Summary
Many Bacteroides transfer factors are mobilizable in Escherichia coli when coresident with the IncP conjugative plasmid RP4, but not F. To begin characterization and potential interaction between Bacteroides mobilizable transfer factors and the RP4 mating channel, both mutants and deletions of the DNA processing (dtr), mating pair formation (mpf) and traG coupling genes of RP4 were tested for mobilization of Bacteroides plasmid pLV22a. All 10 mpf but none of the four dtr genes were required for mobilization of pLV22a. The RP4 TraG coupling protein (CP) was also required for mobilization of pLV22a, but could be substituted by a C-terminal deletion mutant of the F TraD CP. Potential interactions of the TraG CP with relaxase protein(s) and transfer DNA of both RP4 and pLV22a were assessed. Overlay assays identified productive interactions between TraG and the relaxase proteins of both MbpB and TraI from pLV22a and RP4 respectively. The Agrobacterium Transfer-ImmunoPrecipitation (TrIP) assay also identified an interaction between TraG and both RP4 and pLV22a transfer DNA. Thus, mobilization of the Bacteroides pLV22a in E. coli utilizes both RP4 Mpf and CP functions including an interaction between the relaxosome and the RP4 CP similar to that of cognate RP4 plasmid.
Introduction
The dissemination of bacterial antibiotic resistance genes in the environment is thought to be primarily mediated by mobile plasmids and transposons. Many mobile elements have a broad host range and are detectable in a number of different bacterial genera. Their transfer occurs primarily via conjugation; however, the molecular mechanisms contributing to specificity (or lack thereof) that result in broad host-range DNA exchange remain to be determined. What is clear is that ultimately this rapid and efficient DNA dissemination results in widespread gene flow and transmission of antibiotic resistance and other DNA in the environment (Boucher et al., 2007). It has now become increasingly apparent that one such environment is the human gut where many species of bacteria coexist, evolve and adapt primarily utilizing horizontal gene transfer mechanisms (Xu et al., 2007).
RP4 is a highly promiscuous transferable plasmid with the ability to mobilize itself and other plasmids and transposons to a variety of different organisms, including other Gram-negative bacteria, Gram-positive bacteria and yeasts (Heinemann and Sprague, 1989; Trieu-Cuot et al., 1993; Bates et al., 1998; Chen et al., 2005; Schroder and Lanka, 2005). Conjugative transfer of RP4 requires the formation of a relaxosome and a mating channel. The relaxosome is a single-strand RP4 DNA-protein complex formed by the covalent linkage of the RP4-encoded relaxase TraI to the transferring RP4 DNA. Accessory proteins are encoded by three other genes (dtr genes). The mating channel is encoded by 10 mpf genes, the traF pilin support gene, and traG, which encodes a coupling protein (CP). (Haase et al., 1995; Cabezon et al., 1997; Szpirer et al., 2000; Schroder et al., 2002; Gilmour et al., 2003). Key among all these gene products is TraG, as it plays a ‘gatekeeper’ role as the first point of contact between the relaxosome and the mating channel (Waters et al., 1992; Balzer et al., 1994; Llosa et al., 1994; Hamilton et al., 2000). For many transfer systems including the F and Agrobacterium Ti plasmids, the CP appears to be highly selective for the respective (cognate) relaxosome (Sastre et al., 1998; Hamilton et al., 2000), while for others, including RP4 and R388, the CP can recognize and interact with either its own relaxosome or those of closely related plasmids (Hamilton et al., 2000). The precise molecular mechanism(s) of relaxosome recognition by the CP have not been fully defined; specific CP protein/relaxosome interactions have been reported for only a few plasmid systems (Szpirer et al., 2000; Schroder et al., 2002).
Bacteroides sp., members of the Bacteroidetes phylum, also transfer DNA primarily via conjugation, with numerous plasmids, transposons and other mobilizable transfer factors able to utilize a putative mating channel encoded by any one of several large chromosomally encoded conjugative transposons (Shoemaker et al., 2001; Hecht, 2004). While there have been some studies elucidating the requirements for relaxosome formation in Bacteroides sp., little is known about mating channel formation (Sitailo et al., 1998; Vedantam et al., 2006).
In addition to transfer within Bacteroides sp., many, if not all, of the Bacteroides transfer factors are also mobilizable within and from Escherichia coli when coresident with either RP4 or R751 (a closely related plasmid), but not when coresident with the fertility factor F (Novicki and Hecht, 1995; Wang et al., 2000; Bonheyo et al., 2001; Bass and Hecht, 2002; Atmakuri et al., 2004). The mechanism by which RP4 mobilizes and interacts with Bacteroides transfer factors has not been previously studied.
To begin to understand this process, we chose pLV22a, a 4.2 kb cryptic but ubiquitous Bacteroides fragilis plasmid that is mobilized by both conjugative transposons in Bacteroides and either RP4 or R751 in E. coli, similar to other Bacteroides transfer factors (Novicki and Hecht, 1995). We have previously shown that pLV22a encodes all three of its required mobilization proteins, MbpA, MbpB and MbpC in E. coli, but did not know which genes and proteins of the IncP plasmids were required for its mobilization nor if there was a direct interaction with the RP4 CP. In this study, we report that only the 10 core mating channel genes and the TraG CP of RP4 are required for pLV22a mobilization. In addition, a truncated mutant of the F TraD CP was also able to substitute for the TraG CP to mobilize pLV22a. Using both overlay and the transfer DNA immunoprecipitation (TrIP) assays, we identified that RP4 TraG interacts with both the pLV22a relaxase protein and the transferring DNA, similar to that of its own cognate molecule.
Results
pLV22a encodes its own DNA-processing functions sufficient for mobilization in E. coli
Two HB101 donors were used in filter mating experiments to determine whether any of the four RP4 dtr genes were required for mobilization of pLV22a in E. coli. One donor contained the fully transfer-proficient pDB126 plasmid consisting of the RP4 Tra1 region traJ, traI, traH, traK, traG, traF, oriT and the 10 Tra2 mpf core genes cloned into a ColD replicon, a kind gift of Erich Lanka (Balzer et al., 1994)., The second donor lacked the four dtr genes, but did contain pML123 encoding the 10 Tra2 mpf core genes and pML100 encoding only traF and traG (Lessl et al., 1993). Both donors were transformed with pTJ5a, a pLV22a construct cloned into pACYC184 (Novicki and Hecht, 1995) (Table 1). Quantitative filter mating assays were carried out between the two donors and the isogenic HB101 Nxr recipient. Table 2 shows that the mobilization frequencies of pTJ5a from either of the two donor strains gave similar results, indicating that RP4 dtr functions were not required for mobilization of pLV22a (in pTJ5a). As expected, transfer of pDB126 occurred at a frequency of 10−1 per input donor, while pML123 did not transfer (data not shown).
Table 1.
Plasmids and strains used in this study.
