A novel mechanism of ovulation may control interactions among the circadian system, kisspeptin signaling, and a GnRH gating mechanism.
Abstract
In spontaneously ovulating rodents, the preovulatory LH surge is initiated on the day of proestrus by a timed, stimulatory signal originating from the circadian clock in the suprachiasmatic nucleus (SCN). The present studies explored whether kisspeptin is part of the essential neural circuit linking the SCN to the GnRH system to stimulate ovulation in Syrian hamsters (Mesocricetus auratus). Kisspeptin neurons exhibit an estrogen-dependent, daily pattern of cellular activity consistent with a role in the circadian control of the LH surge. The SCN targets kisspeptin neurons via vasopressinergic (AVP), but not vasoactive intestinal polypeptide-ergic, projections. Because AVP administration can only stimulate the LH surge during a restricted time of day, we examined the possibility that the response to AVP is gated at the level of kisspeptin and/or GnRH neurons. Kisspeptin and GnRH activation were assessed after the administration of AVP during the morning (when AVP is incapable of initiating the LH surge) and the afternoon (when AVP injections stimulate the LH surge). Kisspeptin, but not GnRH, cellular activity was up-regulated after morning injections of AVP, suggesting that time-dependent sensitivity to SCN signaling is gated within GnRH but not kisspeptin neurons. In support of this possibility, we found that the GnRH system exhibits pronounced daily changes in sensitivity to kisspeptin stimulation, with maximal sensitivity in the afternoon. Together these studies reveal a novel mechanism of ovulatory control with interactions among the circadian system, kisspeptin signaling, and a GnRH gating mechanism of control.
Despite the established role of the circadian system in regulating ovulation across species (1–3), the precise neurochemical pathways by which a daily timing signal initiates the preovulatory LH surge remain to be fully elucidated. In mammals, the orchestration of circadian rhythms is controlled by a master pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus (4, 5). Circadian rhythms are endogenously generated (6, 7) and synchronized to the external environment via direct neural projections from intrinsically photosensitive retinal ganglion cells to the circadian clock in the SCN (8). The SCN communicates to hypothalamic cell phenotypes driving reproductive function through extensive direct and indirect neural projections (2, 9, 10). On the day of proestrus, in most spontaneously ovulating rodent species, the preovulatory LH is initiated by the SCN late in the afternoon (11–14). Perturbations of SCN output signaling pathways or intrinsic clock activity lead to gross deficits in female rodent ovulatory function and fecundity (15–18).
Two SCN-derived neurochemical pathways have been implicated in the initiation of the LH surge. The first is a monosynaptic pathway whereby SCN-derived, vasoactive intestinal polypeptide (VIP) secreting neurons project directly to GnRH cells (19). GnRH neurons targeted by VIP express FOS around the time of the LH surge and antisense oligonucleotides directed against VIP attenuate and delay the LH surge in estradiol-treated animals (20, 21). Despite these corroborating lines of evidence, other studies indicate equivocal effects of exogenous VIP administration, with VIP inhibiting GnRH in some instances and playing an excitatory role in others (20, 22). Additionally, VIP cells contact only a small percentage of GnRH cells (∼5–20%) across rodent species (9, 23), well below the percentage activated at the time of the surge (24). Importantly, GnRH neurons do not express estrogen receptor (ER)-α (25, 26), the ER subtype responsible for the positive feedback effects of estradiol on the LH surge (27). The fact that SCN-derived VIP projections cannot fully account for the LH surge suggests the existence of an additional mechanism(s) of circadian control that coordinate with timed VIP stimulation of the GnRH system.
The anteroventral periventricular nucleus (AVPV) is a critical neural locus for the initiation of the LH surge. Lesions of the AVPV eliminate estrous cyclicity (28). Furthermore, neurons within the AVPV project to GnRH cells and exhibit FOS expression coinciding with the timing of the LH surge (29). The SCN projects to the AVPV providing a neural pathway for temporally controlling cell populations driving GnRH activity (30, 31). SCN cells targeting the AVPV express vasopressin (AVP) (32, 33), and AVP injections produce surge-like LH levels in SCN-lesioned, ovariectomized (OVX), estradiol-treated rats (34). Finally, ERα-expressing neurons within the AVPV are direct targets of the SCN (33), potentially integrating circadian signals and positive feedback actions of estrogen.
Although abundant evidence indicates that the AVPV is necessary for circadian initiation of ovulation in rodents, the specific neural pathway(s) and cellular phenotype(s) involved in this process have not been fully characterized. The stimulatory neuropeptide, kisspeptin, provides an attractive target for further exploration. Kisspeptin and Kiss1 the gene encoding kisspeptin peptide, are expressed in the AVPV across species (35–37) and play a significant role in positively regulating the reproductive axis (reviewed in Ref. 38). Exogenous kisspeptin administration potently induces LH release and up-regulates FOS expression in GnRH neurons (35, 39). A large percentage of kisspeptin-immunoreactive (ir) neurons in the AVPV express ERα and Kiss1 mRNA is up-regulated by estradiol administration in OVX animals (40). Finally, Kiss1 cells express FOS at the time of the LH surge in naturally cycling and OVX, estradiol-treated rats (41, 42). Together these results suggest that Kiss1 neurons in the AVPV participate in estrogen-positive feedback and are positioned to receive circadian clock input.
An additional layer of complexity in exploring the role of the circadian system in LH surge initiation is that administration of SCN neuropeptides induces the surge only within a narrow time window (43), suggesting additional temporal control at SCN target loci. The means by which this timed gating mechanism controls responsiveness of the hypothalamic-pituitary-gonadal axis to SCN communication remains to be investigated. The gating of SCN information flow may be controlled within the AVPV, at the level of GnRH neurons, or a combination of both loci. The present studies explored the role of AVP and kisspeptin signaling in the timing of the LH surge using several approaches. First, we examined whether kisspeptin neurons in the AVPV are targets of vasopressinergic SCN input. Next, we asked whether the kisspeptin system exhibits a daily pattern of neuronal activity consistent with a role in ovulation and whether any emergent pattern is estrogen sensitive. Finally, we explored the possibility that this circuit is responsible for time-dependent sensitivity of the reproductive axis to SCN signaling by assessing whether: 1) kisspeptin cells within the AVPV respond in a time-dependent manner to AVP stimulation, 2) GnRH neurons display time-dependent sensitivity to kisspeptin signaling, or 3) both kisspeptin and the GnRH systems coordinate to gate the timed initiation of the LH surge. If time-dependent sensitivity to upstream circadian signaling is controlled at the level of the AVPV, then one would expect kisspeptin cells to exhibit daily changes in sensitivity to AVP stimulation. Alternatively, if the gating of control occurs within GnRH cells, then one would expect the GnRH system to display daily sensitivity in response to both AVP and kisspeptin administration.
