Abstract
Mice deficient in lysyl oxidase-like1 protein (LOXL1−/−) develop pelvic organ prolapse (POP). We sought to determine the impact of LOXL1−/− on the biomechanical properties of the vagina and its supportive tissues tested as a complex. Tissues of nulliparous LOXL1−/− and age-matched wild type (WT) mice were tested to failure to obtain load-distension curves. Data were compared utilizing one-way analysis of variance and appropriate post hoc tests. The groups demonstrated different biomechanical behavior, with LOXL1−/− animals displaying a 31% decrease in ultimate load at failure (p=0.001). Experimental disruption of specific levels of support in WT mice failed to generate load-distension curves similar to the LOXL1−/− mice indicating a global instead of a site-specific tissue defect. The decrease in the ultimate load at failure in the LOXL1−/− mice suggests mechanically weaker tissues. LOXL1 mutation results in a global defect in connective tissues and correlates with altered biomechanical behavior of the vagina and supportive tissues.
Keywords: Pelvic floor, Elastin, Prolapse, LOXL1
Introduction
Pelvic organ prolapse is a prevalent costly condition that negatively impacts the quality of life of women [1]. The etiology of pelvic organ prolapse has not been clearly defined, although recent data suggest that genetic predispositions may contribute to this multifactorial condition [2, 3]. In addition to the levator ani muscles, the supportive connective tissues of the vagina play a primary role in maintaining the proper position of the pelvic organs [4], and the structural integrity of these tissues is essential for the prevention of the prolapse [5]. Despite a well-known contribution of the levator ani muscles to the support of the pelvic floor, a recent study demonstrated that a large proportion of women with prolapse had no evidence of levator ani muscle injury [6], again pointing towards multiple factors involved in pelvic floor support. Women with congenital defects in connective tissue are found to be at increased risk for the development of prolapse [7, 8]. Previous studies have also demonstrated abnormalities in the histomorphology and ultrastructure of vaginal supportive connective tissues in women with prolapse relative to women without prolapse [9, 10]; however, from these studies, it is impossible to distinguish between causes and effects of prolapse. Consequently, there has been little data from studies of human tissue that have advanced our knowledge regarding the pathogenesis of prolapse.
Vaginal support arises from the continuous, highly interdependent sheet of connective tissue attachments between the vagina and the pelvic sidewall and levator ani muscles [4, 11]. Disruptions of or damage to the supportive connective tissues and injury to the vaginal wall are thought to be two mechanisms by which prolapse is initiated. The vagina and its supportive connective tissues contain a fibrillar component comprised of collagen and elastin. This fibrillar component is thought to contribute the most to the biomechanical behavior of these tissues, with collagen primarily responsible for the tensile strength and elastin affording the tissues' ability to extend and recoil [12]. While the turnover of most elastin fibers in the body is exceedingly slow, elastin in the reproductive tract rapidly remodels throughout the reproductive lifespan, particularly following childbirth [13]. However, in women with pelvic organ prolapse, a marked decrease in elastin production has been found in the vaginal supportive tissues [12].
The lysyl oxidases (LOX) and lysyl oxidases-like (LOXL) enzymes comprise a family of enzymes that crosslink immature elastin via the hydroxylation of lysine residues, thereby forming a more stable mature protein [14]. Mechanically compromised elastic tissues such as those found in Menkes' syndrome and lathyrism are associated with decreased activity of LOX, demonstrating that adequate crosslinking is essential for proper elastin function [15,16]. Mammalian genomes have up to five LOX family members encoding the prototypic LOX and LOX-like proteins 1 through 4 (LOXL1, LOXL2, LOXL3 and LOXL4); however, their individual roles in elastogenesis remain unclear [17]. Recently, LOXL1 has been shown to co-localize with elastic fibers and to be essential for elastic fiber homeostasis [18]. Mice with LOXL1 deficiency (LOXL1−/−) develop pelvic organ prolapse following parturition due to a failure to replenish mature crosslinked elastin fibers [19]. In addition, the expression of LOXL1 has been shown to diminish with age [19].
Although there is good evidence from these studies that a genetic defect in elastin crosslinking predisposes to pelvic organ prolapse, there is no functional data demonstrating the impact of LOXL1 deficiency on the vagina and its supportive tissues. Therefore, the aim of this study was to compare the biomechanical properties of the vagina and its supportive tissues in LOXL1-deficient mice relative to the wild type mice independent of parity. In addition, we investigated the functional impact of age by comparing young and old wild-type mice. We hypothesized that a genetic defect in elastogenesis represented in the LOXL1-deficient mice, predisposes to prolapse secondary to biomechanically weaker tissues independent of parturition. To test our hypothesis, we utilized nulliparous LOXL1−/−mice.