| Strain or plasmid | Relevant genotype, phenotype or characteristic(s)a | Reference |
|---|---|---|
| E. coli HB101 | Smr | Sambrook et al. (1989) |
| E. coli HB101 Nxr | Nxr resistant derivative of HB101 | Balzer et al. (1994) |
| pLV22a | LV22; Mob+ (Bacteroides sp. clinical isolate) | Novicki and Hecht (1995) |
| pACYC184 | Mob−, Cmr, Tcr | Sambrook et al. (1989) |
| pTJ5a | pLV22a inserted into EcoRI site of pACYC184; Mob+ and Tcr | Novicki and Hecht (1995) |
| pML123 | RP4-Mpf function from Tra2 region in ColD vector; Cmr | Lessl et al. (1993) |
| pML123mtrbB5 | Nonpolar mutation (MURFI linker) in trbB of pML123 | Haase et al. (1995) |
| pML123mtrbC45 | Nonpolar mutation (MURFI linker) in trbC of pML123 | Haase et al. (1995) |
| pML123mtrbD45 | Nonpolar mutation (MURFI linker) in trbD of pML123 | Haase et al. (1995) |
| pML123mtrbE402 | Nonpolar mutation (MURFI linker) in trbE of pML123 | Haase et al. (1995) |
| pML123mtrbG145 | Nonpolar mutation (MURFI linker) in trbG of pML123 | Haase et al. (1995) |
| pML123mtrbH13 | Nonpolar mutation (MURFI linker) in trbH of pML123 | Haase et al. (1995) |
| pML123mtrbI135 | Nonpolar mutation (MURFI linker) in trbI of pML123 | Haase et al. (1995) |
| pML123mtrbJ180 | Nonpolar mutation (MURFI linker) in trbJ of pML123 | Haase et al. (1995) |
| pML123mtrbL184 | Nonpolar mutation (MURFI linker) in trbL of pML123 | Haase et al. (1995) |
| pML100 | RP4 traF and traG from Tra1 region in pMB1; Apr | Lessl et al. (1993) |
| pDB126 | Reconstituted RP4 mpf, dtr, oriT region plus traF and traG in a ColD vector; Cmr | Balzer et al. (1994) |
| pDB127 | Reconstituted RP4 mpf, dtr, oriT region plus traF in a ColD vector; traG0; Cmr | Balzer et al. (1994) |
| pBS140 | traG in pMB1 expression vector: Apr | Balzer et al. (1994) |
| pWP471 | traF in a pMB1 expression vector: Apr | Waters et al. (1992) |
| pET-Mob | Mob of pBHR1 in pET21a(+): Apr | Szpirer et al. (2000) |
| pET-TraG(His)6 | traG(His)6 in pET21a(+): Apr | Szpirer et al. (2000) |
| pET-(His)10MbpB | (His)10 MbpB in pET19b(+): Apr | L. Sitailo and D.W. Hecht, unpublished |
| pTrc99A-traD | traD in a pTrc99A: Apr | Lee et al. (1999) |
| pTrc99A-traD-E620 | ΔC -traD in a pTrc99A: Apr | Lee et al. (1999) |
Apr, Cmr, Nxr, Spr, Smr, Knr and Tcr indicate resistance to ampicillin, chloramphenicol, naladixic acid, spectinomycin, streptomycin, kanamycin and tetracycline respectively. Mob refers to the phenotype of a plasmid mobilized when coresident with a conjugative plasmid like RP4.
Table 2.
Mobilization of pTJ5a when coresident with RP4 or its derivatives.
| Plasmid(s) in donor straina | RP4 function | Mobilization frequencyb of pLV22a | Mob phenotype of pLV22a |
|---|---|---|---|
| None | N/A (control) | < 1 × 10−8 | − |
| pDB126 | Reconstituted RP4 mpf, dtr, oriT region plus traF and traG | (1.13 ± 0.2 × 10−4)c | + |
| pML123, pML100 | RP4-Mpf function from Tra2 region, RP4 traF and traG from Tra1 | (3.6 ± 1.1 × 10−4)c | + |
| pML123mtrbB5 d, pML100 | trbB0 | < 1 × 10−8 | − |
| pML123mtrbC45, pML100 | trbC0 | < 1 × 10−8 | − |
| pML123mtrbD45, pML100 | trbD0 | < 1 × 10−8 | − |
| pML123mtrbE402, pML100 | trbE0 | < 1 × 10−8 | − |
| pML123mtrbF9, pML100 | trbF0 | < 1 × 10−8 | − |
| pML123mtrbG145, pML100 | trbG0 | < 1 × 10−8 | − |
| pML123mtrbH13, pML100 | trbH0 | < 1 × 10−8 | − |
| pML123mtrbI135, pML100 | trbI0 | < 1 × 10−8 | − |
| pML123mtrbJ180, pML100 | trbJ0 | < 1 × 10−8 | − |
| pML123mtrbL184, pML100 | trbL0 | < 1 × 10−8 | − |
The donor strain was HB101 Smr, containing plasmid pTJ5a Tcr (pLV22a cloned in pACYC184).
Mobilization frequency is defined as the number of mobilized plasmid (pLV22a) transconjugants per input donor (HB101 Smr). Transconjugants were selected for expression of Tc (from pTJ5a) and Nx (from HB101 Nxr) resistance.
Mean of three experiments ± standard error of the mean.
Details of pML123 based mutants, see Haase et al. (1995).
Note: N/A means not applicable.
All 10 core RP4-mpf genes are required for the mobilization of pLV22a
Lanka and colleagues have previously shown that disruption of any of 10 core genes encoding the RP4-Mpf will affect the integrity of the RP4 mating channel and loss of RP4 transfer (Lessl et al., 1993; Haase et al., 1995). We predicted that pLV22a would also likely require most, if not all, of the mpf genes for successful mobilization. Non-polar insertion mutations of mpf genes from pML123 (kindly provided by Erich Lanka) were substituted for pML123 in donor strains to test for mobilization of pTJ5a in trans. Ten HB101 donor strains were created, each containing pML100 and one of 10 pML123 mutants consisting of a single insertion linker mutation [14 bp multiple reading frame insertion (MURFI) linker] in one of the mpf genes (Haase et al., 1995). The 10 donor strains were then transformed with pTJ5a and tested for mobilization along with an eleventh donor containing pML123, pML100 and pTJ5a as a positive control. Table 2 shows that pTJ5a was mobilized at the expected frequency of 10−4 when both wild-type pML123 and pML100 were provided in trans. However, pTJ5a mobilization was not detected from any of the 10 mpf mutant donors, indicating that all 10 core mpf functions are required for both RP4 transfer and pLV22a mobilization.
RP4-TraG coupling protein is required for the mobilization of pLV22a
In addition to the core mpf genes, we presumed that RP4 TraG CP was also required for mobilization of pLV22a in E. coli. To test this, we compared mating results between three pTJ5a containing donors that also included either pDB127 (a traG0 non-polar deletion derivative of pDB126), pDB127 plus pBS140 (expressing TraG in a non-induced Ptac/lacIq vector), or pDB126 (positive control) (Waters et al., 1992; Balzer et al., 1994). Table 3 shows that neither pTJ5a nor pDB127 were mobilized when traG was absent, while both plasmids were mobilizable at expected frequencies when TraG CP was provided in trans on pBS140. This confirms that pLV22a requires the TraG CP for mobilization in E. coli.
Table 3.
TraG coupling protein is required for pTJ5a mobilization.
| Plasmid(s) in donor straina | Description | Transfer frequencyb RP4/input donor | Transfer phenotype | Mobilization frequencyc of pLV22a | Mob phenotype |
|---|---|---|---|---|---|
| None | Control | N/A | N/A | < 1 × 10−8 | − |
| pDB126 | RP4 mpf+ and dtr+ | (3.4 ± 0.3 × 10−1)d | + | (1.1 ± 0.2 × 10−4)d | + |
| pDB127 | RP4 mpf+ and dtr+:traG0 | < 1 × 10−8 | − | < 1 × 10−8 | − |
| pDB127, pBS140 | RP4 mpf+ and dtr+:traG0 + TraG | (0.9 ± 0.2 × 10−1)d | + | (3.0 ± 1.1 × 10−4)d | + |
All donors (HB101 Smr) contained plasmid pTJ5a Tcr (pLV22a cloned in pACYC184).
Transfer frequency is equal to the number of transferred plasmid (pDB126/pDB127) transconjugants per input donor (HB101 Smr). Transconjugants were selected for expression of Cm (from pDB126/pDB127) and Nx (from HB101 Nxr) resistance.
The mobilization frequency of pLV22a is determined in the same experiment which is used to determine RP4 transfer frequency.
Mean of three experiments ± standard error of the mean.
Note: N/A means not applicable.
The truncated F TraD coupling protein can substitute for TraG in the mobilization of pLV22a using the RP4 mating channel
In addition to itself, F plasmid is also able to mobilize (albeit inefficiently), the unrelated RSF1010 plasmid at ∼100 000-fold lower frequency. However, Sastre et al. showed that when a 140 C-terminal amino acid deletion derivative of F TraD CP, F-ΔCTraD, was substituted for wild-type TraD, mobilization of RSF1010 increased by up to 1000-fold. However, there was also a concomitant 10 000-fold decrease in F transfer (Fig. 1) (Sastre et al., 1998; Beranek et al., 2004). The authors concluded that the deleted C-terminal amino acids were responsible for recognition and interaction with the relaxosomes. We observed that pLV22a shares similar features with RSF1010 that includes three DNA-mobilization genes, that both belonging to the IncQ family of relaxases (Francia et al., 2004), and that both plasmids are mobilizable when coresident with RP4. To test whether either TraD or F-ΔCTraD could recognize and substitute for TraG CP using the RP4 mating channel, donors containing pDB127 (traG0) with either wild-type TraD (pTrc99A-traD+) or F-ΔCTraD (pTrc99A-traD-E620) in trans were used to test for mobilization of pTJ5a. Figure 1 shows that pTJ5a was in fact mobilizable when coresident with F-ΔCTraD, but not wild-type TraD using the RP4 mating channel, indicating that F-ΔCTraD can substitute for TraG in the mobilization of pLV22a using the RP4 conjugation system. In contrast, pDB127 was not transferred when F-ΔCTraD was substituted for TraG, indicating that the TraD deletion mutant cannot complement TraG for this conjugative plasmid.