Materials and Methods
Animals
Adult (>60 d of age), female LVG Syrian hamsters (Mesocricetus auratus) (n = 91) were used. Hamsters purchased from Charles River (Wilmington, MA) at 4–5 wk of age were housed in translucent propylene cages (48 × 27 × 20 cm) and provided with ad libitum access to food and water at all times. Animals were maintained in a colony room at 23 ± 1 C with a 24-h light: dark cycle (14 h light, 10 h dark) with lights on at 0700 h and lights off at 2100 h. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Berkeley.
Examination of the circadian pattern of kisspeptin activation
To determine whether kisspeptin expression is coordinated with the timing of the LH surge, we examined the activational state of AVPV kisspeptin neurons over the course of the day in ovariectomized hamsters given empty implants or implants containing estradiol (44). Before ovariectomy, estrous cyclicity was monitored by daily examination of vaginal discharge (45), and only females with regular 4-d estrous cycles were retained for study. To examine the pattern of kisspeptin cell activation independent of fluctuations in peripheral sex steroids, females were bilaterally OVX (n = 48) under isoflurane anesthesia. To determine whether daily changes in kisspeptin expression are estrogen dependent, animals were either treated with a SILASTIC capsule (Dow Corning Corp., Midland, MI; 10 mm length, 1.45 mm inner diameter, 1.93 mm outer diameter) containing powdered undiluted 17β-estradiol (n = 24) or an empty capsule (n = 24). In estrogen-implanted hamsters, this treatment generates estradiol concentrations comparable with those seen on the day of proestrus and results in daily LH surges at the same time each day, approximately 4 h before the onset of darkness (46). Two weeks after the ovariectomy and capsule implantation, hamsters were perfused (as described below) at either zeitgeber time (ZT) 7, 11, 13, and 16 (darkness onset at ZT 14) (n = 6/group) and brains were collected for histological analysis.
Examination of kisspeptin cells for SCN-derived input and receptor expression
To determine whether the SCN sends AVPergic or VIPergic projections to kisspeptin cells in the AVPV, adult Syrian hamsters (n = 5) were perfused on the day of proestrus at ZT 11. Brains were collected and stained for either kisspeptin and AVP (n = 5) or kisspeptin, and VIP using immunofluorescence (n = 5) and fiber contacts onto kisspeptin cells were evaluated as described below. To ensure that any fibers found within the AVPV contacting kisspeptin cell bodies originate from the SCN, five additional hamsters received bilateral electrolytic SCN lesions. Under deep anesthesia induced with a ketamine cocktail (21 mg ketamine, 2.4 mg xylazine, and 0.3 mg acepromazine per milliliter injected ip in a dose of 0.34 ml per 100 g body mass), the head was shaved and positioned in a stereotaxic apparatus (David Kopf Instruments, Tujanga, CA) and the hamster prepared for aseptic surgery. Lesions were aimed at the following coordinates: 0.9 mm anterior to bregma, 0.3 mm lateral to midline, and 7.8 mm below dura (31). Bilateral radio frequency lesions were made by applying 25 mV for 15 sec using a Radionics model RFG-4A Research RF lesion generator (Radionics, Burlington, MA) and stainless steel electrodes insulated with Epoxylite (The Epoxylite Corp., Irvine, CA), excluding the tip (0.20 mm). Lesioned brains were collected 24 h after surgery after perfusion as described below. Finally, to examine the coexpression of the vasopressin receptor, V1a, every fourth section was double labeled immunohistochemically for kisspeptin peptide and V1a protein.
Examination of kisspeptin and GnRH activation after timed central administration of vasopressin
Adult hamsters (n = 24) were OVX and implanted with SILASTIC capsules (Dow Corning) containing powdered 17β-estradiol as described above. After a 1-wk recovery period, a unilateral guide cannula (6 mm; Plastics One, Roanoke, VA) was implanted, aimed at the lateral ventricle. Coordinates for implantation were 0.6 mm lateral and 1.5 mm posterior to bregma and 4.5 mm ventral from the surface of dura mater. To maintain patency, dummy cannulas were attached to guide cannulae after surgery. After a 1-wk recovery period, cannulae placements were confirmed by assessing drinking behavior in response to angiotensin II injections (10 ng in 5 μl sterile saline). Because AVP fibers, but not VIP fibers (see Results), targeted kisspeptin cells, we focused on the role of this peptide in stimulating the LH surge. AVP (n = 12) (2 ng per 5 μl) or saline vehicle (n = 12) was administered early in the day at ZT 1 (when AVP is ineffective at stimulating the GnRH system) and in the afternoon at ZT 11 (when AVP stimulates the LH surge). Hamsters were perfused 1 h after injection and brains were collected and double labeled for kisspeptin/FOS and GnRH/FOS using immunofluorescence as described below.
Examination of GnRH activation after timed kisspeptin administration
To determine whether the GnRH system responds in a time-sensitive manner to kisspeptin and whether the GnRH system requires the presence of estradiol for the response to exogenous kisspeptin, hamsters were OVX and treated with empty (n = 36) SILASTIC capsules (Dow Corning) or capsules containing estradiol (n = 36). One week after surgery, kisspeptin (kisspeptin-10 (mouse); Phoenix Pharmaceuticals, Burlingame, CA) was injected (ip; 2 or 4 nmol) at ZT 1 or ZT 11 and hamsters perfused 1 h after injection. Brains were collected and double labeled for GnRH/FOS as described below.
Perfusion and histology
For brain collection, hamsters were deeply anesthetized with sodium pentobarbital (200 mg/kg) and perfused transcardially with approximately 150 ml of 0.9% saline, followed by 300–400 ml of 4% paraformaldehyde in 0.1 m PBS (pH 7.3). Double-labeled immunofluorescence was performed on every fourth, 40-μm coronal section. Sections were incubated for 48 h at 4 C with a rabbit polyclonal antikisspeptin-10 antiserum (1:2000; generated by J.D.M.), previously shown to bind with high affinity to kisspeptin neurons in the AVPV and arcuate nuclei and exhibit minimal cross-reactivity to related RFamide (Arg-Phe-NH2) peptides (47, 48), or rabbit anti-FOS (1:50,000; Santa Cruz Biotechnology, Santa Cruz, CA) and normal goat serum diluted at 1:1000 with 0.1% phosphate buffer containing Triton X-100 for 48 h. After incubation in the first primary antibody, brains were incubated for 1 h in biotinylated goat antirabbit (1:300; Vector Laboratories, Burlingame, CA) and then in avidin biotin complex for 1 h. For kisspeptin or FOS, the signal was amplified with biotinylated tyramide solution (0.6%) for 30 min as previously described (49). Cells were then labeled by using Cy-2 conjugated streptavidin (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA) as the fluorophore. After labeling the primary antibody, the sections were incubated in either a rabbit anti-GnRH antiserum (LR-5; 1:20,000; a generous gift from Dr. R. Benoit, McGill University Health Centre, Montreal, Quebec), rabbit anti-FOS antibody (1:5,000; Santa Cruz Biotechnology), a guinea pig anti-AVP antibody (1:10,000; Santa Cruz Biotechnology), a rabbit anti-V1a (1:500; Santa Cruz Biotechnology), or a guinea pig anti-VIP antibody (1:500; Santa Cruz Biotechnology) with 0.1% phosphate buffer containing Triton X-100 for 48 h (49). The second primary antibody was labeled with CY-3 donkey antirabbit (GnRH, V1a, FOS; 1:200; Jackson ImmunoResearch Laboratories) or CY-3 anti-guinea pig (AVP, VIP; 1:200; Jackson ImmunoResearch Laboratories) as the secondary antibody/fluorophore.