Materials and methods
Animals
Animals were kept in static micro-isolator filter top cages with Aspen Sani-Chip bedding. The animals were fed a daily diet of Purina Mills rodent diet, formulation 2000. Water was provided ad libitum. Mice were housed under a 12-hour light cycle (lights on, 6:00AM to 6:00PM) at 22°C. The conditions for the wild-type (WT) and the LOXL1-deficient mice (mutant) were the same. A total of 37 female mice, including 3-month-old nulliparous WT mice of a C57B16 background (Hilltop Lab Animals, Scottsdale, PA; N = 22), 17-month-old nulliparous WT mice of a C57B16 background (Hilltop Lab Animals, Scottsdale, PA; N = 6), and 17-month-old nulliparous homozygous LOXL1 deficient mice of a 129/Sv and C57BL/6 background (Washington University School of Medicine, Department of Internal Medicine/Dermatology, St. Louis, MO; N = 9) were sacrificed according to IACUC guidelines. LOXL1-deficient mice were generated as previously described [20]. The severity of rectal and vaginal prolapse was assessed through gross examination prior to sacrifice. The weight, total vaginal length, and genital hiatus measurements were obtained for each animal. Total vaginal length was measured with a calibrated measuring device which was gently inserted in the vagina of the anesthetized mouse and advanced until the posterior fornix was reached. Genital hiatus was measured with the same measuring device from the anterior to the posterior aspect of the vaginal introitus in the midline. Five mutant mice with intact vaginal supportive connective tissues were used for biomechanical testing along with ten 3-month-old and six 17-month-old WT mice. Four mutant animals had developed gross disruptions of the vaginal supportive connective tissues identified upon dissection and, therefore, were not used for biomechanical testing. Vaginal tissue from these animals and four WT mice were utilized for histomorphological analysis. To evaluate the impact of age on biomechanical properties of the pelvic tissues in WT mice, six age-matched WT animals were tested at the age of 17 months.
An additional twelve C57B16 WT mice (Hilltop Lab Animals, Scottsdale, PA) were surgically manipulated after sacrifice to distinguish whether a deficiency in LOXL1−/− was primarily impacting specific levels of support vs. having a global impact on the pelvic tissues. For conceptual purposes, vaginal supportive tissues have been subdivided into three levels in the human female: the uterosacral ligaments (level I), the paravaginal attachments (endopelvic fascia) connecting the lateral vaginal walls to the arcus tendineous fascia pelvis (level II), the perineal membrane, and the perineal body (level III) [21]. In the rodent, three analogous levels of vaginal support have been established [22]. Despite existence of similar anatomical structures, the exact role and vulnerability of pelvic support structures might not be analogous between humans and rodents.
To determine whether disruption of a specific level of support was contributing to the biomechanical behavior of the vagina–supportive tissue complex in LOXL1−/− mice, specific levels of support were disrupted in WT mice. We hypothesized that if LOXL1−/− deficiency was primarily affecting a specific level of vaginal support, we should be able to mimic the biomechanical behavior by experimentally disrupting tissue support at that site. In this way, level I support was severed in four WT mice by transecting the uterosacral ligament at the level of the uterus; level II support was disrupted in another four WT animals by separating the paravaginal attachments from the pelvic wall. In an additional four WT mice, both levels I and II were transected. Following disruption of each level of support, we proceeded with biomechanical testing as described below.
Histological analysis
Full-thickness vagina of four LOXL1-deficient mice with severe and moderate prolapse along with four 3-month-old WT mice vaginas were excised and imbedded in Optimal Cutting Temperature media (Sakura Finetek Inc., Torrance, CA), cut into 5–7-μm sections with Cryostat and stained with Masson's trichrome for examination of gross morphologic features.
Biomechanical testing
All mice were tested according to a protocol previously developed by us in which the vagina and its supportive tissues are tested as a complex with all of the insertion and attachment points of the complex left intact [22]. It is important to point out that our ability to perform a controlled multidirectional test in this system is limited, as the pelvis encases the tissues of interest. The goal of the test are to quantitate the bio-mechanical behavior of the vagina–supportive tissue complex in response to one specific loading conditioning, i.e., downward distension along its longitudinal axis. The data obtained from this test are most relevant to understanding the function of these tissues in response to loads that might be generated by prolonged increases in intra-abdominal pressure such as chronic coughing, heavy lifting, and valsalva.