Fig. 1.
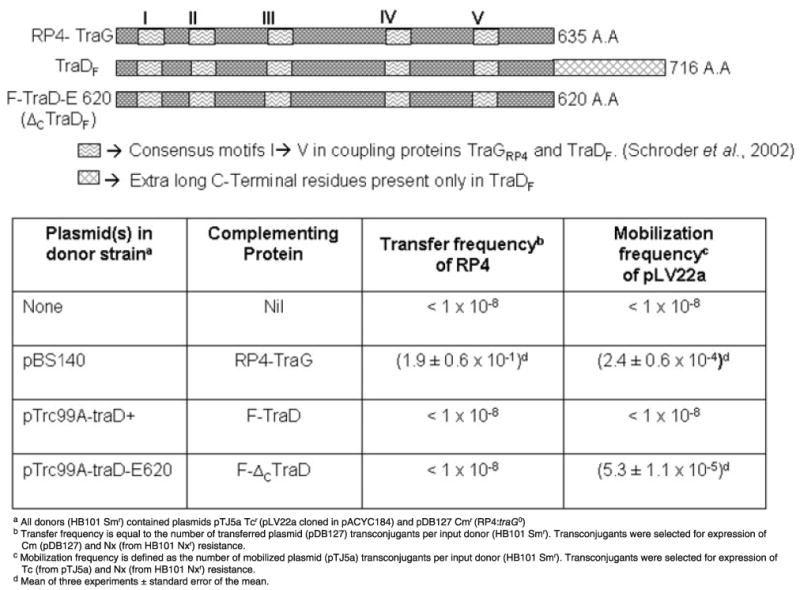
Truncated C-Terminal TraD coupling protein substitutes for TraG in mobilization of pLV22a. The inset shows a schematic alignment of TraG, TraD and F-ΔCTraD mutant in the C-terminal region. Squiggly boxes represent the five conserved motifs present in known coupling proteins including TraG and TraD. TraG and TraD differ primarily by an additional 80 C-terminal amino acids (diamond boxes) in TraD that were deleted in F-ΔCTraD. Frequencies of transfer of RP4 or mobilization of pLV22a when coresident with wild type or TraG, TraD, or F-ΔCTraD are shown in the table.
TraG interacts with B. fragilis MbpB, E. coli RP4 TraI and Bordetella Mob relaxase proteins
During conjugation, the relaxosome is formed by a relaxase protein catalysing site and strand-specific nicking at the nick site in the oriT. This reaction is followed by covalent binding to the 5′ end of the transfer DNA that remains bound during transfer through the mating channel. Several studies support that it is the relaxase that is first recognized by the CP (Sastre et al., 1998; Szpirer et al., 2000; Schroder et al., 2002; Lu and Frost, 2005). This is thought to be followed by a non-specific, but spatial interaction between DNA and CP required for DNA-dependent ATPase activity resulting in transfer DNA pumped through the mating channel (Moncalian et al., 1999; Schroder et al., 2002; Tato et al., 2005). Schroder et al. (2002) has previously shown an in vitro interaction between RP4 TraG CP and TraI relaxase using a surface plasmon resonance assay, while Szpirer et al. (2000) used an overlay assay to detect an interaction between TraG CP and Bordetella pBHR1 Mob relaxase (Szpirer et al., 2000; Schroder et al., 2002). The interaction between TraG CP and RP4 relaxase has not been previously shown using an overlay assay. We tested whether an overlay assay could also detect an in vitro interaction between TraG CP and MbpB as well as RP4 using Mob as a control. In brief, we expressed and purified histidine (His)-tagged MpbB (pET-(His)10MbpB) and His-tagged TraG (pET-TraG(His)6) under non-denaturing conditions, electrophoresed the proteins using SDS-PAGE and stained with GelCode blue (Pierce Biotech, Rockford, IL). Horse-radish peroxidase (HRP)-conjugate anti-His monoclonal antibody (Qiagen, Valencia, CA) and MbpB and TraG polyclonal antibody were used to confirm detection and purity of proteins in a Western blot (Fig. S1).
To first test for an in vitro interaction between TraG and MbpB, non-His-tagged TraG, BSA and non-His-tagged TraF were transferred to a nitrocellulose membrane overnight using a non-denaturing transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). The use of the non-denaturing buffer removes any traces of SDS, and has been shown, at a minimum, to partially restore the native state of the transferred proteins (Burgess et al., 2000). Both TraF (an RP4 inner membrane protein) (Grahn et al., 2000) and BSA were used as negative controls. The membrane was overlaid with purified native (His)10MbpB protein, blocked and washed (see methods). The blot was then incubated with HRP-conjugate anti-His antibody (Qiagen, Valencia, CA) and probed with peroxidase substrate (ECL, GE Healthcare Bio-Sciences, Piscataway, NJ). Figure 2A shows a single band in lane 2 corresponding to the 72 kDa-overexpressed TraG band seen on the acrylamide gel run in parallel (not shown), indicating a direct interaction between TraG and MbpB. No interaction was seen between MbpB and TraF, BSA, or cleared lysate. We then reversed, in part, the assay by immobilizing several MbpB preparations on a membrane and overlaid with purified TraG(His)6. In this experiment, cell lysates from non-overexpressed non-His-tagged MbpB pTJ5a (lane 2), overexpressed MpbB (pET(His)10MbpB) (lane 3), purified (His)10MbpB (lane 4) and overexpressed TraF (lane 7), were electrophoresed along with other controls, transferred to a nitrocellulose blot and treated as described earlier. The blot was then overlaid with purified TraG(His)6 and incubated with HRP-conjugate anti-His antibody, as described earlier. Bands corresponding to MbpB protein (∼30 kDa) were identified in lanes 2, 3 and 4, corresponding to pJT5a (not overexpressed MbpB, lighter band), overexpressed MbpB and purified MbpB protein respectively (Fig. 2B). No interaction with TraF (19 kDa) was detected, although this polyclonal preparation did identify a number of non-specific bands common to lanes 2, 3, 5, 6 and 7, as previously reported by Lanka et al. (Ziegelin et al., 1991). An additional band (∼45 kDa) was also seen in lane 2 that did not appear in the other lanes, and appears to represent an additional non-specific interaction between TraG antiserum and an unknown protein encoded by pTJ5a. This band was not seen when testing for TraG interactions using anti-His antibody against a His-tagged TraG protein (Fig. 3A, lane 6).
Fig. 2.
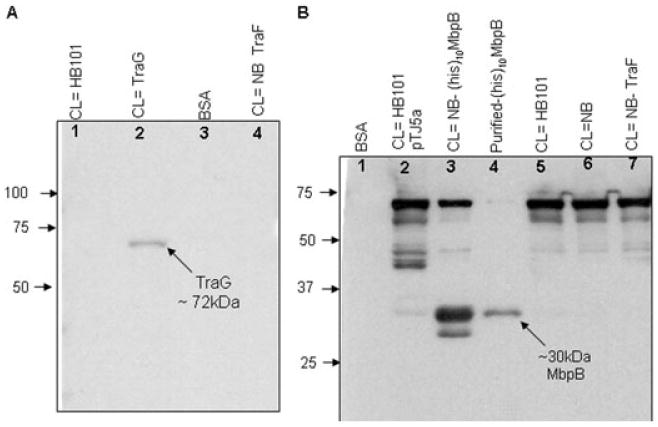
Overlay assay to determine direct TraG–MbpB interaction.
