — | ↓aE,A | — | |
| Body composition | Fat tissue | ↓ | ↓aE,A | ↓ |
| Fat % | ↓ | ↓aE,A | — | |
| Lean tissue | ↓ | — | ↓ | |
| Lean % | — | — | — | |
| Neuropeptides | CRH | — | — | ↑ |
| Oxytocin (parvocellular) | — | ↓aA | — | |
| Oxytocin (magnocellular) | — | — | ↑aL | |
| Vasopressin (parvocellular) | — | — | — | |
| Vasopressin (magnocellular) | — | — | — |
Summary of the impact of chronic stress on adolescent and adult animals. Up or down arrows indicate a significant increase or decrease, respectively, in response to chronic stress compared with age-matched control animals.
Age difference in the magnitude of the effect of CVS. P < 0.05 vs. early adolescent (E), late adolescent (L), or adult (A) animals.
Discussion
The ability to cope with stress has major implications for health, especially during critical stages of development. In this study, we tested the vulnerability of adolescent animals to the challenges of a chronic stress paradigm. Late adolescent rats were selectively vulnerable (i.e., effects of CVS were exaggerated relative to early adolescent or adult animals) to the somatic effects of chronic stress, showing disproportionate increases in basal corticosterone, thymic involution and loss of body fat. Stress in late adolescence also decreased expression of oxytocin in the PVN, consistent with loss of a stress-protective peptide (20). Despite the enhanced susceptibility to the central and somatic effects of chronic stress, late adolescent animals were resistant to the behavioral effects of chronic stress. Thus, adolescent development represents a time period with unique resistance and vulnerability to stress exposure.
Within each age group, chronic stress animals demonstrated adrenal hypertrophy, thymic involution, reduced body weight gain, and decreased body fat. These effects are consistent with previous observations in both adult (21) and adolescent animals (22). Previous work by Isgor et al. demonstrated that the somatic effects of chronic stress (4 weeks) in adolescent animals (4- to 8-week-old rats) were specific to stressor type (i.e., adrenal ratio, thymus ratio, and body weight were only affected by physical, but not social, stressors). Our chronic stress paradigm incorporated both physical and social stressors, suggesting that interspersed stress modalities are sufficient to produce somatic changes.
By comparing the relative effects of chronic stress between age groups, we see that the impact of chronic stress on somatic indices was age specific, with late adolescent animals demonstrating greater thymic involution and a larger decrease in body fat. This enhanced susceptibility to chronic stress did not occur in early adolescent animals. The change in basal corticosterone was also specific to late adolescence and could be a driving factor for the age-dependent differences in the somatic impact of chronic stress (i.e., chronic stress exposure led to a greater increase in glucocorticoids in late adolescent animals and that the increased glucocorticoids were responsible for the exaggerated effects observed within this age group). The difference in stress susceptibility between early and late adolescent animals suggests that even within the period of adolescent development, there is an age-specific window regarding the vulnerability to chronic stress.
To assess depression-like behavior the FST was used to measure the amount of time rats demonstrate immobility in the face of a stressful challenge. In adult animals, we observed an increase in immobility in animals exposed to chronic stress. Of interest, adolescent animals did not show an increase in immobility after chronic stress, suggesting a resistance to the effect of chronic stress on depression-like behavior. Previous work using the elevated plus maze has also shown that juvenile animals are resistant to the behavioral effects of chronic stress (23), although the basis of this behavioral protection is unknown. It is possible that the neural pathways responsible for chronic stress-induced behavioral changes in adults are the following: 1) protected during adolescence; 2) not fully developed yet in adolescent animals; and/or 3) altered in adolescent animals, yet behavioral changes are not observed because adolescent behavior involves different neural and endocrine mechanisms than adults (24, 25).
In addition to the behavioral response, the FST also allowed us to measure the HPA axis response to a novel stressor following chronic stress exposure. Within each age group there was at least one time point after the FST where chronic stress animals demonstrated an exaggerated response. In humans, depressed adolescents have an exaggerated cortisol response to stress (26). Consistent with this observation in adolescent humans, we saw that chronic stress during adolescence results in an exaggerated corticosterone response.