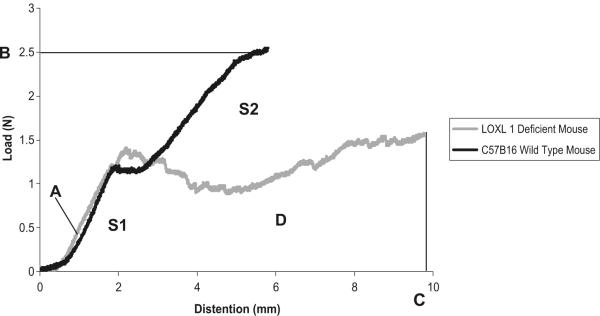
After sacrifice, the mice were dissected down to the level of the pelvis to expose the vagina and supportive tissues. The hind limbs were disarticulated at the acetabulum and the spine was disarticulated above the L1 vertebra as previously described [22]. The specimens were wrapped in gauze, sealed in plastic bags, and maintained on ice until testing. The specimens were kept moist with physiologic saline during dissection and testing. Prior to testing, the specimen were mounted in a custom-made testing device as previously described in detail [22]. Prior to each test, the specimens were preloaded to 0.1N, followed by ten cycles of preconditioning at a velocity of 5 mm/min between 0 and 0.75 mm distension. A uniaxial load (load applied along the longitudinal axis of the vagina) to failure test was conducted for each specimen following preconditioning at the same velocity. Data points were collected every 0.02 s using software provided by Enduratec. The load–distension curves were generated from each load to failure test (Fig. 5). Four parameters describing the biomechanical properties of the vagina–supportive tissue complex are derived from the curve: linear stiffness (N/mm), ultimate load at failure (N), maximal distension (mm), and energy absorbed to failure (N–mm) (Fig. 5). The peak of the curve corresponds to failure of the specimen. Linear stiffness (A), which is the maximum slope of the curve measured in the linear region over a 1-mm range of distension; ultimate load at failure (B), represented as y-intercept corresponding with the peak of the curve; maximal distension at failure (C), represented as the x-intercept corresponding with the peak of the curve; and energy absorbed to failure, which is the area under the load–distension curve (D) (Fig. 5). The linear stiffness and maximum distension generally describe the ability and degree to which the specimen can deform in response to a defined load. Specimens that are easily deformable have low linear stiffness and generally distend more in response to the same force. The ultimate load at failure describes the maximal force that the specimen is able to withstand prior to disruption, while the energy absorbed simply describes the energy expended by the specimen to resist deformation. Given that the test is not multidirectional, it does not perfectly reproduce heterogeneous forces that contribute to the development of prolapse. The goal of this test was to quantitate the biomechanical behavior of the vagina and supportive tissues as a complex in response to one specific loading condition, i.e., downward distension along the longitudinal axis of the vagina. The data obtained from this test is most relevant to understanding the function of these tissues in response to loads that might be generated by increased intra-abdominal pressure in women.
Fig. 5.
Representative load–distension curves generated following a load to failure test in WT and LOXL1−/−mice. Before each test, the specimens were preloaded and preconditioned. Linear stiffness (A), is the slope of the curve measured in the linear region of the curve; ultimate load at failure (B); maximal distension at failure (C); and energy absorbed to failure is the area under the load–distension curve (D)
Data analysis and statistics
The data were imported and analyzed in Excel (Excel, Microsoft Corp, Redmond, WA). Biomechanical properties of the vagina and its supportive tissues were derived from the load–distension curve as previously described [22].
Statistical analyses were performed using SPSS statistical software version release 14.0.1 (SPSS Inc., Chicago, IL). Statistical tests were two-sided at the 0.05 significance level. Since the skewness and kurtosis values did not indicate a departure from symmetry in the data distribution, one-way analysis of variance was used to evaluate differences in the means of the biomechanical properties between the WT mice, the LOXL1−/− mice, and the WT mice with disruption of levels of support. Post-hoc pairwise comparisons between the WT mice and all other groups were made using Dunnett's multiple comparisons procedure. Linear regression was used to evaluate the association between biomechanical variables of interest and weight. One-way analysis of variance was used to compare 3-month-old WT mice, 17-month-old WT mice, and LOXL1−/− animals. Post-hoc pairwise comparisons were made using the Sidak multiple comparisons procedure. Based on preliminary data from the 3-month-old WT mice, four and five animals per group were required to have 80% power to detect a 30% difference in load and distension, respectively, at the 0.05 significance level.