A. In vitro TraG–MbpB interaction determined by overlay with (His)10MbpB followed by detection using anti-His antibody. The blot containing cell lysate (CL) with non-His-tagged TraG (pBS140) was incubated with purified (His)10MbpB and probed with HRP-conjugate anti-His antibody. A band at the predicted size for TraG (∼72 kDa) is detected only in the lane containing overexpressed TraG (lane 2). Lane 1, CL HB101 with no plasmid; lane 2, CL with non-His-tagged TraG expressed from plasmid pBS140 (HB101); lane 3, Pure BSA; lane 4, CL with TraF expressed from plasmid pWP471 in Nova Blue (NB) cells.
B. In vitro TraG–MbpB interaction determined by overlay (reciprocal) with TraG(His)6 followed by detection using TraG antiserum. The blot containing CL with either His-tagged MbpB [pET-(His)10MbpB] or non-His-tagged MbpB (from pTJ5a) was incubated with purified TraG(His)6 and probed with TraG antiserum. Signals corresponding to ∼30–33 kDa (both types of MbpB) band were picked in three lanes containing MbpB, i.e. lane 2, CL expressing MbpB from pTJ5a; lane 3, CL expressing (His)10MbpB from pET-(His)10MbpB in NB cells; and lane 4, purified (His)10MbpB protein. No bands corresponding to this size were present in negative control lanes, i.e. lane 1, pure BSA; lanes 5 and 6, CL from HB101 and NB cells both with no plasmid; and lane 7, CL expressing TraF from pWP471, NB cells.
Fig. 3.
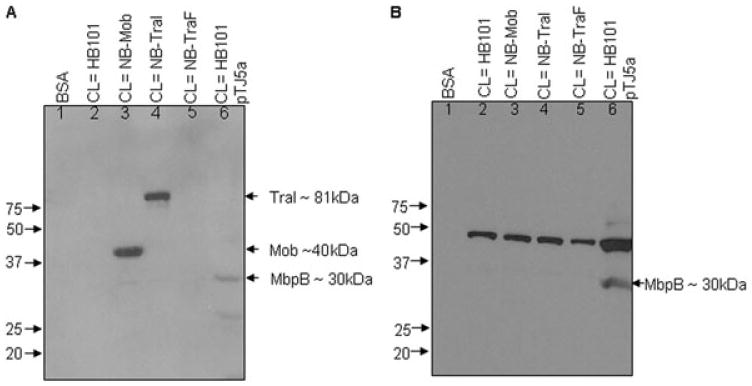
Overlay assay to determine interaction of TraG(His)6 with three relaxase proteins: Mob of pBHR1, TraI of RP4 and MbpB of pLV22a.
A. A blot containing cell lysates with non-His-tagged overexpressed Mob from pET-Mob (lane 3), non-His-tagged overexpressed TraI from pDB173 in NB cells (lane 4) and non-His-tagged, non-overexpressed MbpB from pTJ5a in HB101 (lane 6) was incubated with purified TraG(His)6 and probed with HRP-conjugate anti-His antibody. Cell lysate of non-overexpressed MbpB was three times that of Mob and TraI. Signals were detected with molecular weight corresponding to Mob (∼40 kDa) from pBHR1 of Bordetella (lane 3), TraI (∼81 kDa) from RP4 of E. coli (lane 4) and MbpB (30 kDa) from pLV22a of Bacteroides (lane 6). Lane 1, BSA; lane 2, cell lysate HB101 (no plasmid); lane 5, cell lysate of TraF overexpression vector in NB cells.
B. The blot from (A) was stripped and re-probed using MbpB antiserum, confirming the location of 30 kDa MbpB band.
On a separate blot, we tested whether TraG interacts with RP4 TraI, its cognate relaxase with the Bordetella pBHR1 Mob (Szpirer et al., 2000) and pLV22a MbpB. In this experiment, cleared lysates of overexpressed TraI, Mob and TraF (negative control), as well as non-overexpressed MbpB from pTJ5a, were electrophoresed and blotted, overlaid with purified TraG(His)6, and incubated with anti-His monoclonal antibody as described earlier. Figure 3A shows bands corresponding to Mob (∼40 kDa), TraI (∼81 kDa) and MpbB (∼30 kDa) detected in lanes 3, 4 and 6, respectively, while no bands were seen in lanes with negative controls. To confirm that the 30 kDa band seen in lane 6, Fig. 3A was MbpB, the blot was stripped and incubated with MbpB antiserum identifying the same 30 kDa band (Fig. 3B). Thus, we were able to identify a direct interaction between TraG CP and MbpB relaxase both when overexpressed and at low levels of native expression.
RP4-TraG coupling protein interacts with both RP4 transfer DNA and pLV22a transfer DNA
Having identified a direct interaction between TraG CP and MbpB in vitro, we hypothesized that an interaction between TraG CP and pLV22a transfer DNA was also occurring. To test for this, we used a biochemical approach by adapting the TrIP assay described for Agrobacterium (Cascales and Christie, 2004). The TrIP assay was developed and successfully used to study the translocation pathway of transfer DNA as it passes through the VirB mating channel encoded by its cognate Ti plasmid system. In Agrobacterium, the VirD4 CP interaction with transfer DNA from the relaxosome is detected by cross-linking protein and transfer DNA followed by immunoprecipitation (IP). Polymerase chain reaction (PCR) amplification is then used to detect transfer DNA with the amplified transfer DNA fragment found in the supernatant (S) fraction of any cell containing the relaxosome as well as in the IP fraction that contains the CP. We adapted this assay to E. coli for cell lysates containing pDB126 and pTJ5a. This was followed by IP of TraG, using TraG antiserum. Both the IP and S fractions were used to amplify DNA with transfer DNA-specific primers to both RP4 (from pDB126) and pLV22a (from pTJ5a). Because cells can produce both RP4 (pDB126) and pLV22a (pTJ5a) relaxosomes, we expected to amplify both cognate RP4 and pLV22a transfer DNA in both the IP and S fractions. As a negative control, primers to amplify adk genomic DNA that should not interact with TraG CP were used in the S and IP fractions.
Figure 4A shows results of a multiplex PCR assay to detect both pLV22a and genomic DNA in the S and IP fractions. As expected, both pLV22a transfer DNA and genomic DNA were detected in the S fraction, while only pLV22a transfer DNA was detected in the IP fraction. This indicated that an interaction between TraG CP and transfer DNA was occurring that was not seen with genomic DNA. Using multiplex PCR, we were also able to show an interaction between TraG CP and RP4 transfer DNA (Fig. 4B) As predicted, PCR products corresponding to the expected products were seen in both the S and IP fractions, indicating an interaction with both cognate RP4 and pLV22a transfer DNA from the same donor cells.
Fig. 4.
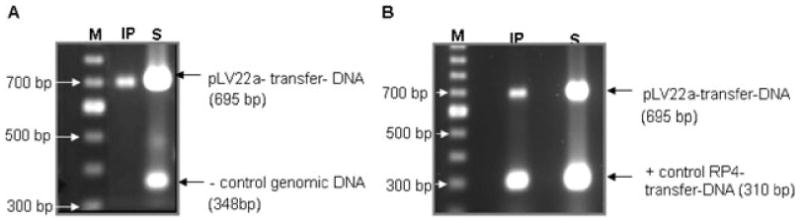
TrIP assay detects the interaction of TraG with transfer DNA from pLV22a and RP4 using TraG antiserum.
A. Multiplex PCR of pLV22a transfer DNA and genomic DNA from supernatant (S) and immunoprecipitation (IP) fractions of HB101 cells containing pDB126 and pTJ5a. The S fraction shows a 348 bp PCR product from genomic DNA and a 695 bp PCR product from pLV22a transfer DNA. The IP fraction shows only a PCR product of pLV22a transfer DNA. PCR product of genomic DNA is not seen in the IP fraction (negative control).
B. Multiplex PCR of pLV22a transfer DNA and genomic DNA from S and IP fractions of HB101 cells containing pDB126 (wt-RP4) and pTJ5a (wt-pLV22a). The S fraction shows a 310 bp PCR product from RP4 transfer DNA and a 695 bp PCR product from pLV22a transfer DNA. The IP fraction shows both PCR products of pLV22a transfer DNA as well as RP4 transfer DNA. PCR product of RP4 transfer DNA in the IP fraction serves as a positive control. M, 100 bp marker; IP, immunoprecipitation using TraG antiserum; S, supernatant which serves as input DNA.
