Abstract
HPV-DNA integration into cellular chromatin is usually a necessary event in the pathogenesis of HPV-related cancer; however, the mechanism of integration has not been clearly defined. Breaks must be created in both the host DNA and in the circular viral episome for integration to occur, and studies have shown that viral integration is indeed increased by the induction of DNA double strand breaks. Inflammation generates reactive oxygen species, which in turn have the potential to create such DNA strand breaks. It is plausible that these breaks enable a greater frequency of HPV-DNA integration, and in this way contribute to carcinogenesis. Consistent with this idea, co-infections with certain sexually transmitted diseases cause cervical inflammation, and have also been identified as cofactors in the progression to cervical cancer. This article examines the idea that inflammation facilitates HPV-DNA integration into cellular chromatin through the generation of reactive oxygen species, thereby contributing to carcinogenesis.
Keywords: bacterial co-infection, cervical cancer, DNA damage, HPV, inflammation, integration, oropharyngeal cancer, oxidative stress, papillomavirus, viral
Human papillomaviruses
Human papillomaviruses (HPVs) are a group of circular, dsDNA viruses that infect epithelial cells [1], and are divided into more than 100 different genotypes based on sequence differences within their L1 gene [2]. Of the 100 genotypes of HPV, at least 30 are sexually transmitted and infect the genital areas of both men and women. A subset of these genotypes causes anogenital warts, which can be either benign or cancerous. Consequently, HPV types are designated ‘low risk’ or ‘high risk’ (HR), based on whether they are known to cause benign or cancerous lesions [3]. Virtually all cases of cervical and anogenital cancer are caused by approximately 15 HR genotypes of HPV [4–7]. Of the HR genotypes, HPV16, HPV18, HPV31 and HPV33 are associated with 90% of all cases of cervical cancer, and HPV DNA is present in more than 90% of premalignant and malignant squamous lesions of the uterine cervix [8,9]. HPV type 16 is the most prevalent type and is associated with more than 50% of all cases of cervical cancers [10,11].
Papillomavirus genomes consist of double-stranded circular DNA of approximately 8 kb in size, containing approximately eight open reading frames (ORFs), which are transcribed as polycistronic messages from a single DNA strand. The genome can be divided into three regions: an early region, a late region and a long control region. The early region encodes six common ORFs: E1, E2, E4, E5, E6 and E7. The late region, positioned downstream of the early region, encodes the ORFs for the L1 and L2 capsid proteins. The long control region holds the origin of replication and several transcription factor binding sites but lacks any protein-coding function.
During the normal lifecycle, the viral genome exists in an episomal form in the basal cells of the epithelium at approximately 50–100 copies per cell [12,13]. Episomal expression of the viral oncogenes E6 and E7 is tightly regulated, with high-level expression observed only in suprabasal postmitotic cells [14]. At this stage, the viral oncogenes induce unscheduled re-entry into the S-phase of the cell cycle and activate the host replication machinery needed for viral genome amplification [15]. Viral oncogene expression in these ‘productive infections’ does not cause cancer, as these cells are destined to be lost from the cervical squamous epithelium. For neoplastic progression to occur, viral oncogene expression must remain high throughout the epithelium [16], and in the vast majority of cases, this high-level expression occurs due to integration of truncated viral genomes that have lost the ability to downregulate expression of these oncogenes. This event is not a normal part of the viral lifecycle, as it is accompanied by the loss of one or more viral genes needed for synthesis of an infectious virion.
Immune response to HPV infections
An immunocompetent host has several lines of defense against viral infection. Host immunity consists of a partnership between the innate (phagocytes, soluble proteins) and adaptive (antibody, cytotoxic effector cells) immune responses. The innate immune response detects pathogens and acts as the first line of defense. It has no specific memory for pathogens, but can activate the adaptive immune system, which can then mount a specific response to a particular pathogen [17]. In the case of HPV infection, the host immune response is generally limited. The primary reason for this is that the virus infects basal epithelial cells, which are shielded from circulating immune cells during the initial stages of infection. Additionally, the HPV lifecycle is nonlytic, so that the typical immune response observed upon cell death as a result of inflammation does not occur. HPV DNA is only sufficiently amplified to a level where the virus and its proteins can be detected by the host immune surveillance cells in suprabasal keratinocytes, so that a functionally active immune response can only be generated during the later stages of HPV infection.