Results
Gross pathology
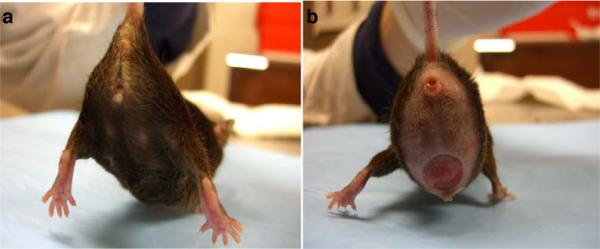
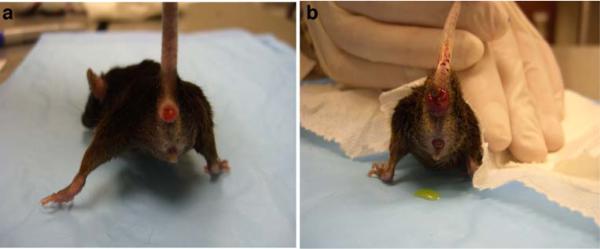
The organs involved and the severity of the prolapse varied in the LOXL1-deficient mice. Of the nine LOXL1-deficient animals, three had severe, one had moderate, and five had minimal to no prolapse with the majority of animals exhibiting a prominent perineal bulge (Fig. 1b). Transanal rectal prolapse was a common finding, occurring in four animals (Fig. 2). Prolapse of the remaining pelvic organs into the vagina (bladder, uterus, or bowel) was observed in five animals (Fig. 1). Vaginas of the LOXL1−/− mice were circumferentially distended and appeared thinned out, resulting in a genital hiatus (GH) that was larger than in the WT controls: 0.51 cm ± 0.50 in the mutant animals vs. 0.2 cm in the WT mice. However, this difference did not reach statistical significance (p = 0.07) (Table 1). Upon dissection, the vagina of WT animals was found to be well-supported. Close dissection showed that the vagina and cervix were maintained in position by soft tissue attachments to the posterior pelvis (level I). The remaining vagina was supported by paravaginal attachments to the sidewall and pubocaudalis muscle (level II) and to the perineum (level III). The uterine cervix of controls was at the level of the pubic symphysis and the urethra was identified along the length of the cervix. With intact vaginal support, the bladder was positioned above the level of the pubic symphysis and above the uterine cervix. The anatomy of the vagina and its supportive tissues in our study was consistent with that described previously by others [23]. Mutant mice with prolapse were noted to have prolapse of the uterus, bladder, and vaginal tissue with uterine horns descending to the level of the pubic symphysis, with notable disruption of the supportive tissues. In three LOXL1-deficient mice with severe prolapse, the urethra was obstructed, resulting in a distended bladder with hydroureter and hydronephrosis (Fig. 3). Weight varied between the groups, with the 17-month-old WT animals being the heaviest (p < 0.001) followed by the LOXL1 knockouts. The difference in weights in the young and old animals is due to the progressive increase in weight of mice with age. We attributed the failure of the knockout mice to achieve the weight of their age-matched controls to poor growth associated with the mutation.
Fig. 1.
Gross anatomy of the LOX1−/−female mouse demonstrating varying degrees of prolapse: a no visible sign of prolapse and b severe prolapse
Fig. 2.
Gross anatomy of the LOX1−/−female mouse demonstrating moderate a and severe b rectal prolapse
Table 1.
Comparison of weight and genital hiatus measurements in wild-type (WT) and mutant mice
| Property | 3-month-old WT(n=10) | 17-month-old WT (n=6) | 17-month-old LOXL1−/−mice (n=9) | p value* |
|---|---|---|---|---|
| Genital hiatus (cm) | 0.20a | 0.20 | 0.51±0.50 | 0.07 |
| Weight (g) | 22.23±1.56 | 45.70±3.51 | 28.72±3.03 | <0.001 |
p value obtained from a t test
Data are presented as mean±standard deviation
Fig. 3.
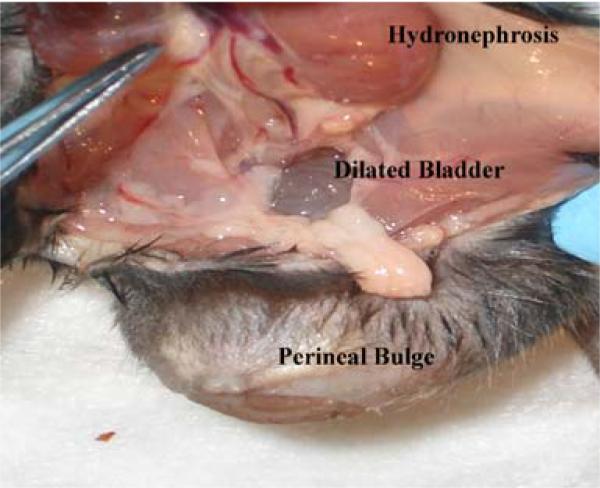
Gross anatomy of the LOX1−/−female mouse. Severe prolapse contains vagina, uterus, and bladder. Similar to humans, the bladder distension leads to hydronephrosis, as a result of bladder outlet obstruction caused by severe prolapse
Histology
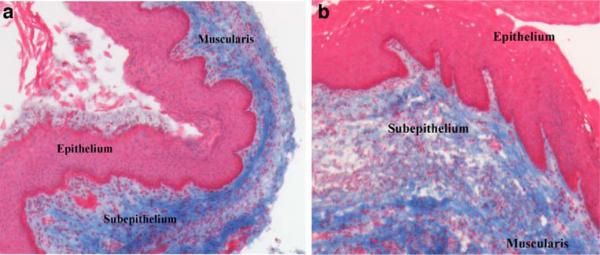
Masson's trichrome-stained full-thickness sections of the vagina were examined for overall histomorphology with light microscopy. The vaginal wall circumference in the LOXL1−/− mice was larger than that in the WT animals. The four layers of the vagina were well delineated in the WT mice (Fig. 4a); however, in the LOXL1−/− mice, the subepithelial and muscularis layers of the vagina appeared disorganized, with areas of disruption (Fig. 4b). Trichrome staining revealed areas of discontinuity in the subepithelial layer with variable thickness in the mutant animals not observed in the WT mice. The disruption of subepithelial connective tissue and the smooth muscle layer of the vaginal wall suggested a potential site of tissue weakness in LOXL1−/− mice. An increased cellular infiltrate was also observed within the subepithelial layer of the mutant vaginas (Fig. 4b). The histological analysis of the vaginal tissue of 17-month-old LOXL1 deficient mice was compared to the 3-month-old WT mice since the latter were considered our gold standard for anatomy. After confirmation of equivalent biomechanical properties in 3- and 17-month-old WT mice, histological analyses were not repeated in the 17-month-old WT animals.