To ensure that transfer DNA PCR products were not from non-specific protein coimmunoprecipitation reactions, the TrIP assay was repeated using single PCR assays when TraG was provided in trans. IP was performed using anti-His monoclonal antibody (Qiagen, Valencia, CA) to precipitate TraG(His)6 expressed by pET-TraG(His)6. Figure 5A again shows PCR products corresponding to RP4 transfer DNA (Fig. 5A, lanes 1 and 2) and pLV22a transfer DNA (Fig. 5A, lanes 3 and 4) seen in both the S and IP fractions. However, when TraG was not provided in trans, PCR products corresponding to RP4 and pLV22a transfer DNA were only identified in the S fraction (Fig. 5B), indicating that TraG was required for IP of transfer DNA from either RP4 or pLV22a. Several additional control experiments to detect for background signal or for non-specific interaction between TraG CP and genomic DNA in the IP fraction were performed with negative results (see Fig. S2 for an example).
Fig. 5.
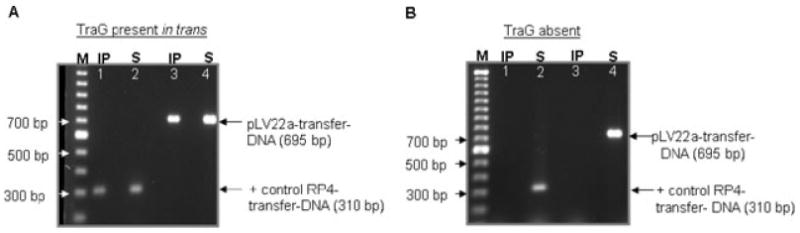
TrIP assay detects an interaction of TraG with transfer DNA from pLV22a and RP4 when TraG is present in trans.
A. Single PCR of pLV22a transfer DNA and genomic DNA from S and IP fractions of HB101 cells containing pDB127 (RP4:traG0), pET21-TraG(His)6 and pTJ5a. The S fractions shows a 310 bp PCR product from RP4 transfer DNA (lane 2) and a 695 bp PCR product from pLV22a transfer DNA (lane 4). The IP fraction when TraG(His)6 was expressed in trans gave PCR products of pLV22a transfer DNA (lane 3) and RP4 transfer DNA (lane 1). PCR product of RP4 transfer DNA in the IP fraction serves as a positive control.
B. Single PCR of pLV22a transfer DNA and genomic DNA from S and IP fractions of HB101 cells containing only pDB127 (RP4:traG0) and pTJ5a. The S fractions shows a 310 bp PCR product from RP4 transfer DNA (lane 2) and a 695 bp PCR product from pLV22a transfer DNA (lane 4). No PCR products of pLV22a transfer DNA (lane 3) or RP4 transfer DNA (lane 1) were detected in the absence of TraG(His)6 expression in the IP fractions. M, 100 bp marker; IP, Immunoprecipitation using anti-His antibody (Qiagen); S, Supernatant which serves as input DNA.
Discussion
In this study, we have shown that the mobilization of the Bacteroides pLV22a transfer factor by RP4 in E. coli requires the core mating channel genes and CP of RP4, the expression of pLV22a dtr functions in E. coli, and a direct interaction between the TraG CP and MbpB relaxase proteins. Although the mobilization of Bacteroides plasmids and transposons has been described since 1979, there is still much to be learned about the biochemistry of Bacteroides sp. conjugal transfer (Privitera et al., 1979; Robillard et al., 1985; Whittle et al., 2002). This is in large part due to limited or no homology between Bacteroides conjugative elements and those of other characterized systems. Importantly, all reported Bacteroides transfer factors are mobilizable by either CTnDOT and/or related elements in Bacteroides and the broad host range IncP plasmids in E. coli. Considering that Bacteroides belong to the Bacteroidetes phylum that is phylogenetically distant from the proteobacteria E. coli, it is rather remarkable that both conjugation systems support mobilization of the same transfer factors, and presumably share similarities that are yet to be determined (Gherna and Woese, 1992; Garrity et al., 2007).
An important observation from this study was the identification of direct interactions between RP4-TraG CP and MbpB relaxase, and RP4-TraG CP and TraI relaxase using an overlay assay. This is the first time this technique has demonstrated the interaction between TraG CP and its cognate TraI, previously only shown using a surface plasmon resonance assay (Schroder et al., 2002). This approach has been successfully used to demonstrate the interaction between TraG CP and pBHR1 which is confirmed in this work, and may be a useful tool for detecting interactions between TraG CP and other relaxases (Szpirer et al., 2000). In all experiments, this interaction was observed regardless of which protein was immobilized and partially renatured.
The ability of TraG CP to interact with both cognate TraI relaxase and MbpB from the Bacteroidetes phylum is somewhat surprising considering the lack of homology between the two conjugation systems. RP4 TraG CP does not have homology with any known Bacteroides putative or known conjugative proteins. In the conjugative transposon CTnDOT, only one coding sequence, OrfG, was found to have any significant homology to conjugation proteins (Bonheyo et al., 2001). Among E. coli, OrfG has limited homology to TraC (F) and TrbE (RK2) (Frost et al., 1994; Lanka and Wilkins, 1995; Dang and Christie, 1997; Middleton et al., 2005). However, OrfG does appear to be highly conserved among other Bacteroides transfer factors including TraG (CTn341) and BctA (pBF4 and BTF-37) (Morgan and Macrina, 1997; Vedantam and Hecht, 2002; Bacic et al., 2005; Hecht et al., 2007) and also exhibits low similarity to VirB4 from the Ti plasmid of Agrobacterium (Dang and Christie, 1997; Middleton et al., 2005). Whether OrfG is a Bacteroides CP-like protein remains to be determined.
The interaction of TraG with not only its own cognate relaxase, but also two non-related relaxase proteins suggests there is a common feature of the relaxase proteins. Recent publications by Vergunst et al. (2005) and Nagai et al. (2005) have identified the presence of several clustered positively charged residues in the C-termini of relaxase proteins that are speculated to provide a ‘substrate recognition signal’ for the CP. MbpB also has clusters of positively charged residues in the C-terminus, as well as other putative Bacteroides relaxase proteins including MocA (Tn4399), BmgA (cLV25) and MobBs (CTnDOT, CTn341). All of these proteins have a continuous stretch of positively charged ‘patch’ of C-terminal residues. Future work needs to be done to determine if these positive charge amino acids play any significant role in CP recognition.
We presume that the TraG CP makes initial contact with the pLV22a relaxosome with some specificity for the relaxase as is the case for RP4 and RSF1010. However, we also found that the C-terminally truncated TraD CP from F, F-ΔCTraD, but not wild-type TraD, was able to substitute for TraG CP using the RP4 mating channel. F-ΔCTraD was previously shown to mobilize RSF1010 at 100 000-fold higher frequency using the F mating channel, suggesting that the specificity of the TraD CP could be broadened, albeit at a cost to F transfer (Sastre et al., 1998). In our experiments, not only did F-ΔCTraD allow mobilization of pLV22a, it also interacted with and utilized the RP4 mating channel. However, although the F-ΔCTraD substitution did allow for mobilization of pLV22a, it did not mobilize the conjugative RP4 plasmid. This observation is consistent with previous reports that conjugative plasmids, such as IncP, F and R388, only transfer their own or closely related transfer DNA. The mechanism by which F-ΔCTraD could recognize pLV22a but not RP4 is unknown (Cabezon et al., 1997). The interchangeability and successful functional interaction of the F-ΔCTraD CP simultaneously with two unrelated transfer factors is the first such report, and warrants further study.
Another test of E. coli CP interaction with a Bacteroides relaxosome used the TrIP assay. We adapted the TriP assay to E. coli to test whether TraG CP interacts with pLV22a transfer DNA in vivo. Recovery of both pLV22a and RP4 transfer DNA PCR products from the TraG coimmunoprecipitation fractions supported such an interaction. The only previous report of such an interaction utilized an electrophoretic mobility shift assay (Schroder et al., 2002). We also showed that the interaction between TraG CP and pLV22a transfer DNA was not random, although the precise nature of it remains unknown. Christie et al. 2004 have shown that transfer DNA, as part of the relaxosome and covalently bound to the relaxase, interacts not only with the CP but also with other mating channel proteins as it traverses the membrane (Atmakuri et al., 2004; Cascales and Christie, 2004). Any interaction between pLV22a transfer DNA and other RP4 mating channel proteins remains to be determined.