Several control procedures were implemented to ensure the specificity of immunohistochemical labeling. First, all antibodies were preadsorbed with their respective ligands for 24 h before tissue application. This procedure eliminated staining in all cases. Because kisspeptin is an RFamide peptide with a C terminus structure similar to that of RFamide-related peptide (RFRP), the kisspeptin antibody was preadsorbed with RFRP peptide to examine potential cross-reactivity. This procedure did not result in a change in the pattern or intensity of kisspeptin labeling, indicating that the antibody does not exhibit cross-reactivity with this peptide.
Light microscopy
Sections were examined using the standard wavelengths for CY-2 (488 nm) and CY-3 (568 nm) using a Zeiss Z1 microscope (Thornwood, NY). Every fourth section through the AVPV (AVP/kisspeptin, VIP/kisspeptin, or kisspeptin/FOS) and the medial septum (MS)/diagonal band of Broca (DBB), and medial preoptic area (MPOA; GnRH/FOS) were assessed. The transition from MS/DBB to the MPOA was considered to occur at the beginning of the organum vasculosum of the lamina terminalis, a region clearly defined by GnRH labeling. For light microscopy, kisspeptin cells identified as having AVP or VIP contacts or expressing FOS or GnRH cells expressing FOS were digitally captured at ×400 (fiber contacts) or ×200 (FOS) in 8-bit gray scale using a cooled charge-coupled device camera (Zeiss). Each label was captured as a single image without moving the position of the stage or plane of focus between captures. Images were superimposed digitally. Brain areas were examined by two independent observers using Photoshop software (Adobe Systems, Inc., San Jose, CA) to view CY-2 and CY-3 channels independently. Variation between observers was 1.4% for single-cell analysis and 3.7% for double-labeled analyses. Kisspeptin or GnRH cells with a clear nucleus were quantified using single-channel analysis. Cells were considered to be double labeled if FOS was expressed in the cell nucleus but not beyond the borders of each predefined nuclear area. All kisspeptin cells identified as having putative AVP or VIP contacts were examined using confocal analyses (see below). For FOS expression, only those kisspeptin and GnRH cells with a visible nucleus in which FOS expression was localized to the nucleus were counted as double-labeled cells.
Confocal microscopy
Cells were observed under a Zeiss Axiovert 100TV fluorescence microscope (Carl Zeiss) with a Zeiss LSM 510 laser-scanning confocal attachment. The sections were excited with an argon-krypton laser using the standard excitation wavelengths for CY-2 and CY-3. Stacked images were collected as 0.5 μm (fiber assessment) or 1.0 μm (FOS expression) multitract optical sections. Using the LSM 3.95 software (Zeiss), red and green images of the sections were superimposed. Kisspeptin or GnRH cells in a given brain region were examined through their entirety. To examine SCN contacts, kisspeptin cells with putative AVP or VIP contacts were scanned though the extent of each cell in 0.5 μm increments at ×400. Only those cells in which the AVP- or VIP-labeled fiber contacted a kisspeptin cell in the same 0.5-μm scan were counted as close contacts. Cells characterized as double labeled for FOS/kisspeptin or FOS/GnRH at the conventional microscopy level were confirmed in the same manner to ensure that FOS was expressed within the cells rather than in overlapping cells in the same light microscopic field of view. Likewise, cells classified as single labeled were assessed to ensure that the conventional microscopy strategy did not result in false-negative results. At least 10% of cells quantified using conventional microscopy were assessed in confocal scans for FOS colabeling. Regions of the brain with putative double label identified at the light level were scanned in 1.0-μm steps at ×400.
Statistics
Data were analyzed using SigmaStat software (Aspire Software, Intl., Ashburn, VA) for all studies. Data for kisspeptin cell counts, FOS expression in kisspeptin cells, and GnRH cells were analyzed using 2 × 4 (hormonal condition × time of day) ANOVA for those studies assessing the change in activation over the course of the day. Total cell counts and FOS expression in GnRH and kisspeptin cells were analyzed using 2 × 2 (treatment × time of day) AVOVA for studies after the injection of AVP. Data for total cell counts and FOS expression in GnRH cells were analyzed using 2 × 2 × 2 ANOVAs (hormonal condition × treatment × time of day) for studies after the injection of kisspeptin. Group differences were evaluated using Tukey honestly significant difference tests. Differences were considered significant if P < 0.05.
Results
The daily activational state of AVPV kisspeptin cells is coordinated with the LH surge and is estradiol dependent
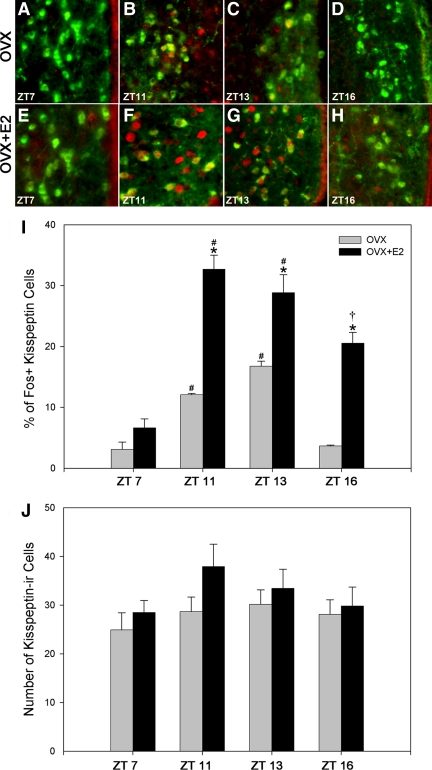
Daily fluctuations in kisspeptin neuronal cell activity were assessed at time points before, during, and after the time of the LH surge (ZT 7, 11, 13, 16) in OVX hamsters provided with either empty (Fig. 1, A–D) or estradiol-filled (Fig. 1, E–H) capsules. Syrian hamsters held in a 14-h light, 10-h dark cycle express LH concentrations on proestrous that peak 4 h before onset of darkness, with an initial increase in LH around 6 h before darkness and cessation of the surge approximately 2 h before lights out (13, 50). In hamsters provided with empty capsules and those with capsules containing estradiol, the percentage of kisspeptin cells expressing FOS exhibits a significant daily pattern consistent with a circadian-controlled mechanism participating in LH surge initiation (Fig. 1I). Neuronal activation was low in the early afternoon (ZT 7), increased markedly around the time of the LH surge (ZT 11 and ZT 13), and decreased 2 h after lights out (ZT 16). OVX hamsters exhibited a significantly lower percentage of kisspeptin cells expressing FOS than OVX + estradiol treated females at each time point measured (P < 0.05 in each case), with the exception of (ZT 7 (Fig. 1I). The total number of kisspeptin-ir cells was not impacted by time of day or hormonal condition (P > 0.05 in each case; Fig. 1J).