Fig. 4.
Masson's trichrome-stained full-thickness mouse vagina demonstrating different appearance of the 3 main layers of vaginal wall in a WT (a) and LOX1−/−mice (b). ×10
Biomechanics
As shown in Fig. 5, the shapes of the load–distension curves of the LOXL1−/− mice were clearly distinct from those of the WT mice demonstrating overall different biomechanical behavior. Specifically, the load–distension curves of 3-month-old WT mice were highly reproducible with two distinct linear regions of increasing force with increasing distension. The first ranged from 1 to 2.5 mm of distension (S1) and the second from 3 to 8.5 mm of distension (S2). This likely represented partial failure and engagement of multiple tissue groups over the range of distension, which can be seen in WT mice with disrupted levels of support. In the load–distension curves representing the mutants, the first linear region of increasing force to 2.5 mm of distension (S1) was also present. However, contrary to WT mice, the second linear region (S2) was absent. Thus, following the first collective resistance to downward distension, the mechanism providing for the second linear region did not engage. Consequently, instead of achieving a second linear region, the curve tended to plateau. For quantitative analysis, values for ultimate load at failure, linear stiffness, maximum distension at failure, and energy absorbed were calculated and compared between the two groups. By this method, we found that in comparison to 3-month-old WT mice, the LOXL1 knockouts displayed a 31% decrease in ultimate load at failure (p = 0.001; Table 2), but no significant difference in linear stiffness (p = 0.99), maximum distension (p = 0.97), or energy absorbed (p = 0.63). The failure of the second region of the load–distension curve of the knockout mice to achieve linearity precluded a quantitative comparison of S2 regions other than the demonstration that the region was not present in the knockout animals.
Table 2.
Biomechanical parameters of the load–distension curves of 3-month-old WT, 17-month-old WT mice, and 17-month-old LOXL1−/−demonstrating decrease in ultimate load at failure in LOXL1−/−mice relative to WT mice, independent of age
| Categories of mice | Ultimate load at failure (N) | Linear stiffness (N/mm) | Maximal distension (mm) | Energy absorbed (N–mm) |
|---|---|---|---|---|
| 3-month-old WT (n=10) | 2.78±0.20a | 1.18±0.15 | 6.83 ±1.05 | 9.23 ±2.04 |
| 17-month-old WT (n=6) | 2.61±0.36 | 1.12±0.11 | 5.15±0.81 | 6.33±1.08 |
| LOXL1−/− (n=5) | 1.91±0.50 | 1.20±0.17 | 7.18±2.68 | 7.71±4.19 |
| *p values | ||||
| 3-month-old WT vs. 17-month-old WT | 0.72 | 0.82 | 0.14 | 0.11 |
| 3-month-old WT vs. L0XL1−/− | 0.001 | 0.99 | 0.97 | 0.63 |
| 17-month-old WT vs. LOXL1−/− | 0.008 | 0.75 | 0.12 | 0.76 |
p values are generated from post hoc pairwise comparisons using Sidak's multiple comparisons procedure
Data are presented as mean±standard deviation
Since it was possible that some of the differences in the biomechanical behavior of the knockout mice were due to the effects of aging, we then tested the tissues of the 17-month-old WT mice for comparison to the mutant animals. The load–distension curves of 17-month-old WT mice were highly reproducible, exhibiting the same behavior as the curves of 3-month-old WT mice, with the same two distinct linear regions of increasing force with increasing distension. Quantitative analysis of the biomechanical parameters derived from the curves of 17-month-old WT mice did not differ significantly in ultimate load at failure (p = 0.72), linear stiffness (p = 0.82), maximal distension (p = 0.14), or energy absorbed to failure (p = 0.11) when compared to 3-month-old WT animals (Table 2). These results demonstrate that the age of the animals had very little overall impact on the biomechanical behavior of vagina–supportive tissue complex. Therefore, the differences observed in load–distension curves of mutant and WT animals are due to the LOXL1 mutation and not age per se. When 17-month-old mice were compared to mutant animals, a 27% decrease in ultimate load at failure (p = 0.008), similar to the one seen between LOXL1-deficient and 3-month-old WT mice, was again demonstrated (Table 2). The experimental groups differed by weight (Table 1); however, weight of the animals was not correlated with any of the biomechanical parameters (p > 0.05).