In conclusion, we not only have begun to understand the requirements for pLV22a mobilization in E. coli¸ but also have established that interactions between this transfer factor and the required RP4 TraG CP are similar to those of cognate relaxosome. These similarities support the broad host range nature of RP4 in the recognition of non-cognate transfer systems, such as those of Bacteroides, that may contribute to gene flow among many different bacteria including those found in the gut. How RP4 specifically recognizes non-cognate transfer systems, especially given the diversity and lack of homology among Bacteroides transfer factors, remains to be determined.
Experimental procedures
Strains and plasmids
Strains and plasmids used in this study are listed in Table 1. Media, antibiotic levels and growth conditions for E. coli have been previously described (Haase et al., 1995; Novicki and Hecht, 1995). Cells were grown in Luria–Bertani (LB) medium (1% w/v tryptone, 0.5% w/v yeast extract, 1% sodium chloride) and titrated on LB plates. When appropriate, antibiotics were added as follows: ampicillin 200 μg ml−1; nalidixic acid 30 μg ml−1; chloramphenicol 25 μg ml−1; streptomycin 50 μg ml−1; kanamycin 50 μg ml−1; and tetracycline 10 μg ml−1.
E. coli to E. coli mating experiments
Quantitative E. coli to E. coli filter mating experiments were performed by mixing log-phase cultures of donor E. coli HB101 Smr containing RP4 and the mobilizable plasmid and isogenic E. coli recipient HB101 Nxr cells (donor to recipient ratio 1:9), as previously described (Novicki and Hecht, 1995). The cells were pelleted and resuspended in 100 μl of phosphate buffered saline (PBS) (8 mM Na2HPO4, 2 mM NaH2PO4, 145 mM NaCl, pH 6.9) and plated onto a sterile 0.22 μM acetate-plus filters (GE Osmonics, Minnetonka, MN) which are supported on plain LB agar plates. LB agar plates were incubated for 3 h at 37°C to minimize secondary mobilization events. Cells were resuspended and serially diluted with PBS, with transconjugants selected by plating on LB agar plates with appropriate antibiotics. The mobilization frequencies were calculated by dividing the number of colonies (transconjugants) in the selection plate by the number of input donor cells.
Western blots
Proteins were visualized by Western blots as described previously (Ziegelin et al., 1991). In brief, 25 ml of LB medium was inoculated with 500 μl of overnight culture cells and grown at 37°C in a shaking water bath. Where induction was required, a 1 mM final concentration of isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the culture and continued incubation for another 3 h. An OD600 of 1.00 cells were harvested by centrifugation and lysed with 30 μl of cracking buffer (15% (w/v) glycerol, 100 mM Tris-Cl pH 6.9, 5% (w/v) SDS, 1 M β-mercaptoethanol). The mixture was incubated first for 5 min at 60°C and then for another 5 min at 100°C. Cleared lysate was obtained by centrifugation at 80 000 g for 30 min at 25°C. About 5 μl of cleared lysate were electrophoresed using a SDS-PAGE gel followed by overnight transfer to a 0.2 μm of nitrocellulose membrane (Bio-Rad, Hercules, CA). The blot was then blocked overnight using casein blocking buffer (Pierce Biotech, Rockford, IL), and then incubated for 60 min with primary antibody prepared in the same blocking buffer solution. The blot was washed thrice in Tris-buffered saline (TBS)-Tween buffer [50 mM Tris-Cl, 150 mM NaCl, pH 7.5 and 0.1% (v/v) of Tween 20]. Final detection of proteins was accomplished by incubating the blot for 45 min with horse-radish peroxidase-labelled secondary antibody (POD-HRP, Roche, Indianapolis, IN) and chemiluminescent substrate luminol (ECL system, GE Biosciences, Piscataway, NY). Light emitted by the substrate is captured using an CLxposure film (Pierce Biotech, Rockford, IL).
Purification of histidine-tagged MbpB and TraG
An N-terminal (His)10-tagged recombinant protein of MbpB was expressed using pET-(His)10MbpB (pET19b + construct) (Novagen, LaJolla, CA) from E. coli strain BL21 (DE3). Overnight culture of BL21 (DE3) carrying pET-(His)10MbpB was used to inoculate a fresh 50 ml of LB medium with ampicillin. At log phase, the culture was inoculated with IPTG to a final concentration of 1 mM and incubated overnight at 37°C. The cells were resuspended in 5 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 15 mM imidazole, pH 8.0). Following 30 min incubation with lysozyme (1 mg ml−1) the cells were sonicated using ten 10s bursts with a 10s cooling period between each burst. The cell lysate was incubated with 500 μl of 50% Ni-NTA resin (Qiagen, Valencia, CA) slurry at 4°C for 1 h. The lysate–resin mixture was loaded into a polypropylene column (Qiagen, Valencia, CA) and washed twice with 4 ml of wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0). The pure MbpB protein was eluted using 10 fractions of 500 μl of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0). Pooled eluates were dialysed using 10 000 MWCO Slide-A-Lyzer® Dialysis Cassette (Pierce Biotech, Rockford, IL) for sequential dialysis to remove salt and imidazole. The protein were analysed by both Gel-Code blue (Pierce Biotech, Rockford, IL) or by Western blotting following separation on a 12% SDS-PAGE gel. A BCA™ Protein Assay kit (Pierce Biotech, Rockford, IL) was used for protein quantification. Overexpression and purification of TraG(His)6 protein was performed according to the protocol from plasmid pET-TraG(His)6, as described by Szpirer et al. (2000).
Transfer-ImmunoPrecipitation (TrIP) assay
In vivo cross linking
We adapted the TrIP assay from Agrobacterium to E. coli (Cascales and Christie 2004). Ten millilitres of LB medium was inoculated with overnight HB101 (E. coli) culture (1:50 dilution). The log-phase cells were induced with 1 mM IPTG when appropriate and incubated for 3.5 h post induction. A final of 2.5 OD600 of cells from each sample were harvested, washed twice with 5 ml of 20 mM sodium phosphate pH 6.9 and resuspended in 1.5 ml of 20 mM sodium phosphate buffer, pH 6.9. In vivo step-by-step cross-linking was performed by sequential addition of formaldehyde to reach a final concentration of 1% (five steps: 0.05%, 0.1%, 0.2%, 0.5% and 1%). Each time the mixture was incubated for 15 min without shaking at room temperature. The cells were washed in 1 ml of sodium phosphate buffer and the pellet stored in −80°C.
Cell lysis
The pellet obtained above was first thawed in ice for 30 min and resuspended in 50 μl of Tris EDTA SDS (TES) buffer (50 mM Tris-Cl pH 6.8, 2 mM EDTA, 1% β-mercaptoethanol, 1% SDS) followed by incubation for 30 min at 37°C with shaking. To this, mixture 225 μl of ice cold non-ionic polygycol 1(NP1) buffer (150 mM Tris-Cl pH 8.0, 0.5 M sucrose, 10 mM EDTA) was added followed by lysozyme to a final concentration of 1 mg ml−1. After incubating on ice for 3 h, the mixture was shaken at 37°C for 30 min Triton X-100 was added to a final concentration of 4% (v/v) and incubated for 15 min at room temperature. Complete, EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN) was added and incubated overnight first for 15 min at 37°C rocking then on wheel at 4°C. To the mixture 800 μl of NP1 buffer was added and the cleared cell lysate was collected by centrifugation at 14 000 g for 15 min at 4°C.