Fig. 1.
AVPV kisspeptin cellular activity follows a daily pattern of expression coincident with the LH surge. The percentage of AVPV kisspeptin-ir cells expressing FOS increases in the afternoon and peaks at ZT 11, around the time of the LH surge, and decreases thereafter. The daily pattern of expression is robust in OVX+estradiol (E2) hamsters, with the magnitude of this daily pattern at each time point significantly attenuated in the absence of estradiol, excluding ZT 7. A–D, Low-power photomicrographs of kisspeptin-ir cells expressing FOS in OVX hamsters at ZT 7 (A), around the time of the GnRH surge at ZT 11 (B) and ZT 13 (C), and 2 h after lights out at ZT 16 (D). E–H, Low-power photomicrographs of kisspeptin-ir cells expressing FOS in OVX+E2 hamsters at ZT 7 (E), around the time of the GnRH surge at ZT 11 (F) and ZT 13 (G), and 2 h after lights out at ZT 16 (H). I, Mean (±sem) percentage of kisspeptin-ir cells expressing FOS at various time points in OVX and OVX+E2 hamsters. J, Mean (±sem) number of kisspeptin-ir cells at various time points in OVX and OVX+E2 females. #, Significantly greater than kisspeptin cells expressing FOS at ZT 7 and ZT 16 within the same hormonal treatment (P < 0.05); *, significantly greater than kisspeptin cells expressing FOS in OVX hamsters within the same time point (P < 0.05); †, significantly greater than kisspeptin cells expressing FOS at ZT 7 within the same hormonal treatment.
Kisspeptin cells in the AVPV are contacted by SCN-derived AVP, but not VIP, fibers
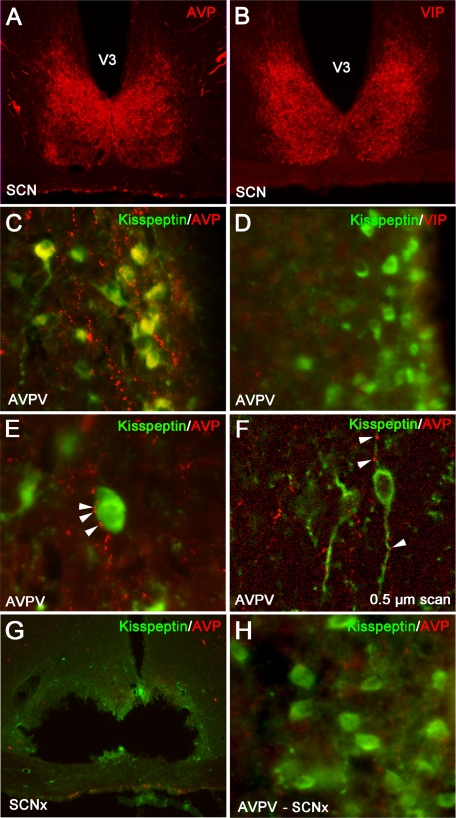
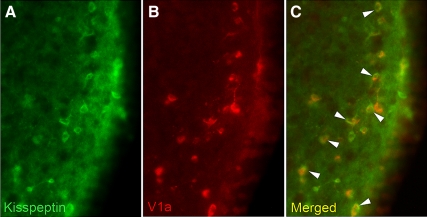
AVPV kisspeptin cells received extensive contacts (37.7 ± 6.3%) from AVP-ir fibers (Fig. 2C). All contacts at the light level were confirmed to be in the same 0.5 μm plane by confocal microscopy (Fig. 2F). Cells not exhibiting contacts at the light microscopic level did not have contacts at the confocal level. Conversely, in both light and confocal analyses, kisspeptin-ir neurons in the AVPV did not receive any contacts from VIPergic fibers (Fig. 2D), despite prominent VIP-ir fiber staining in relevant brain loci (MPOA, SCN, subparaventricular zone; Fig. 2A). Given that AVP-ir, but not VIP-ir, fibers contact AVPV kisspeptin cells, we sought to determine whether this cell population expresses the main AVP receptor subtype, V1a. We found that 42 ± 7.4% of kisspeptin-ir cells exhibited V1a-ir labeling (Fig. 3). To assess whether AVP-ir contacts on kisspeptin cell bodies in the AVPV originate from the SCN, electrolytic lesions were directed at the SCN, and double-labeled immunocytochemistry was performed for kisspeptin and AVP. Hamsters with lesions sparing the SCN served as controls. AVP-ir fiber appositions on kisspeptin cell bodies were eliminated in SCN-lesioned hamsters (Fig. 2, G and H). Importantly, SCN lesions eliminating AVP-ir contacts on kisspeptin cells spared the supraoptic nucleus and paraventricular nucleus (Fig. 2G).
Fig. 2.
Kisspeptin-ir cells in the AVPV receive SCN-derived fiber contacts expressing AVP-ir but not VIP-ir. A and C, Low-power photomicrographs of AVP-ir in the SCN (A) and in the AVPV (C), in which kisspeptin cell bodies receive extensive AVP-ir fiber contacts. B and D, Low-power photomicrographs of VIP-ir in the SCN (B) and in the AVPV (D), in which VIP-ir is virtually nonexistent around kisspeptin-ir cell bodies. E, High-power photomicrograph showing several presumptive AVP-ir terminal boutons on a kisspeptin-ir cell body. Arrows are indicative of close contacts. F, Confocal image (0.5 μm scan taken at ×400) confirming AVP-ir contacts upon kisspeptin-ir cell body and processes. Arrows are indicative of presumptive boutons. G and H, Low-power photomicrographs of AVP-ir in an SCN-lesioned hamster, at the level of the SCN (G), in which AVP-ir is maintained in the paraventricular nucleus and supraoptic nucleus, and in the AVPV (H), in which AVP-ir contacts upon kisspeptin-ir cell bodies is virtually eliminated after SCN lesions, confirming the AVP-ir fibers contacting kisspeptin-ir cell bodies originates from the SCN in C, and see Results.
Fig. 3.
Kisspeptin cells in the AVPV express the V1a receptor. Low-power photomicrographs of kisspeptin-ir cells in the AVPV (A), V1a-ir cells in the AVPV (B), and the merged image showing overlap between kisspeptin-ir and V1a-ir (C).