In addition, the virus employs several specific mechanisms that downregulate host innate and cell-mediated immunity and thus facilitate host immune evasion and persistent infection. For example, E6 and E7 interfere with the expression of Toll-like receptors that recognize pathogen-associated molecular patterns, thereby disrupting this aspect of the immune response. Toll-like receptor-9 transcription in particular is inhibited in cells expressing HPV16 E6 and E7 [18]. In addition, HR-HPV viruses downregulate IFN-α-inducible gene expression [19].
Despite these challenges, most HPV infections are eventually successfully cleared, due primarily to a strong localized cell-mediated immune response by the host [20]. For example, it has been observed that regressing warts are infiltrated by T lymphocytes and macrophages [21]. In immuno suppressed individuals, a higher prevalence of HPV-induced lesions and HPV-related tumors is observed, thereby emphasizing the importance of the immune system in counteracting HPV infection [22]. However, in a subset of infected individuals, the infection is not cleared, and the virus is allowed to persist. It is this subset of infected individuals who are at risk for developing HPV-associated cancer.
HPV-related cancers
Human papillomaviruses are primarily known as the causative agent of nearly all cases of cervical cancer. Infection with HPV is the most common sexually transmitted disease (STD) in the USA, and data indicate that it is particularly prevalent in women aged 20–24 years [23]. Cervical cancer is the second most common cancer in women around the globe. In total, 83% of all cases of cervical cancer occur in developing countries, where it accounts for approximately 15% of all cancers in women [24]. Most cases of cervical cancer are squamous cell carcinomas; adenocarcinomas are far less common.
High-risk HPV infection has also been implicated in the development of a number of other cancers, including head and neck squamous cell carcinoma. In head and neck squamous cell carcinoma, the overall prevalence of HPV is 25.9%, with the highest prevalence (35.6%) seen in oropharyngeal tumors [25]. HPV infection has been implicated in 50% of vulvar squamous cell carcinomas and 60% of vaginal squamous cell carcinomas [26]. In addition, HPV-DNA was detected in more than 40% of penile cancers [24]. In a study examining the incidence of anal cancer in men in Denmark and Sweden, HPV-DNA was found in 83% of men with anal cancer [27]; a US study found the corresponding number in US men to be 90% [28]. These data provide evidence that HPV is responsible for a considerable health burden worldwide, as these viruses are involved in the etiology of a significant percentage of anogenital and oropharyngeal cancers. Therefore, there remains a need to explore the mechanisms of HPV-associated cancer progression, and in particular, the incompletely understood process of HR-HPV integration.
HPV integration
Although 95% of patients with precancerous lesions of the cervix harbor HPV, only a small fraction of these eventually progress to invasive carcinoma [29]. Three premalignant stages, cervical intraepithelial neoplasia (CIN)1, CIN2, and CIN3, precede development of invasive carcinoma. CIN1 lesions typically regress spontaneously, with only a few lesions progressing to CIN2/CIN3 and eventually to invasive carcinoma [30]. It is therefore clear that although HR-HPV infection is a necessary event in cervical carcinogenesis, it is not sufficient for the pathogenesis of cervical cancer. Progression of cervical cancer in HPV-infected women is tightly linked to integration status, and the frequency with which HR-HPV is found integrated in cervical cancers is consistently high. For example, it has been reported that 100% of HPV18-, 80% of HPV16- and 81% of HPV31-positive cancers show viral integration [31,32]. It is important to note that while integration of the HR-HPV genome is observed in the vast majority of cases of cervical cancer, a small percentage of cervical cancers do develop while the HPV-DNA remains in episomal form [33]. In these instances an elevation in the episome copy number is observed, which is accompanied by an increase in viral oncogene expression. This step accomplishes, albeit less efficiently, the same effect as HPV integration; namely, the high-level expression of viral oncogenes leading to cell transformation [34].
Integration of the HR-HPV genome, therefore, is usually considered a necessary event in the progression to cervical and other anogenital cancers, with an increase in the presence of integrated viral DNA correlating with disease progression [35–41]. Evidence indicates that the HPV genome is present in episomal form in early low-grade lesions (such as CIN1 and CIN2), while integration of the viral genome is observed in advanced stages of precancer and invasive carcinoma, suggesting that integration of HR-HPV genomes into the host genome occurs relatively late in the pathogenesis of cancer [32,42]. Furthermore, inflammation-mediated DNA damage frequently precedes the genomic abnormalities caused by HPV oncoproteins [43], thereby suggesting that HPV integration is involved in neoplastic progression.