Immunoprecipitation
To preclear the cell lysate, 40 μl of Protein A-Sepharose CL4B (GE Healthcare Bio-Sciences, Piscataway, NJ) slurry was added to the entire volume of cell lysate. The mixture was incubated at room temperature tumbled end over end and centrifuged to remove the bead slurry. The S was incubated overnight at 4°C with either TraG antiserum or anti-His antibody coupled to Protein A-Sepharose CL4B. The S fraction was separated from the pellet by centrifugation. Then the pellet was washed twice with NP1 buffer containing 1% Triton X-100 and once with NP1 buffer containing 0.1% Triton X-100. Finally to the pellet 50 μl of 10 mM Tris-Cl (pH 6.8) was added and incubated overnight at 65°C. The IP fraction was eluted from the beads by centrifugation at 14 000 g for 5 min at RT.
Polymerase chain reaction
The S and IP fraction were analysed in parallel to determine transfer DNA PCR product. Three sets of primers were designed; first set to amplify part of pLV22a transfer DNA corresponding to mbpB gene (mbpB-For: 5′-CCGGGTGTGAGTTTGTTCC-3′ and mbpB-Rev: 5′-GCAGTAGCCACCGAGATAGC-3′) to give a PCR product of size 695 bp, as a positive control a second set to amplify part of RP4 transfer DNA corresponding to trbE gene (trbE-For: 5′-CCGAGGTTCATCAGCTCTTC-3′ and trbE-Rev: 5′-TGCAGTTCCTGAGCACCAAG-3′) to give a PCR product of size 310 bp. As a negative control, a third set to amplify part of a genomic gene ‘adk’ (adk-For: 5′-TTGGTATTACCCGCTTCTGC-3′ and adk-Rev: 5′-CTGCCGTAATGGTTTCCTGTTG-3′) to give a PCR product of size 348 bp. PCR amplification was performed using Green Go Taq™ polymerase mixture (Promega, Madison, WI). Two microlitres of IP fraction and 2 μl of S fraction (1:100 dilution) were used as DNA template and the PCR cycles were as below:
| Denaturation: 95°C 2 min | 1× | |
| Denaturation: 95°C 1 min | ||
| Annealing: 55°C 1 min | 30× | |
| Extension: 72°C 45 s | ||
| Final Extension: 72°C 5 min |
An equal volume of PCR products (25 μl) was loaded in a 1.2% agarose gel to fractionate the product by electrophoresis and visualized by staining the gel with 0.5 μg ml−1 ethidium bromide and images taken using an automated CCD camera (AlphaImager®), from AlphaInnotech (San Leandro, CA).
Overlay assay
Total cell lysate
25 ml of LB medium was inoculated with 500 μl of overnight culture cells and grown at 37°C in a shaking water bath. Where induction was required, a 1 mM final concentration of IPTG was added to the culture with continued incubation for another 3 h. An OD600 of 1.00 cells were harvested by centrifugation and lysed with 30 μl of cracking buffer (15% (w/v) glycerol, 100 mM Tris-Cl pH 6.9, 5% (w/v) SDS, 1 M β-mercaptoethanol). The mixture was incubated initially for 5 min at 60°C followed by an additional 5 min at 100°C. Cleared lysate was obtained by centrifugation at 80 000 g for 30 min at 25°C.
Transfer of cell lysate to blot
For the assay, the first 5 μl of cell lysate overexpressing the ‘prey’ proteins were electrophoresed on a SDS-PAGE gel and transferred on to a 0.2 μm of nitrocellulose blot (Bio-Rad, Hercules, CA). The proteins were transferred overnight using a non-denaturing transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). To remove any traces of SDS, incubating blot for extended period (for transferring, washing and blocking) with this buffer removes any traces of SDS. Eliminating most or all SDS from the transfer buffer during the transfer process allows for partial renaturation of transferred proteins on the blot allowing better detection in the overlay assay (Burgess et al., 2000).
Overlay with purified proteins
Following transfer of cell lysate with either relaxase or CPs, the blot was washed twice in transfer buffer and blocked overnight with blocking buffer (Qiagen, Valencia, CA). Following three washes with TBS buffer (50 mM Tris-Cl, 150 mM NaCl, pH 7.5) the blot was overlaid with the purified native relaxase or CPs at a concentration of 1 μg ml−1 diluted in 20 ml of blocking buffer. Following 3 h of incubation, blots are washed twice in TBS-Tween buffer [50 mM Tris-Cl, 150 mM NaCl, pH 7.5 and 0.1% (v/v) of Tween 20], then incubated with primary antibody to the overlayed protein. This was followed by three washes in TBS-tween buffer. Productive interaction between the relaxase and CP were then detected and analysed by incubating the blot for 45 min with horse-radish peroxidase-labelled secondary antibody (POD-HRP, Roche, Indianapolis, IN) and chemiluminescent substrate luminol (ECL system, GE Healthcare Bio-Sciences, Piscataway, NJ). The light emitted by the substrate is captured using an CLxposure film (Pierce, Rockford, IL).
Supplementary Material
Acknowledgments
We gratefully acknowledge Erich Lanka for providing all RP4 constructs, tra1 and tra2 mutant plasmids and TraG antiserum. We also thank Cedric Szpirer and Beth Traxler for the gift of pET-TraG(His)6 and traD plasmids respectively. Our sincere appreciation to Peter Christie and Krishnamohan Atmakuri for helpful discussions and guidance for the success of TrIP assay. We also thank Gayatri Vedantam for her critical review of this manuscript. This work was supported by the Department of Veterans Affairs, Merit Review 006 and National Institutes of Allergy and Infectious Diseases AI050122.
Footnotes
Supplementary material: This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.2007.05967.x (This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Atmakuri K, Cascales E, Christie PJ. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol Microbiol. 2004;54:1199–1211. doi: 10.1111/j.1365-2958.2004.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic M, Parker AC, Stagg J, Whitley HP, Wells WG, Jacob LA, Smith CJ. Genetic and structural analysis of the Bacteroides conjugative transposon CTn341. J Bacteriol. 2005;187:2858–2869. doi: 10.1128/JB.187.8.2858-2869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer D, Pansegrau W, Lanka E. Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4. J Bacteriol. 1994;176:4285–4295. doi: 10.1128/jb.176.14.4285-4295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass KA, Hecht DW. Isolation and characterization of cLV25, a Bacteroides fragilis chromosomal transfer factor resembling multiple Bacteroides sp. mobilizable transposons. J Bacteriol. 2002;184:1895–1904. doi: 10.1128/JB.184.7.1895-1904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S, Cashmore AM, Wilkins BM. IncP plasmids are unusually effective in mediating conjugation of Escherichia coli and Saccharomyces cerevisiae: involvement of the tra2 mating system. J Bacteriol. 1998;180:6538–6543. doi: 10.1128/jb.180.24.6538-6543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek A, Zettl M, Lorenzoni K, Schauer A, Manhart M, Koraimann G. Thirty-eight C-terminal amino acids of the coupling protein TraD of the F-like conjugative resistance plasmid R1 are required and sufficient to confer binding to the substrate selector protein TraM. J Bacteriol. 2004;186:6999–7006. doi: 10.1128/JB.186.20.6999-7006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonheyo G, Graham D, Shoemaker NB, Salyers AA. Transfer region of a Bacteroides conjugative transposon, CTnDOT. Plasmid. 2001;45:41–51. doi: 10.1006/plas.2000.1495. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Labbate M, Koenig JE, Stokes HW. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 2007;15:301–309. doi: 10.1016/j.tim.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burgess RR, Arthur TM, Pietz BC. Mapping protein–protein interaction domains using ordered fragment ladder far-western analysis of hexahistidine-tagged fusion proteins. Methods Enzymol. 2000;328:141–157. doi: 10.1016/s0076-6879(00)28396-1. [DOI] [PubMed] [Google Scholar]
- Cabezon E, Sastre JI, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- Cascales E, Christie PJ. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science. 2004;304:1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Christie PJ, Dubnau D. The ins and outs of DNA transfer in bacteria. Science. 2005;310:1456–1460. doi: 10.1126/science.1114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang TA, Christie PJ. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J Bacteriol. 1997;179:453–462. doi: 10.1128/jb.179.2.453-462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia MV, Varsaki A, Garcillan-Barcia MP, Latorre A, Drainas C, de la Cruz F. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol Rev. 2004;28:79–100. doi: 10.1016/j.femsre.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Frost LS, Ippen-Ihler K, Skurray RA. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity G, Lilburn T, Cole J, Harrison S, Euzeby J, Tindall B. Introduction to the Taxonomic Outline of Bacteria and Archaea (TOBA) Release 7.7. 2007 [WWW document]. URL http://www.taxonomicoutline.org.