In prepubertal animals the corticosterone response remains elevated longer and takes more time to return to baseline, compared with adult animals (8, 27). Consistent with this finding, our results show that the corticosterone levels in adult animals begin to return toward baseline quicker than adolescent animals. This can be observed in Fig. 3D where the adult corticosterone response peaks at 40 min and then starts to return to baseline. In contrast, in adolescent animals, the peak in corticosterone at 40 min remains elevated or continues to increase over the next 30 min. Our results also show that the peak corticosterone response was more modest in adolescent animals than adults, and that by 2 h after stressor initiation, both adolescent and adult animals had returned to basal values. Thus, aspects of the corticosterone response of prepubertal animals begins to be modified during adolescent development.
Chronic stress causes facilitation of HPA axis responses to novel stressors in adulthood (28–30). Our data confirm these previous observations, with adult animals exhibiting a significant exaggeration of the integrated stress response to swim. Although both adolescent and adult animals had an increased corticosterone response to stress compared with age-matched controls, the data suggest that this effect of chronic stress is more pronounced in adults, implying a level of resistance to stress facilitation within adolescent animals. However, the corticosterone response to FST data must be interpreted in light of the behavioral response to FST. Only adult animals showed increased FST immobility after CVS. Therefore, it is possible that the perceived experience of the stressor, in terms of intensity or salience, differed among age groups. Groups may have also differed in their ability to cope with the physical demands of the swim test. Thus, the age difference in the corticosterone response to FST may be due to a difference in perception of the stress experience, or the demands of the stressor, and as such may not be the result of a differential effect of chronic stress on HPA axis reactivity per se.
The hypothalamus is the key site for integration and execution of stress signaling (31). To quantify the effect of chronic stress on neuropeptide expression in putative stress-regulatory regions, we measured CRH, vasopressin, and oxytocin mRNA in the paraventricular and supraoptic nuclei. We observed a main effect of stress on CRH and vasopressin and a main effect of age on oxytocin and vasopressin.
The expression of CRH was stress sensitive, consistent with other studies (15, 32). Our results did not reveal an age difference in CRH expression although previous work has shown that prepurbertal males have greater CRH expression than adult males (33). Our results suggest that by postnatal d 49, the expression of CRH mRNA is similar to adult levels.
In contrast to CRH, where expression was not age-dependent, there was a main effect of age on the level of vasopressin and oxytocin mRNA. The expression of vasopressin increased across the period of adolescence and into adulthood, suggesting an increasing role for vasopressin in developing animals. The increase in vasopressin during adolescence is consistent with previous work showing that vasopressin expression increased in the SON and PVN from age 4 weeks to age 10 weeks (34). It is possible that changes in testosterone levels during adolescent development contributed to the changes in vasopressin levels observed in this article (35, 36).
The pattern of oxytocin expression, at least in some regions, demonstrated an inverse U-pattern (i.e., increase from early to late adolescence and a decrease from late adolescence to adulthood). Our observation of increased oxytocin levels from age 49 d to age 64 d fits with the previous observation that oxytocin expression is higher in 70-d-old animals compared with 28-d-old animals (34). The age specific up-regulation of oxytocin during late adolescence suggests that oxytocin plays a more important role during this developmental stage. This observation is particularly interesting in light of the fact that late adolescence was the only age at which oxytocin expression was susceptible to the effects of chronic stress. Indeed, it could be the loss of oxytocin, a proposed stress protective neuropeptide, that renders the late adolescent animal more susceptible to the effects of chronic stress.