Integration typically results in the increased expression and stability of transcripts encoding the viral oncogenes E6 and E7, which are known to inactivate and/or accelerate the degradation of numerous cellular proteins, including retinoblastoma protein (E7) and p53 (E6) [44–46]. The E2 ORF has been identified as the preferential site of integration because it is more commonly disrupted or deleted than any other site [47]. The E2 protein negatively regulates E6 and E7 expression; therefore, loss of this ORF during integration results in increased expression of the transforming E6 and E7 oncoproteins [48]. Thus, integration of the HPV genome results in the enhanced, deregulated expression of the viral oncogenes, E6 and E7, which are responsible for cellular transformation. In addition, it is thought that viral DNA integration disrupts critical cellular genes [49,50]. Both of these factors would contribute to neoplastic progression.
Relatively little is known of the process whereby HPV genomes become integrated into that of the host. However, several studies have suggested that DNA damage and agents that can induce DNA damage may play a role in HPV integration. A 2007 study in W12 cells, which stably maintain HPV16 episomes, demonstrated that when double strand breaks (DSBs) are generated due to Ku70 depletion, new HPV16 viral integration events occurred. Ku70 is a crucial mediator of DSB nonhomologous end joining [51]. In addition, a study by Someya et al. showed that the activity of DNA-dependent protein kinase, an important molecule involved in DNA-DSB repair, was significantly lower in patients with cervical cancer than in normal volunteers [52]. These data indicate that DSBs may be associated with HPV16 episome loss and integration in cervical cancer [53]. In the case of another DNA virus, hepatitis B, that causes hepatocellular cancer and in which viral DNA integration coincides with severe dysplasia, studies have shown that integration frequency increases with DNA damage [54]. Therefore, it is reasonable to expect that the integration of viral DNA into that of the host would be enhanced by damage to both the viral episome and the host DNA, as this would create a site for integration.
Inflammation-mediated DNA damage therefore provides a potential mechanism by which HPV integration could occur in the progression of cervical cancer. Indeed, inflammation has been implicated in the progression of a variety of cancers, and it has been suggested that the excessive amounts of reactive oxygen and nitrogen species (ROS and RNS) produced during chronic inflammation play a role in carcinogenesis by promoting DNA damage [55]. In the case of HPV-associated cancers, inflammation would also facilitate the integration of the viral genome by inducing breaks in both the viral and host genomes. In this article, we explore the evidence for this hypothesis.
Cofactors for HPV oncogenesis
Several cofactors, in addition to HPV infection, have been associated with the progression to cancer. Smoking, long-term use of oral contraceptives and parity have all been suggested to serve as cofactors for the development of cervical cancer [56,57]. Among these cofactors, the case for smoking as a cofactor is perhaps the strongest, as evidence shows that it precedes the development of cervical precancer and cancer, and increases the risk of developing cervical cancer in HPV-positive women [58,59]. Smoking is known to induce inflammation [60]. Smoking leads to DNA-adduct formation and thus DNA damage, a possible mechanism for cancer development. High parity may also be mechanistically linked, as it causes cervical trauma and cellular oxidative and nitrosative stress, all of which can lead to DNA damage and contribute to cancer progression [61]. Women with seven or more full-term pregnancies are at higher risk of developing cervical cancer than are those with only one or two, indicating that as the number of full-term pregnancies increases so does the risk of developing cervical cancer [56,62]. Women also increase their risk of cervical cancer through the long-term use of oral contraceptives [63–65].
Another cofactor involved in the development of cervical cancer is co-infection with other STDs, either viral or bacterial in nature. Such infections can cause inflammation, and in HPV-infected women, cervical inflammation is associated with cervical neoplasia [66,67]. It is important to note that this inflammation is not typically due to HPV itself, in part because the immune system is largely ineffective against HPV. HPV infects keratinocytes, which are distant from immune centers and have a naturally short lifespan. In addition, the virus does not need to destroy the cell, and so inflammation is not typically triggered [20]. Despite this, high levels of inflammatory mediators are observed in cervical cancer. For example, cyclooxgenase (COX)-2, an enzyme responsible for prostaglandin formation, is overexpressed in cervical cancer [68,69]. COX are a family of enzymes that catalyze the formation of prostaglandins from arachidonic acid [70]. The COX-2 isoform is induced in response to inflammatory factors and is expressed in early-stage premalignant lesions, including cervical tissues [70]. One study found that 100% of the cervical cancer samples tested showed COX-2 expression, compared with only 7.7% in normal samples [71]. Also, NF-κB, a master transcription factor that is essential for promoting inflammation-associated carcino-genesis [72,73], is overexpressed in cervical lesions co-expressing Chlamydia trachomatis and HPV.