- Gherna R, Woese CR. A partial phylogenetic analysis of the ‘flavobacter-bacteroides’ phylum: basis for taxonomic restructuring. Syst Appl Microbiol. 1992;15:513–521. doi: 10.1016/S0723-2020(11)80110-4. [DOI] [PubMed] [Google Scholar]
- Gilmour MW, Gunton JE, Lawley TD, Taylor DE. Interaction between the IncHI1 plasmid R27 coupling protein and type IV secretion system: TraG associates with the coiled-coil mating pair formation protein TrhB. Mol Microbiol. 2003;49:105–116. doi: 10.1046/j.1365-2958.2003.03551.x. [DOI] [PubMed] [Google Scholar]
- Grahn AM, Haase J, Bamford DH, Lanka E. Components of the RP4 conjugative transfer apparatus form an envelope structure bridging inner and outer membranes of donor cells: implications for related macromolecule transport systems. J Bacteriol. 2000;182:1564–1574. doi: 10.1128/jb.182.6.1564-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J, Lurz R, Grahn AM, Bamford DH, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CM, Lee H, Li PL, Cook DM, Piper KR, von Bodman SB, et al. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J Bacteriol. 2000;182:1541–1548. doi: 10.1128/jb.182.6.1541-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht DW. Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis. 2004;39:92–97. doi: 10.1086/421558. [DOI] [PubMed] [Google Scholar]
- Hecht DW, Kos IM, Knopf S, Vedantam G. Characterization of BctA, and a mating apparatus protein required for transfer of the Bacteroides fragilis conjugal element, BTF-37. Res Microbiol. 2007 doi: 10.1016/j.resmic.2007.06.004. in press. [DOI] [PubMed] [Google Scholar]
- Heinemann JA, Sprague GF., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989;340:205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- Lanka E, Wilkins BM. DNA processing reactions in bacterial conjugation. Annu Rev Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- Lee MH, Kosuk N, Bailey J, Traxler B, Manoil C. Analysis of F factor TraD membrane topology by use of gene fusions and trypsin-sensitive insertions. J Bacteriol. 1999;181:6108–6113. doi: 10.1128/jb.181.19.6108-6113.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M, Balzer D, Weyrauch K, Lanka E. The mating pair formation system of plasmid RP4 defined by RSF1010 mobilization and donor-specific phage propagation. J Bacteriol. 1993;175:6415–6425. doi: 10.1128/jb.175.20.6415-6425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M, Bolland S, de la Cruz F. Genetic organization of the conjugal DNA processing region of the IncW plasmid R388. J Mol Biol. 1994;235:448–464. doi: 10.1006/jmbi.1994.1005. [DOI] [PubMed] [Google Scholar]
- Lu J, Frost LS. Mutations in the C-terminal region of TraM provide evidence for in vivo TraM–TraD interactions during F-plasmid conjugation. J Bacteriol. 2005;187:4767–4773. doi: 10.1128/JB.187.14.4767-4773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton R, Sjolander K, Krishnamurthy N, Foley J, Zambryski P. Predicted hexameric structure of the Agrobacterium VirB4 C terminus suggests VirB4 acts as a docking site during type IV secretion. Proc Natl Acad Sci USA. 2005;102:1685–1690. doi: 10.1073/pnas.0409399102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncalian G, Cabezon E, Alkorta I, Valle M, Moro F, Valpuesta JM, et al. Characterization of ATP and DNA binding activities of TrwB, the coupling protein essential in plasmid R388 conjugation. J Biol Chem. 1999;274:36117–36124. doi: 10.1074/jbc.274.51.36117. [DOI] [PubMed] [Google Scholar]
- Morgan RM, Macrina FL. bctA: a novel pBF4 gene necessary for conjugal transfer in Bacteroides spp. Microbiology. 1997;143:2155–2165. doi: 10.1099/00221287-143-7-2155. [DOI] [PubMed] [Google Scholar]
- Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci USA. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novicki TJ, Hecht DW. Characterization and DNA sequence of the mobilization region of pLV22a from Bacteroides fragilis. J Bacteriol. 1995;177:4466–4473. doi: 10.1128/jb.177.15.4466-4473.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G, Dublanchet A, Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979;139:97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- Robillard NJ, Tally FP, Malamy MH. Tn4400, a compound transposon isolated from Bacteroides fragilis, functions in Escherichia coli. J Bacteriol. 1985;164:1248–1255. doi: 10.1128/jb.164.3.1248-1255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sastre JI, Cabezon E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol. 1998;180:6039–6042. doi: 10.1128/jb.180.22.6039-6042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder G, Lanka E. The mating pair formation system of conjugative plasmids-A versatile secretion machinery for transfer of proteins and DNA. Plasmid. 2005;54:1–25. doi: 10.1016/j.plasmid.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Schroder G, Krause S, Zechner EL, Traxler B, Yeo HJ, Lurz R, et al. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: inner membrane gate for exported substrates? J Bacteriol. 2002;184:2767–2779. doi: 10.1128/JB.184.10.2767-2779.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker NB, Vlamakis H, Hayes K, Salyers AA. Evidence for extensive resistance gene transfer among Bacteroides spp. & among Bacteroides and other genera in the human colon. Appl Environ Microbiol. 2001;67:561–568. doi: 10.1128/AEM.67.2.561-568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitailo LA, Zagariya AM, Arnold PJ, Vedantam G, Hecht DW. The Bacteroides fragilis BtgA mobilization protein binds to the oriT region of pBFTM10. J Bacteriol. 1998;180:4922–4928. doi: 10.1128/jb.180.18.4922-4928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer CY, Faelen M, Couturier M. Interaction between the RP4 coupling protein TraG and the pBHR1 mobilization protein Mob. Mol Microbiol. 2000;37:1283–1292. doi: 10.1046/j.1365-2958.2000.02077.x. [DOI] [PubMed] [Google Scholar]
- Tato I, Zunzunegui S, de la Cruz F, Cabezon E. TrwB, the coupling protein involved in DNA transport during bacterial conjugation, is a DNA-dependent ATPase. Proc Natl Acad Sci USA. 2005;102:8156–8161. doi: 10.1073/pnas.0503402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P, Derlot E, Courvalin P. Enhanced conjugative transfer of plasmid DNA from Escherichia coli to Staphylococcus aureus and Listeria monocytogenes. FEMS Microbiol Lett. 1993;109:19–23. doi: 10.1111/j.1574-6968.1993.tb06137.x. [DOI] [PubMed] [Google Scholar]
- Vedantam G, Hecht DW. Isolation and characterization of BTF-37: chromosomal DNA captured from Bacteroides fragilis that confers self-transferability and expresses a pilus-like structure in Bacteroides spp. and Escherichia coli. J Bacteriol. 2002;184:728–738. doi: 10.1128/JB.184.3.728-738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantam G, Knopf S, Hecht DW. Bacteroides fragilis mobilizable transposon Tn5520 requires a 71 base pair origin of transfer sequence and a single mobilization protein for relaxosome formation during conjugation. Mol Microbiol. 2006;59:288–300. doi: 10.1111/j.1365-2958.2005.04934.x. [DOI] [PubMed] [Google Scholar]
- Vergunst AC, van Lier MC, Dulk-Ras A, Stuve TA, Ouwehand A, Hooykaas PJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci USA. 2005;102:832–837. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shoemaker NB, Wang GR, Salyers AA. Characterization of a Bacteroides mobilizable transposon, NBU2, which carries a functional lincomycin resistance gene. J Bacteriol. 2000;182:3559–3571. doi: 10.1128/jb.182.12.3559-3571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters VL, Strack B, Pansegrau W, Lanka E, Guiney DG. Mutational analysis of essential IncP alpha plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992;174:6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle G, Shoemaker NB, Salyers AA. The role of Bacteroides conjugative transposons in the dissemination of antibiotic resistance genes. Cell Mol Life Sci. 2002;59:2044–2054. doi: 10.1007/s000180200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 2007;5:e156. doi: 10.1371/journal.pbio.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelin G, Pansegrau W, Strack B, Balzer D, Kroger M, Kruft V, Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1:303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