Oxytocin from the PVN can affect both HPA axis activity and influence autonomic output. Central blockade of oxytocin increases the HPA response to stress (37), suggesting a role as a stress inhibitory neuropeptide. Our results show that it was the parvocellular, not magnocellular, neurons of the PVN that were sensitive to the effects of chronic stress. The parvocellular oxytocinergic neurons of the PVN project to the spinal cord and dorsal vagal complex (38, 39) and have been proposed to excite cardiac sympathetic preganglionic neurons (40). Studies showing that electrical stimulation of the PVN increases oxytocin levels in the spinal cord (41) and that intrathecal oxytocin has cardiovascular effects (40, 42) support the hypothesis of an oxytocin PVN-spinal pathway that can affect the cardiovascular system. Thus, changing oxytocin expression in the parvocellular division of the PVN may alter the cardiovascular response to stress in late adolescent animals.
In addition to its roles in the stress response and reproduction, oxytocin affects behavior. In particular, central oxytocin has been shown to modulate maternal behavior (43), increase interpersonal trust (44), and increase social attachment and affiliative behavior (45). In prairie voles, it was determined that oxytocin action in the nucleus accumbens is critical for affiliative behavior and that the oxytocin-immunoreactive fibers that target the nucleus accumbens originate from the PVN and SON (45). Our data show that during late adolescence the expression of oxytocin in the PVN increases and that chronic stress prevents the increase in oxytocin. Thus, chronic stress during adolescence might curtail appropriate socio-behavioral development by preventing the increase in oxytocin that is critical for the development of social interactions at this stage of life.
In conclusion, the vulnerability to chronic stress changes during adolescent development. During early adolescence, animals are resistant to the behavioral and neuropeptide effects of chronic stress while similar somatic effects of stress were still observed. Late adolescent animals were also resistant to the behavioral effects of chronic stress despite the fact that the neuropeptide and somatic effects of chronic stress were exaggerated in this age group. Thus, there is an increase in the susceptibility to chronic stress following the developmental transition from early to late adolescence.
Supplementary Material
Acknowledgments
We thank Anne Christiansen, Annette DeKloet, Mark Dolgas, Kenny Jones, Eric Krause, Ben Packard, Karen Scott, and Yve Ulrich-Lai for their assistance with animal experiments.
This work was supported by National Institutes of Health Grants MH069725, MH084515, and DK058903.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AUC
- Area under the curve
- AVP
- arginine vasopressin
- CVS
- chronic variable stress
- FST
- forced swim test
- HPA
- hypothalamic-pituitary-adrenal
- KPBS
- potassium PBS
- mPVN
- magnocellular paraventricular nucleus
- pPVN
- parvocellular paraventricular nucleus
- PVN
- paraventricular nucleus
- SSC
- saline sodium citrate buffer.
References
- 1. Burnett S, Blakemore SJ. 2009. The development of adolescent social cognition. Ann NY Acad Sci 1167:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463 [DOI] [PubMed] [Google Scholar]
- 3. Andersen SL. 2003. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18 [DOI] [PubMed] [Google Scholar]
- 4. Casey BJ, Getz S, Galvan A. 2008. The adolescent brain. Dev Rev 28:62–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahl RE. 2004. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann NY Acad Sci 1021:1–22 [DOI] [PubMed] [Google Scholar]
- 6. Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. 2006. Adrenarche in the rat. J Endocrinol 191:301–308 [DOI] [PubMed] [Google Scholar]
- 7. Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. 2004. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 79:125–132 [DOI] [PubMed] [Google Scholar]
- 8. Goldman L, Winget C, Hollingshead GW, Levine S. 1973. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology 12:199–211 [DOI] [PubMed] [Google Scholar]
- 9. Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. 2006. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 147:1664–1674 [DOI] [PubMed] [Google Scholar]
- 10. Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. 1998. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol 107:128–140 [DOI] [PubMed] [Google Scholar]
- 11. Kessler RC, Avenevoli S, Ries Merikangas K. 2001. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry 49:1002–1014 [DOI] [PubMed] [Google Scholar]
- 12. Andersen SL, Teicher MH. 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 31:183–191 [DOI] [PubMed] [Google Scholar]