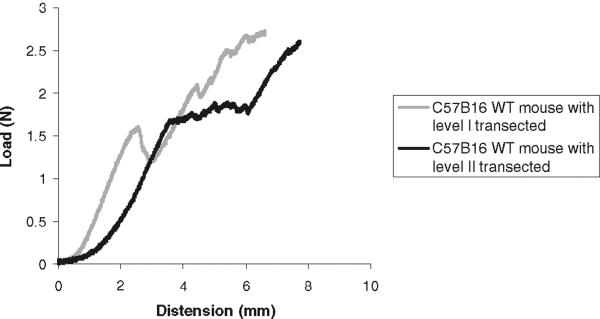
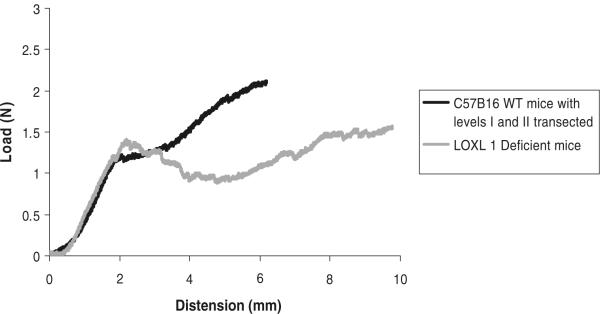
In a previous study, we had demonstrated that failure of the vagina–supportive tissue complex occurs initially at level II support, followed shortly after by level I and then level III [22]. Because the S1 and S2 regions were highly reproducible in the WT animals, we hypothesized that they correspond to progressive disruption of levels II and I support, respectively. In this way, the failure of the knockout mice to achieve a second linear region of increasing force with increasing distension may be due to the absence (or severe attenuation) of a specific level of support. To test our hypothesis, we performed a series of experiments in which we asked whether we could experimentally reproduce the curves of the knockout mice by disrupting level I and II support in the WT mice. The load–distension curves generated after disruption of the levels of vaginal support are shown in Fig. 6. The disruption of a single specific layer of vaginal support did not significantly change biomechanical parameters in the WT mice when compared to WT animals with intact support. On the other hand, simultaneous disruption of both levels I and II resulted in a decrease in the load at failure similar to that of the mutant animals (Table 3). Ultimately, however, the shape of the load–distension curves after transection of levels I and II support differed from that of the LOXL1−/− mice (Fig. 7), indicating that disruption of a specific level of support did not reproduce the effect of mutation on the biomechanical behavior of the vagina–supportive tissue complex. Thus, it is likely that the LOXL1 mutation has a global and not site-specific effect on the connective tissue.
Fig. 6.
Representative load–distension curves generated following a load to failure test in WT mice after transection of level I or II vaginal supportive tissue. Before each test, the specimens were preloaded and preconditioned
Table 3.
Biomechanical parameters of the load−distension curves of 3-month-old WT, LOXL1−/−, and 3-month-old WT mice after disruption of specific levels of support, demonstrating decrease in ultimate load at failure in WT mice with disrupted levels of support similar to LOXL1−/−mice
| Categories of mice | Ultimate load at failure (N) | Linear stiffness (N/mm) | Maximal distension (mm) | Energy absorbed (N–mm) |
|---|---|---|---|---|
| WT (n=10) | 2.78±0.20 | 1.18±0.15 | 6.83±1.05 | 9.23±2.04 |
| LOXL1−/− (n=5) | 1.91±0.50 | 1.20±0.17 | 7.18±2.68 | 7.71±4.19 |
| WT without level I (n=4) | 2.37±0.28 | 1.17±0.10 | 6.56±1.24 | 8.32±1.59 |
| WT without level II (n=4) | 2.82±0.61 | 1.17±0.20 | 6.95±0.42 | 9.52±1.49 |
| WT without levels I and II (n=4) | 1.85±0.25 | 1.24±0.29 | 4.88±1.30 | 5.11±1.93 |
| p values | ||||
| WT vs. LOXL1−/− | 0.001 | >0.99 | 0.99 | 0.68 |
| WT vs. WT without level I | 0.23 | >0.99 | >0.99 | 0.94 |
| WT vs. WT without level II | >0.99 | >0.99 | >0.99 | >0.99 |
| WT vs. WT without levels I and II | 0.001 | 0.97 | 0.13 | 0.04 |
| LOXL1−/− vs. WT without level I | 0.32 | 0.99 | 0.92 | 0.98 |
| LOXL1−/− vs. WT without level II | 0.02 | 0.99 | 0.99 | 0.65 |
| LOXL1−/− vs. WT without levels I and I | >0.99 | 0.99 | 0.17 | 0.39 |
Data are presented as mean+standard deviation, p values are generated from post hoc pairwise comparisons between WT and all other groups were made using Dunnett's multiple comparisons procedure. Similar analysis performed excluding WT and comparing all groups to LOXL1−/−
Fig. 7.