Central vasopressin administration reveals indiscriminate activation of kisspeptin neurons concomitant with gated activation of the GnRH system
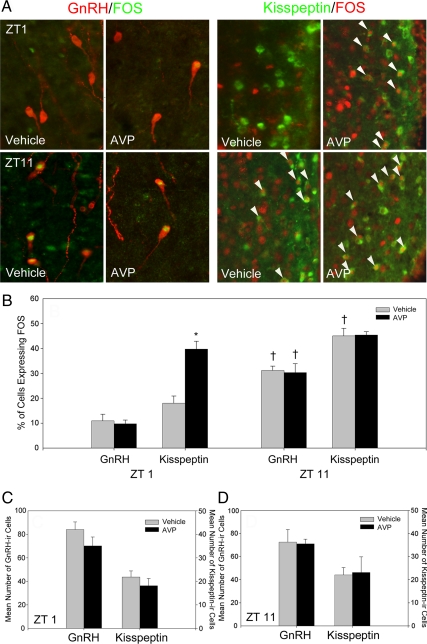
Kisspeptin neuronal activation was assessed following intracerebroventricular injections of saline or AVP at ZT 1 and ZT 11 in SCN-intact, OVX + estradiol females (Fig. 4). At ZT 1, the percentage of kisspeptin-ir cells expressing FOS was significantly increased over saline controls (P < 0.05) (Fig. 4B). At ZT 11, kisspeptin cells were maximally activated, with no differences observed between AVP-treated and saline controls (P > 0.05; Fig. 4B). In contrast, AVP infusions did not increase GnRH cell activation at ZT 1 relative to saline controls (Fig. 4B) (P >0.05). At ZT 11, both GnRH and kisspeptin cells were maximally activated, with no differences between saline or AVP-treated females (P > 0.05 in both cases) (Fig. 4B). Total GnRH-ir and kisspeptin-ir cell counts did not differ significantly across treatments or time periods (P > 0.05 in all cases) (Fig. 4, C, and D), indicating differences in FOS-ir are representative of the proportional changes in activation states in all cases.
Fig. 4.
Central vasopressin administration reveals indiscriminate activation of kisspeptin neurons concomitant with gated activation of the GnRH system. Intracerebroventricular administration of AVP robustly activates kisspeptin-ir cells in the morning (ZT 1), whereas GnRH-ir cells remain inactive at this time point. In the afternoon (ZT 11), both kisspeptin-ir and GnRH-ir cells express high levels of FOS activation after saline or AVP injection. A (left panel), Low-power photomicrographs of GnRH-ir cells expressing FOS after i.c.v. saline (vehicle) or AVP administration at ZT 1 or ZT 11. A (right panel), Low-power photomicrographs of kisspeptin-ir cells in the AVPV expressing FOS after i.c.v. saline (vehicle) or AVP administration at ZT 1 or ZT 11. (B) Mean (±sem) percentage of GnRH-ir and kisspeptin-ir cells expressing FOS after saline or AVP at ZT 1 or ZT 11. C and D, Mean (±sem) total number of GnRH-ir and kisspeptin-ir cells after saline or AVP at ZT 1 (C) or ZT 11 (D). *, Significantly greater than the percentage of kisspeptin cells expressing FOS in hamsters treated with vehicle at ZT 1 (P < 0.05); †, significantly greater than females given the same pharmacological treatment at ZT 1 (P < 0.05).
The GnRH system exhibits time-dependent sensitivity to kisspeptin stimulation
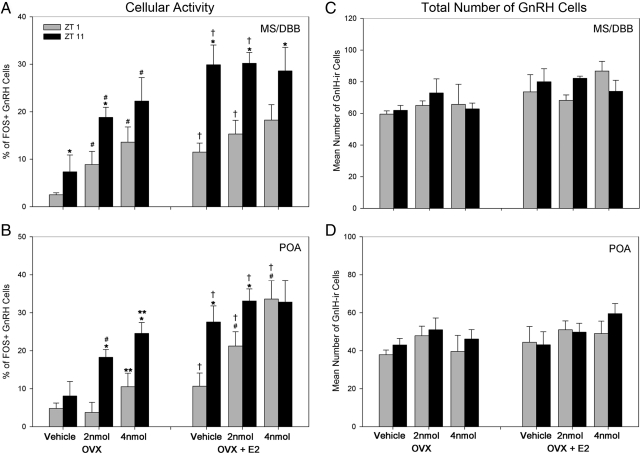
To examine whether the GnRH system is differentially sensitive to kisspeptin treatment at times when the LH surge cannot (ZT 1) or can (ZT 11) be induced, GnRH neuronal activation was assessed after injections of saline or kisspeptin (Fig. 5 and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The estrogen dependence of any differences was examined in OVX females treated with an empty or estradiol-filled capsule. In all cases, excluding the 4nmol dose of kisspeptin in the MS/DBB of OVX hamsters and the preoptic area (POA) of OVX + estradiol hamsters, kisspeptin was more effective at activating the GnRH system at ZT 11 than at ZT 1 (P < 0.05 in each case; Fig. 5, A and B). In both brain loci and both time points, estrogen increased the percentage of GnRH neurons expressing FOS in vehicle-treated controls and 2 nmol kisspeptin-treated hamsters compared with OVX females bearing empty capsules (P < 0.05 in all cases). The presence of estrogen also increased the percentage of GnRH neurons expressing FOS at the 4-nmol dose of kisspeptin relative to OVX females (P < 0.05). In OVX + estradiol hamsters probed at ZT 11, there were no differences among groups in either brain region, suggesting that the GnRH systems is maximally activated at this time due to endogenous stimulation of the GnRH in the presence of estradiol (P > 0.05 in all cases). Finally, the total number of GnRH cells was not affected by time, kisspeptin treatment, or estradiol (P > 0.05 in all cases) (Fig. 5, C and D).
Fig. 5.
The activation of GnRH after kisspeptin administration is time dependent, shows regional differences, and is enhanced by the presence of estradiol. A and B, The percentage of GnRH-ir cells expressing FOS after vehicle, 2 nmol, or 4 nmol kisspeptin administration at ZT 1 or ZT 11 in OVX and OVX+ estradiol (E2) hamsters. A, Mean (±sem) percentage of MS/DBB GnRH-ir cells expressing FOS after kisspeptin administration at ZT 1 or ZT 11 in OVX (left panel) and OVX+E2 (right panel) hamsters. B, Mean (±sem) percentage of POA GnRH-ir cells expressing FOS after kisspeptin administration at ZT 1 or ZT 11 in OVX (left panel) and OVX+E2 (right panel) hamsters. GnRH activation after kisspeptin administration is time dependent in OVX hamsters and is time dependent after 2 nmol kisspeptin in OVX+E2 hamsters. C, Mean (±sem) total GnRH-ir cells in the MS/DBB after kisspeptin administration. D, Mean (±sem) total GnRH-ir cells in the POA after kisspeptin administration. No differences were seen between cell counts, indicating the differences in FOS-ir represent proportional changes in GnRH activation levels. *, Significantly greater than hamsters provided with the same pharmacological treatment, at the same time point, and the same hormonal condition (P < 0.05); #, significantly greater than vehicle controls at the same time point and hormonal condition; **, significantly greater than vehicle controls and 2 nmol kisspeptin-treated hamsters at the same time point and same hormonal condition; †, significantly greater than OVX hamsters at the same time point and pharmacological treatment. All differences are significant at P < 0.05.