Since HPV infection of the cervix by itself is not highly inflammatory, viral (e.g., herpes simplex virus [HSV]) and bacterial (e.g., C. trachomatis) infections serve as the main sources of cervical inflammation. C. trachomatis is a well-known cause of the inflammatory condition, cervitis, and cervical cancer cells infected with C. trachomatis secrete higher levels of proinflammatory cytokines than uninfected cervical cancer cells [74]. Several recent studies have determined that co-infection with either C. trachomatis or HSV is associated with a greater risk of developing cervical cancer [75], and have also pointed to an association between the development of cervical cancer and other STDs such as Neisseria gonorrhoeae [76]. In addition, co-infection of HPV16 with herpesviruses such as cytomegalovirus and EBV, as well as with HSV2, increased the frequency of HPV16 integration [77], and C. trachomatis infection was shown to favor the entry and persistence of multiple HR-HPV types in cervical epithelium [78]. A paper by Schwebke and Zajackowski reported that it was the inflammation caused by such infections, rather than the particular infection itself, that was associated with squamous intraepithelial lesions within the cervix [79]. Infection-induced inflammation has also been shown to increase the risk of other HPV-induced cancers, such as penile cancer, where infection with genital lichen sclerosis increases the risk of neoplasia in HPV-infected men [80,81]. The inflammation produced as a result of such co-infections can induce the generation of ROS, which can in turn contribute to the initiation and progression of cancers through damage to DNA. Thus, factors that affect the generation of ROS, such as smoking and inflammation, may cause DNA damage and affect HPV integration. As such, they may share a common mechanism in inducing severe neoplasia.
Inflammation in cancer
Chronic inflammation has long been established as a factor in the pathogenesis of cancer [82–86]. Chronic inflammation has been implicated in the development of several epithelial cancers such as those of the stomach, colon and bladder. Hepatocellular cancer, the most common type of liver cancer, is a frequent result of years of chronic liver inflammation induced by hepatitis B or C viral infection [87,88]. Inflammation also plays a role in the development of Hodgkin's lymphoma. In this case, the observed inflammation is due to infection with EBV, a lymphotrophic herpes virus that induces the release of inflammatory cytokines and chemokines involved in carcino-genesis [89]. In fact, it is thought that more than 15% of all deaths from cancer can be attributed to an underlying infection or inflammation [90]. The longer the inflammation persists, the higher the risk of cancer.
Inflammation is the body's primary immune response to infection with pathogens, and is characterized by neutrophilic and mononuclear immune cell infiltration, tissue destruction and fibrosis. Typically, an inflammatory response will continue until the pathogens are eliminated, so that continuous infection frequently leads to chronic inflammation. Virchow in 1863 first hypothesized that malignant neoplasms can occur at sites of chronic inflammation. He reasoned that tissue injury, inflammation and increased cell proliferation were caused by various irritants [85,91]. We now know that chronic inflammation does indeed play a multifaceted role in carcinogenesis, and clinical studies point towards it as a driving force in the development of cancer. It can be viewed as a cancer ‘promoter’ since it induces cell proliferation, recruits inflammatory cells, increases ROS leading to oxidative DNA damage, and reduces DNA repair [85]. Inflammation also promotes apoptosis resistance, proliferation, invasion, metastasis and the secretion of proangiogenic and immunosuppressive factors, all of which contribute to carcinogenesis [92].
Inflammation & ROS
The chronic inflammatory response can lead to cell damage and cellular hyperplasia due to overproduction of ROS and RNS. The main sources of ROS in cells are the mitochondria, cytochrome P450 and the peroxisome [93]. Under normal physiological conditions, there is a constant endogenous production of ROS and RNS, both of which play important roles as signaling molecules involved in metabolism, cell cycle and transduction pathways [94,95]. In order to maintain the beneficial effects of ROS, the cell must balance the production of ROS with its removal. During chronic inflammation, this balance is altered. Studies have shown that mitochondrial ROS production increases with viral infection [96], and NO and its derivatives (RNS) are produced in copious quantities in inflamed tissue [97]. In addition, the mechanisms for removing ROS and RNS are downregulated. These increased levels of ROS and RNS can then cause direct and indirect damage to the cell [98].