Representative load–distension curves generated following a load to failure test in WT mice after transection of level I and II vaginal supportive tissues, shown next to representative load–distension curve of LOXL1-deficient mice. Before each test, the specimens were preloaded and preconditioned
Discussion
Although the etiology of pelvic organ prolapse has not yet been defined, recent data demonstrate that genetic and environmental factors leading to altered connective tissue metabolism play a role [2]. In this study of LOXL1-deficient mice, we provide evidence that elastic fibers play a critical functional role in providing support to the vagina and other pelvic organs. Our results suggest a correlation between the disruption of elastic fiber homeostasis and inferior biomechanical properties of pelvic connective tissues. In the studies by Liu et al., in addition to the LOXL1 mutation, parturition was necessary for the development of pelvic organ prolapse [18, 19]. The data from our study support that the elastinopathy, resulting from the LOXL1−/− mutation, has a decompensatory effect on connective tissue supportive capacity independent of parity. Thus, we provide further data that genetic factors leading to altered connective tissue remodeling may predispose to disorders of pelvic support. The most important finding of our study is that the LOXL1 mutation leads to inferior biomechanical properties of the vagina–supportive tissue complex. The decrease in the ultimate load at failure observed in the tissues of the knockout mice suggests mechanically weaker tissues, which is a likely common endpoint of the multiple etiologies that eventually manifest as pelvic organ prolapse.
We were interested in determining whether the two distinct drops in load in the load–distension curves of the WT animals corresponded to a disruption of a specific level of vaginal support. If this was the case, the level of support corresponding to the first region (S1) was intact in the LOXL1-deficient animals while the support corresponding to the second region (S2) was severely compromised. Even though the ultimate load at failure in WT animals with transected levels I and II was comparable to the ultimate load at failure of the LOXL1 knockout mice and statistically different from the load at failure of the WT mice with intact levels of support, close examination of the load–distension curves of the WT mice with disrupted support showed that this experimental intervention did not consistently reproduce the curves of LOXL1-deficient animals. This finding suggests that the altered biomechanical properties in LOXL1-deficient mice are due to a global structural defect in connective tissues and not to a loss of a specific level of vaginal support per se. The global effect of the mutation on the connective tissue likely accounts for the gross and microscopic changes in the LOXL1-deficient animals when compared to the WT mice.
In spite of our observation that biomechanical behavior of the tissues of the WT and LOXL1-deficient mice was distinct, the maximum linear stiffness, which describes the ability of the specimen to deform in response to a given load, was not different between the groups. The failure of the stiffness to differ is attributable to the similarity of the steepest linear region (maximum slope) of the curves, corresponding to the S1 region, in the WT and mutant animals (Fig. 5). It was the second region (S2) which differed between the two groups. Specifically, in the WT mice, in this region of the curve, additional vaginal support tissues began to resist downward distension, producing the second region of increasing force with increasing distension. However, in the LOXL-deficient animals this did not occur, manifesting as a plateauing of the curve after the S1 region. As this portion of the curve never achieved linearity, it was impossible to directly compare it to the WT animals using quantitative analysis. Additionally, as the shape of the plateauing region varied from knockout specimen to specimen, we did not attempt to describe the behavior of the curve in this region other then representing it by the quantitative analysis of the point of tissue failure, which demonstrated a significant decrease in the ultimate load at failure for the LOXL1-deficient mice.
In spite of their nulliparity, close to 80% of the LOXL-deficient mice in this study developed pelvic organ prolapse, which was grossly remarkably analogous to what is clinically observed in humans. Severe prolapse led to bladder outlet obstruction and hydronephrosis in some animals also resembling prolapse complications that occur in women [24]. Our observations are, however, limited to the small colony of knockout animals we possessed. With the exception of the rapid turnover that takes place in the reproductive tract following parturition [13], elastin fibers elsewhere in the body turn over exquisitely slowly throughout the lifespan. Therefore, the mechanism by which the LOXL1 mutation leads to deterioration of the vaginal supportive connective tissues is not clear from this study. Yamamoto et al. found a marked decrease in elastin mRNA and tropoelastin protein in the cardinal ligaments of women with pelvic organ prolapse, suggesting a failure in synthesis in these women [12]. Chen et al. demonstrated a significant decrease in the endogenous inhibitors of elastases with increase in elastolytic activity in vaginal tissue from women with stress urinary incontinence and pelvic organ prolapse compared with controls [25]. Further research on elastin homeostasis in relation to pelvic floor disorders is necessary to better delineate the specific role of a failure of elastogenesis in the pathogenesis of pelvic organ prolapse. If elastinopathy is confirmed as an important contributor to the development of pelvic floor dysfunction, strategies to increase synthesis or decrease degradation of the normal elastic fibers in the reproductive tract will help prevent development of this morbid condition in susceptible individuals. Genetic predisposition leading to quantitative or qualitative elastin deficiencies, combined with environmental factors, can potentially explain the development of pelvic floor disorders in susceptible individuals. Indeed, defects in the structural components of connective tissue result from other genetic diseases, such as cutis laxa or Marfan's syndrome [26, 27]. And even though women with these overt connective tissue abnormalities represent a minority of patients with POP, the high prevalence of pelvic floor dysfunction in these individuals, together with findings from animal studies, suggests that an abnormality in elastin homeostasis might contribute to the development of POP in otherwise phenotypically normal women.