Discussion
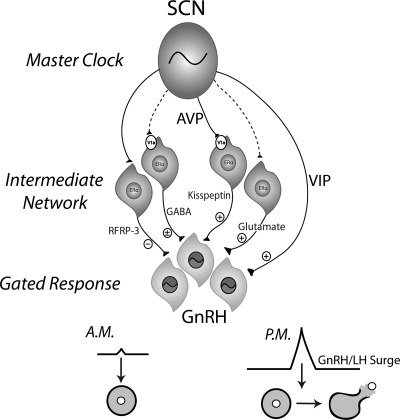
The present findings reveal a hierarchical organization of the hamster ovulatory circuitry involving interactions among the circadian system, a sex steroid integration center, and a final common pathway gating information flow. First, we find that AVPergic cells originating in the SCN target kisspeptin cells in the AVPV, a subpopulation that express the vasopressin receptor subtype, V1a. The AVPV population of kisspeptin neurons exhibits a daily pattern of activation with peak activity coincident with the timing of the GnRH/LH surge (13). The daily pattern of kisspeptin cellular activity is partially dependent on the presence of estradiol; ovariectomy markedly attenuates, but does not abolish, this daily activation. We also found that GnRH neurons act as gatekeepers, restricting the preovulatory LH surge to the late afternoon, at least in part, through daily changes in responsiveness to kisspeptin. More specifically, central administration of AVP increases kisspeptin cellular activation at both time points examined, whereas GnRH neurons are sensitive to AVP administration only during the afternoon, suggesting insensitivity to AVP-induced kisspeptin at this time. Finally, pharmacological studies of OVX and OVX/estrogen-primed hamsters confirm daily changes in GnRH cell responsiveness to kisspeptin that are dependent on the presence of estradiol. Together these findings point to kisspeptin neurons in the AVPV as an integration center for estradiol and circadian signals necessary for the generation of the LH surge and suggest that daily changes in GnRH neuron sensitivity to kisspeptin signaling further ensures appropriate timing of the surge (Fig. 6).
Fig. 6.
Model for the circadian control of the GnRH/LH surge. Proposed model by which several well-characterized, circadian-controlled neurochemicals participate in ovulation. The present studies and previous work indicate that the SCN sends efferent projections to kisspeptin neurons in the AVPV that express the V1a receptor and RFRP-3 (i.e. gonadotropin inhibitory hormone) neurons in the dorsomedial hypothalamus. GABAergic and glutamatergic neurons within the AVPV likely receive SCN input, but these pathways have not been explored (indicated by dashed lines). Together these intermediate signals represent integration sites of circadian and estrogenic input, with all neuronal phenotypes expressing estrogen receptors. GnRH neurons, in turn, receive input from these inhibitory and stimulatory afferents as well as direct VIPergic input from the SCN. In turn, the consequent response of the GnRH system will depend on the sum total of positive and negative influence and on time of day, the latter due to an inherent daily timing mechanism in GnRH cells.
In spontaneously ovulating rodents (e.g. rats, mice, hamsters), the SCN initiates the LH surge on the day of proestrus when estrogen concentrations are elevated (1). That estrogen is permissive for circadian stimulation of the GnRH system is somewhat paradoxical because estrogen negatively regulates GnRH secretion throughout the remainder of the cycle (51). We have previously shown that this feat is accomplished, at least in part, by the removal of inhibitory influences of the mammalian ortholog of avian gonadotropin inhibitory hormone, RFRP-3, at the time of the surge (13). Historically it was believed that direct VIPergic SCN projections targeting GnRH neurons were responsible for the positive arm of the ovulatory circuit (21, 52). However, given the paucity of ER expression in GnRH cells (25, 26), researchers began searching for estrogen-responsive targets of the SCN upstream of the GnRH system. Kisspeptin cells in the AVPV represented a likely candidate because this brain tissue expresses FOS coincident with the surge (29), contains ERs (53), and receives SCN input (30, 31). Likewise, kisspeptin cells are up-regulated by estrogen and express FOS at the time of the LH surge (36, 41), suggesting an important role in LH surge initiation. Whether AVPV kisspeptin cells represent an integration point for circadian/sex steroid signaling and might participate in the gating of circadian communication has not been explored.
Because AVPergic cells in the SCN project to the AVPV across species (32, 33) and AVP can only induce the LH surge during a limited time window in estradiol-implanted rats (43), we asked whether this population of kisspeptin cells might receive AVPergic SCN input and gate responsiveness to this peptide. We find that kisspeptin cells receive AVPergic SCN input and express V1a receptors, providing a direct means of communication from the circadian clock to this cell population. Recent findings in mice indicate that SCN-derived AVP cells project to the kisspeptin system (54), suggesting a common mechanism of control across rodent species. We also found that intracerebroventricular (i.c.v.) injections of AVP increase kisspeptin cellular activity. Importantly, although AVP can initiate the LH surge only during the afternoon in estrogen-treated animals (43), administration of this peptide enhances kisspeptin cellular activity in the morning (Fig. 4). In contrast, although kisspeptin activity was increased by AVP administration in the morning, GnRH activity was not. As expected, given endogenous AVPergic signaling during the afternoon, both GnRH and kisspeptin activity are maximal in both vehicle- and AVP-treated hamsters. This finding suggested that circadian information is not gated at kisspeptin cells but downstream of this cell population. Because GnRH neurons are the major target of kisspeptin (47, 48), this cell population represented a possible gating locus mediating the timing of ovulation.
Using immortalized GT1–7 cells, we documented that GnRH neurons exhibit time-dependent sensitivity to upstream stimulatory signals that initiate the LH surge, including kisspeptin and VIP (55). GnRH cells express the same clock genes driving circadian function at the cellular level (55–57), providing a potential time-keeping mechanism necessary to appropriately phase daily changes in sensitivity to upstream signaling. By administering kisspeptin in both the morning and afternoon to OVX hamsters with and without concurrent estrogen treatment, several findings emerged that enhance our understanding of the mechanisms that time ovulation (Fig. 5). In the morning (ZT 1), GnRH neurons are considerably less responsive to kisspeptin administration than the afternoon (ZT 11), during which treatment reliably enhances GnRH cellular activity. This attenuated responsiveness is unlikely due to ineffective doses of kisspeptin because previous studies indicated that the GnRH system responds robustly to doses as low as 1 nmol in rats and 1 fmol in mice (36, 58). The presence of estradiol generally enhanced GnRH cellular activity in kisspeptin and vehicle-treated hamsters. These findings suggest that the gating of GnRH activation may occur via circadian-mediated differences in kisspeptin receptor expression or high inhibitory tone during the morning. Notably, a reliable dose-response to kisspeptin is observed in the POA population of GnRH neurons of vehicle-treated hamsters at hamsters at ZT 11 but not ZT1 (Fig. 5B). As expected, during the afternoon when endogenous release of AVP and kisspeptin are maximal, estrogen serves a permissive role in GnRH cell activation, leading to elevated GnRH activity, regardless of treatment (Fig. 5B), thereby masking a dose response. Importantly, that OVX hamsters (Fig. 5B, left panel) exhibit a dose response to kisspeptin at ZT 11 further suggests that the endogenous activation of GnRH in estrogen-implanted hamsters accounts for the ceiling effect in estradiol-implanted animals. Whether GnRH gates responsiveness to all upstream signaling represents an important area for further inquiry.