- 13. Pryce CR. 2008. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev 57:596–605 [DOI] [PubMed] [Google Scholar]
- 14. Wulsin AC, Herman JP, Solomon MB. 2010. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology 35:1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. 2006. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 147:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. 2003. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 18:2357–2364 [DOI] [PubMed] [Google Scholar]
- 17. Herman JP. 1995. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: regulation by stress and glucocorticoids. J Comp Neurol 363:15–27 [DOI] [PubMed] [Google Scholar]
- 18. Paxinos G, Watson C. 2005. The rat brain in stereotaxic coordinates. 5th ed: Elsevier Academic Press [Google Scholar]
- 19. Maxwell SE, Delaney HD. 1989. Designing experiments and analyzing data: a model comparison perspective. Belmont, CA: Wadsworth [Google Scholar]
- 20. Neumann ID. 2008. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol 20:858–865 [DOI] [PubMed] [Google Scholar]
- 21. Solomon MB, Jones K, Packard BA, Herman JP. 2010. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol 22:13–23 [DOI] [PubMed] [Google Scholar]
- 22. Isgor C, Kabbaj M, Akil H, Watson SJ. 2004. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14:636–648 [DOI] [PubMed] [Google Scholar]
- 23. McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res 187:228–238 [DOI] [PubMed] [Google Scholar]
- 24. McCormick CM, Mathews IZ. 2009. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Prog Neuropsychopharmacol Biol Psychiatry [DOI] [PubMed] [Google Scholar]
- 25. Nephew BC, Lovelock DF, Bridges RS. 2008. The progesterone receptor and parental behavior in juvenile rats. Dev Psychobiol 50:535–541 [DOI] [PubMed] [Google Scholar]
- 26. Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. 2008. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry 64:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romeo RD. 2010. Adolescence: A central event in shaping stress reactivity. Dev Psychobiol [DOI] [PubMed] [Google Scholar]
- 28. Bhatnagar S, Dallman M. 1998. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 29. Bhatnagar S, Vining C. 2003. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav 43:158–165 [DOI] [PubMed] [Google Scholar]
- 30. Hauger RL, Lorang M, Irwin M, Aguilera G. 1990. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res 532:34–40 [DOI] [PubMed] [Google Scholar]
- 31. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- 32. Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. 1991. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci 11:585–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. 2007. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology 85:199–206 [DOI] [PubMed] [Google Scholar]
- 34. van Tol HH, van den Buuse M, de Jong W, Burbach JP. 1988. Vasopressin and oxytocin gene expression in the supraoptic and paraventricular nucleus of the spontaneously hypertensive rat (SHR) during development of hypertension. Brain Res 464:303–311 [DOI] [PubMed] [Google Scholar]
- 35. Evuarherhe O, Leggett JD, Waite EJ, Kershaw YM, Atkinson HC, Lightman SL. 2009. Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. J Physiol 587:2977–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williamson M, Bingham B, Gray M, Innala L, Viau V. 2010. The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. J Neurosci 30:11762–11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. 2000. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol 12:235–243 [DOI] [PubMed] [Google Scholar]
- 38. Cechetto DF, Saper CB. 1988. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 272:579–604 [DOI] [PubMed] [Google Scholar]
- 39. Swanson LW, McKellar S. 1979. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol 188:87–106 [DOI] [PubMed] [Google Scholar]
- 40. Yang Z, Han D, Coote JH. 2009. Cardiac sympatho-excitatory action of PVN-spinal oxytocin neurones. Auton Neurosci 147:80–85 [DOI] [PubMed] [Google Scholar]
- 41. Pittman QJ, Riphagen CL, Lederis K. 1984. Release of immunoassayable neurohypophyseal peptides from rat spinal cord, in vivo. Brain Res 300:321–326 [DOI] [PubMed] [Google Scholar]
- 42. Riphagen CL, Pittman QJ. 1986. Oxytocin and [1-deamino, 8-D-arginine]-vasopressin (dDAVP): intrathecal effects on blood pressure, heart rate and urine output. Brain Res 374:371–374 [DOI] [PubMed] [Google Scholar]
- 43. Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. 2010. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 151:2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature 435:673–676 [DOI] [PubMed] [Google Scholar]
- 45. Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. 2009. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162:892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.