In conclusion, we demonstrated that the development of pelvic organ prolapse correlates to the altered biomechanical behavior of the vaginal supportive connective tissue complex in mice deficient in LOXL1, independent of parturition. Analysis of age-matched mutant and WT mice demonstrates the role of elastin in the pathogenesis of pelvic organ prolapse independent of aging. Studies like this have a potential to provide necessary background for the development of therapeutic interventions that will tip the balance of elastin production towards synthesis thus improving the biomechanical properties of the vagina–supportive connective tissue complex. Future studies on elastin homeostasis in relation to pelvic floor disorders with increased sample size are necessary to better delineate elastinopathy in the pathogenesis of pelvic organ prolapse.
Acknowledgment
We would like to thank Ian K. Hornstra, MD, for his generous donation of the LOXL1 knockout mice.
Footnotes
Conflicts of interest None.
References
- 1.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–651. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 2.Swift S, Woodman P, O'Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 3.Dietz H, Hansell N, Grace M, Eldridge A, Clarke B, Martin N. Bladder neck mobility is a heritable trait. Br J Obstet Gynaecol. 2005;112:334–339. doi: 10.1111/j.1471-0528.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 4.Goh JTW. Biomechnical and biochemical assessments for pelvic organ prolapse. Curr Opin Obstet Gynecol. 2003;15:391–394. doi: 10.1097/00001703-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol. 2004;190:620–627. doi: 10.1016/j.ajog.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;10:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 7.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers-Danlos syndrome. Am J Obstet Gynecol. 2000;182:1021–1023. doi: 10.1067/mob.2000.105410. [DOI] [PubMed] [Google Scholar]
- 8.Mattox TF, Bhatia NN. The prevalence of urinary incontinence or prolapse among White and Hispanic women. Am J Obstet Gynecol. 1996;146:1255–1259. doi: 10.1016/s0002-9378(96)70443-x. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel B, Denschlag D, Gobel H, Fittkow C, Werner M, Gitsch G, et al. Uterosacral ligament in women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:475–479. doi: 10.1007/s00192-005-1294-5. [DOI] [PubMed] [Google Scholar]
- 10.Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106:953–963. doi: 10.1097/01.AOG.0000182584.15087.dd. [DOI] [PubMed] [Google Scholar]
- 11.Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol. 1993;36:926–38. doi: 10.1097/00003081-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Yamamoto K, Akazawa S, Wakimoto TH, Aoyagi M. Decrease in elastin gene expression and protein synthesis in fibroblasts derived from cardinal ligaments of patients with prolapsus uteri. Cell Biol Int. 1997;21:605–611. doi: 10.1006/cbir.1997.0192. [DOI] [PubMed] [Google Scholar]
- 13.Starcher B, Percival S. Elastin turnover in the rat uterus. Connect Tissue Res. 1985;13:207–215. doi: 10.3109/03008208509152400. [DOI] [PubMed] [Google Scholar]
- 14.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 15.Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem. 2000;275(32):24400–24406. doi: 10.1074/jbc.M003665200. [DOI] [PubMed] [Google Scholar]
- 16.Tang C, Klinman JP. The catalytic function of bovine lysyl oxidase in the absence of copper. J Biol Chem. 2001;276:30575–30578. doi: 10.1074/jbc.C100138200. [DOI] [PubMed] [Google Scholar]
- 17.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like protein 1. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Yun Z, Pawlyk B, Damaser M, Li T. Failure of elastic fiber homeostasis leads to pelvic floor disorder. Am J Path. 2006;168:519–528. doi: 10.2353/ajpath.2006.050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysil oxidase is required for vascular and diaphragmatic development in mice. J Bio Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 21.DeLancey JO. Anatomy and biomechanics of genital prolapse. Clin Obstet Gynecol. 1993;36:897–909. doi: 10.1097/00003081-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Moalli PA, Howden NS, Lowder JL, Navarro J, Debes KM, Abramowitch SD, Woo SL. A rat model to study the structural properties of the vagina and its supportive tissues. Am J Obstet Gynecol. 2005;192:80–88. doi: 10.1016/j.ajog.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, et al. Pelvic organ prolapse in fibulin-5 knockout mice. Pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Path. 2007;170:578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanzi LJ, Chaikin DC, Blaivas JG. The effect of genital prolapse on voiding. J Urol. 1999;161:581–586. [PubMed] [Google Scholar]
- 25.Chen BH, Wen Y, Polan ML. Elastolytic activity in women with stress urinary incontinence and pelvic organ prolapse. Neurourol Urodyn. 2004;23:119–126. doi: 10.1002/nau.20012. [DOI] [PubMed] [Google Scholar]
- 26.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milewicz DM, Urban Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biology. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]