If the LH surge is initiated solely by the integration of estradiol with circadian signaling at AVPV kisspeptin neurons, all downstream effects should reflect the extent of kisspeptin release, and kisspeptin treatment should maximize GnRH cellular activity at any time of day, even in the absence of estrogen. The fact that the addition of estradiol potentiates kisspeptin-stimulated GnRH cellular activity suggests additional, estrogen-dependent mechanisms of stimulatory control. ERs have been localized to the SCN of humans (59), mice (60), and rats (61), suggesting that AVP or VIP neurons might be directly responsive to this sex steroid. Likewise, in addition to the requirement of estradiol to maximize GnRH cellular activity, the GnRH response to kisspeptin stimulation is time dependent, suggesting that the surge is not simply elicited when the GnRH system is stimulated with kisspeptin but that the GnRH system gates daily responsiveness to this peptide. These findings agree with our previous findings of daily changes in the sensitivity of the GnRH system to kisspeptin administration using GT1–7 cells (55), and studies in which central infusion of kisspeptin i.c.v. (62) or into the mPOA (63) fail to advance the onset of the LH surge in either naturally cycling or OVX and estradiol/progesterone-primed female rats.
Recent findings in female mice provide converging support for the present results implicating circadian control of kisspeptin in ovulation. Kiss1/c-fos mRNA is maximally coexpressed to approximately 40% around the time of the GnRH surge when mice are held in constant conditions (64), indicating circadian control of kisspeptin cellular activity. Our immunohistochemical studies indicate a maximum coexpression of kisspeptin/FOS of 32% around the time of the LH surge, slightly lower than the maximal Kiss1/c-fos mRNA coexpression previously reported. The disparity between these findings and the present findings may be the result of posttranscriptional Kiss1 regulation, leading to a lower number of cells expressing the mature peptide. Additionally, it is possible our quantification strategy was more conservative, restricting our quantification to only those FOS-labeled cells with a clear nucleus. In this same report, ovariectomy without estradiol replacement abolished this daily pattern. The latter finding is at odds with the present result, indicating that the rhythm of kisspeptin/FOS is grossly attenuated, but maintained, in OVX hamsters not treated with estradiol. This partial dependence on estrogen is consistent with the daily pattern of LH secretion that persists, albeit with a lower amplitude than estradiol-implanted animals, in OVX Syrian hamsters (50). Whether this disparity is due to species differences or a discrepancy in transcriptional/translational differences in the daily pattern of Kiss1/kisspeptin represents an interesting question for further exploration.
It is likely that other neural loci upstream of the GnRH systems are targets of the SCN involved in the timing of the LH surge. Dual-phenotype neurons expressing γ-aminobutyric acid (GABA)/glutamate within the AVPV, for example, have been implicated in the control of GnRH activity and are regulated by estradiol feedback (65–67). Likewise, GnRH neurons respond to GABA differentially across the day, with excitatory responses in the afternoon (51, 68). Although no direct connections have been reported between the SCN and GABA neurons within the AVPV, the diurnal shift in GABA release (68) and the expression of V1a receptors in GABA neurons within the AVPV (69) point to a potential circadian mechanism regulating this cell population. Furthermore, kisspeptin up-regulates GABA transmission in the AVPV during estrogen-negative (but not positive) feedback, further suggesting a local control within the AVPV (70). In apparent contrast to the present findings, in one previous study, unilateral AVPV administration of a V1aR antagonist did not alter the timing or amplitude of the LH surge in rats (43). However, because the V1aR antagonist was injected unilaterally and the position of the dialysis probe was variable, it is unclear to what extent the full population of kisspeptin cells was impacted. Other studies of rats indicate that bilateral suppression of V1aR attenuates the LH surge in proestrous rats (71). Additionally, in cocultures of POA and SCN, the GnRH surge is coordinated with the rhythm in AVP, but not VIP (72), providing further evidence for an important role of AVP in surge generation.
The present studies reveal a novel, circadian-controlled neurochemical pathway participating in the LH surge. Kisspeptin neurons in the AVPV are targets of AVPergic SCN fibers and express V1a receptors, providing evidence for a direct connection between the master circadian clock and kisspeptin neurons in the AVPV. Kisspeptin neurons exhibit a daily activation pattern coincident with the timing of the GnRH/LH surge that is dependent on the presence of estradiol, further implicating these neurons as integrators of circadian and estrogenic signals necessary for the preovulatory LH surge. AVPV kisspeptin cells are indiscriminately activated by SCN peptidergic signaling, whereas the GnRH system displays time-dependent responsiveness to AVP and kisspeptin stimulation. Together these findings point to a novel mechanisms of ovulatory control whereby circadian and estrogenic signals converge on AVPV kisspeptin cells and kisspeptin signaling is gated by daily changes in GnRH cell sensitivity to this peptide.
Supplementary Material
Acknowledgments
We thank Dr. Irving Zucker for helpful comments and discussion on an earlier version of this paper and Nina Brahme and Diane Liang for excellent technical support.
This work was supported by National Institutes of Health Grant HD050470 (to L.J.K.).
Disclosure Summary: The authors of this manuscript have nothing to disclose.
Footnotes
- AVP
- Arginine vasopressin
- AVPV
- anteroventral periventricular nucleus
- DBB
- diagonal band of Broca
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- ir
- immunoreactive
- i.c.v.
- intracerebroventricular
- MPOA
- medial POA
- MS
- medial septum
- OVX
- ovariectomized
- POA
- preoptic area
- RFRP
- RFamide-related peptide
- SCN
- suprachiasmatic nucleus
- VIP
- vasoactive intestinal polypeptide
- ZT
- zeitgeber time.
References
- 1. de la Iglesia HO, Schwartz WJ. 2006. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153 [DOI] [PubMed] [Google Scholar]
- 2. Kriegsfeld LJ, Silver R. 2006. The regulation of neuroendocrine function: timing is everything. Horm Behav 49:557–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christian CA, Moenter SM. 2010. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 31:544–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore RY, Eichler VB. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206 [DOI] [PubMed] [Google Scholar]
- 5. Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. 1987. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7:1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ralph MR, Foster RG, Davis FC, Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978 [DOI] [PubMed] [Google Scholar]
- 8. Morin LP, Allen CN. 2006. The circadian visual system, 2005. Brain Res Rev 51:1–60 [DOI] [PubMed] [Google Scholar]
- 9. Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. 1997. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384:569–579 [DOI] [PubMed] [Google Scholar]
- 10. Horvath TL. 1997. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells, including those producing dopamine. Endocrinology 138:1312–1320 [DOI] [PubMed] [Google Scholar]
- 11. Legan SJ, Karsch FJ. 1975. A daily signal for the LH surge in the rat. Endocrinology 96:57–62 [DOI] [PubMed] [Google Scholar]
- 12. Mahoney MM, Sisk C, Ross HE, Smale L. 2004. Circadian regulation of gonadotropin-releasing hormone neurons and the preovulatory surge in luteinizing hormone in the diurnal rodent, Arvicanthis niloticus, and in a nocturnal rodent, Rattus norvegicus. Biol Reprod 70:1049–1054 [DOI] [PubMed] [Google Scholar]
- 13. Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. 2008. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149:4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chappell PE. 2005. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol 17:119–130 [DOI] [PubMed] [Google Scholar]
- 15. Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. 2004. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 14:1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nunez AA, Stephan FK. 1977. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol 20:224–234 [DOI] [PubMed] [Google Scholar]
- 17. Wiegand SJ, Terasawa E. 1982. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404 [DOI] [PubMed] [Google Scholar]
- 18. Gray GD, Söderstein P, Tallentire D, Davidson JM. 1978. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology 25:174–191 [DOI] [PubMed] [Google Scholar]
- 19. van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. 1993. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol 5:137–144 [DOI] [PubMed] [Google Scholar]
- 20. Harney JP, Scarbrough K, Rosewell KL, Wise PM. 1996. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137: 3696–3701 [DOI] [PubMed] [Google Scholar]
- 21. van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. 1994. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134:2636–2644 [DOI] [PubMed] [Google Scholar]
- 22. Weick RF, Stobie KM. 1992. Vasoactive intestinal peptide inhibits the steroid-induced LH surge in the ovariectomized rat. J Endocrinol 133:433–437 [DOI] [PubMed] [Google Scholar]
- 23. Kriegsfeld LJ, Silver R, Gore AC, Crews D. 2002. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J Neuroendocrinol 14:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee WS, Smith MS, Hoffman GE. 1990. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA 87:5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. 1998. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36:357–378 [DOI] [PubMed] [Google Scholar]
- 26. Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. 2001. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- 27. Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. 2006. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiegand SJ, Terasawa E, Bridson WE, Goy RW. 1980. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157 [DOI] [PubMed] [Google Scholar]
- 29. Le WW, Berghorn KA, Rassnick S, Hoffman GE. 1999. Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140:510–519 [DOI] [PubMed] [Google Scholar]
- 30. Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. 1995. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res 689:254–264 [DOI] [PubMed] [Google Scholar]
- 31. Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. 2004. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol 468:361–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leak RK, Moore RY. 2001. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol 433:312–334 [DOI] [PubMed] [Google Scholar]
- 33. de la Iglesia HO, Blaustein JD, Bittman EL. 1995. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6:1715–1722 [DOI] [PubMed] [Google Scholar]
- 34. Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. 1999. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- 35. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 36. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 37. Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. 2007. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav 52:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 40. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 41. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. 2006. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 43. Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. 2001. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901:109–116 [DOI] [PubMed] [Google Scholar]
- 44. Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. 1999. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- 45. Orsini MW. 1987. Citation classic—the external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster, Mesocricetus auratus. Cc/Agr Biol Environ 7:18–18 [Google Scholar]
- 46. Norman RL, Blake CA, Sawyer CH. 1973. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- 47. Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. 2010. Mapping of kisspeptin fibres in the brain of the pro-oestrus rat. J Neuroendocrinol 22:1101–1112 [DOI] [PubMed] [Google Scholar]
- 48. Mikkelsen JD, Simonneaux V. 2009. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 30:26–33 [DOI] [PubMed] [Google Scholar]
- 49. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. 2006. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stetson MH, Watson-Whitmyre M, Matt KS. 1978. Cyclic gonadotropin release in the presence and absence of estrogenic feedback in ovariectomized golden hamsters. Biol Reprod 19:40–50 [DOI] [PubMed] [Google Scholar]
- 51. Petersen SL, Ottem EN, Carpenter CD. 2003. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778 [DOI] [PubMed] [Google Scholar]
- 52. Horvath TL, Cela V, van der Beek EM. 1998. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res 795:277–281 [DOI] [PubMed] [Google Scholar]
- 53. Herbison AE. 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kallo I. 2010. Evidence for suprachiasmatic vasopressin neurons innervating kisspeptin neurons in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol 22:1032–1039 [DOI] [PubMed] [Google Scholar]
- 55. Zhao S, Kriegsfeld LJ. 2009. Daily changes in GT1–7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology 89:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chappell PE, White RS, Mellon PL. 2003. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1–7 cell line. J Neurosci 23:11202–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hickok JR, Tischkau SA. 2010. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology 91:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2005. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 146:1689–1697 [DOI] [PubMed] [Google Scholar]
- 59. Kruijver FP, Swaab DF. 2002. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 75:296–305 [DOI] [PubMed] [Google Scholar]
- 60. Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. 2003. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology 144:2055–2067 [DOI] [PubMed] [Google Scholar]
- 61. Su JD, Qiu J, Zhong YP, Chen YZ. 2001. Expression of estrogen receptor -α and -β immunoreactivity in the cultured neonatal suprachiasmatic nucleus: with special attention to GABAergic neurons. Neuroreport 12:1955–1959 [DOI] [PubMed] [Google Scholar]
- 62. Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. 2008. Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149:1627–1637 [DOI] [PubMed] [Google Scholar]
- 63. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. 2009. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology 150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. 2009. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leranth C, MacLusky NJ, Sakamoto H, Shanabrough M, Naftolin F. 1985. Glutamic acid decarboxylase-containing axons synapse on LHRH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539 [DOI] [PubMed] [Google Scholar]
- 66. Jarry H, Leonhardt S, Schwarze T, Wuttke W. 1995. Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 62:479–486 [DOI] [PubMed] [Google Scholar]
- 67. Christian CA, Pielecka-Fortuna J, Moenter SM. 2009. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod 80:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Christian CA, Moenter SM. 2007. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kalamatianos T, Kalló I, Goubillon ML, Coen CW. 2004. Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: effects of oestrogen. J Neuroendocrinol 16:525–533 [DOI] [PubMed] [Google Scholar]
- 70. Pielecka-Fortuna J, Moenter SM. 2010. Kisspeptin increases γ-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 151:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F. 1999. Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats. Neurosci Lett 260:37–40 [DOI] [PubMed] [Google Scholar]
- 72. Funabashi T, Shinohara K, Mitsushima D, Kimura F. 2000. Gonadotropin-releasing hormone exhibits circadian rhythm in phase with arginine-vasopressin in co-cultures of the female rat preoptic area and suprachiasmatic nucleus. J Neuroendocrinol 12:521–528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.