Chronic inf lammation causes the overproduction of ROS and RNS by increasing prosta glandin levels, which in turn induce the expression of proinflammatory cytokines such as IL-1, IL-6, TNF-α and IFN-γ [99,100]. These proinflammatory cytokines are then responsible for increasing the production of these free radicals through protein kinase-mediated signaling pathways; these effects are seen in both phagocytic and nonphagocytic cells. The ROS and RNS produced in this way can then interact with multiple types of macromolecules [101], including the DNA in mitotic cells, to induce permanent genetic mutations such as point mutations, gene deletions and gene rearrangements. Cells normally respond to increases in ROS and RNS by activating their antioxidant systems, which reduce the levels of ROS and RNS and begin to repair the damage by activating genes responsible for DNA repair. However, in chronic inflammation, this DNA damage accumulates and is not repaired. Compounding the problem, inflammation also reduces the levels of antioxidant enzyme defense in cells [102]. Several studies have shown a decrease in the three main antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase in cervical cancer [103–105]. This reduction in the antioxidant defense further exacerbates ROS-induced DNA damage. Thus, chronic inflammation induces oxidative stress, a characteristic of inflammatory diseases, with its associated deleterious effects in cells.
Free radicals react with all components of the cell to form stable adducts. At present, there are more than 100 known oxidized DNA products. ROS-induced DNA damage includes ss and/or dsDNA breaks, DNA base modifications, DNA intrastrand adducts and DNA–protein cross-links [106]. DNA damage can cause either arrest or induction of transcription, induction of signal transduction pathways, replication errors and genomic instability, all processes associated with the development of cancer [107,108]. NO and superoxide (O2-) react to form peroxynitrite (ONOO-), a highly reactive species that induces nitrosative and oxidative DNA damage. Peroxynitrite mediates the formation of 8-oxo-7,8-dihydro-2′-deoxyguanosine [109] and 8-nitroguanine [110,111], two of the most common base modifications [112], and thus potential biomarkers of inflammation-related carcinogenesis [108,113]. 8-hydrodeoxyguanosine content was observed to be higher in cervical dysplastic HPV-positive cells as compared with HPV-positive normal cells [114]. 8-nitroguanine is a mutagenic substance that preferentially causes G–T transversions [110,115,116]. These G–T transversions have been observed in vivo both in the ras gene and in the p53 tumor suppressor gene [117], indicating that DNA damage mediated by RNS and ROS may contribute to carcinogenesis via both the activation of protooncogenes and the inactivation of tumor suppressor genes. Oxidative damage to mitochondria can also contribute to carcinogenesis. For example, numerous mutations and altered expression of mitochondrial genes have been identified in various human cancers [118,119], and fragments of mtDNA have been found inserted into genomic DNA, suggesting an additional mechanism for oncogene activation [83]. Because mtDNA codes for enzymes important in respiration, damage to this DNA can cause mitochondrial respiratory chain dysfunction, thus increasing the production of hydroxyl radicals, which in turn cause additional oxidative damage to DNA [120,121].
Proteins are susceptible to oxidation by free radicals, more so than any other cell component. For example, the oxidation of SH groups on cysteine reduces the activity of various enzymes as well as the synthesis of glutathione, a major intracellular free radical scavenger that functions as part of the antioxidant defense system [122]. Lipid oxidation produces aldehydes and lipid peroxides. At low, nontoxic concentrations these molecules act as signaling transducers of ROS-mediated reactions, allowing them to modulate several cell functions, including gene expression and cell proliferation [123]. At high concentrations, however, they react with proteins, DNA and phospholipids to generate a variety of intra- and inter-molecular toxic covalent adducts that lead to the propagation and amplification of oxidative stress [124]. The most abundant ROS lipid-derived product found under conditions of oxidative stress is 4-hydroxynonenal (HNE), which promotes oxidative alterations of DNA and induces apoptosis [125–127]. HNE is directly involved in cell cycle regulation, and also causes mutations in p53 gene expression [128]. In addition, HNE forms etheno adducts with DNA and upregulates COX-2 expression. COX-2, an enzyme responsible for prostaglandin formation, is known to be upregulated in HPV-related cancers, such as those found in the head and neck [129]. This increased COX-2 expression in turn induces enhanced ROS production [130]. Therefore, the ROS and RNS released in copious amounts as a consequence of inflammation can initiate a set of amplification reactions that lead to even higher levels of these mediators, thus causing severe damage to cellular DNA.
Model for inflammation-induced HPV integration in cells
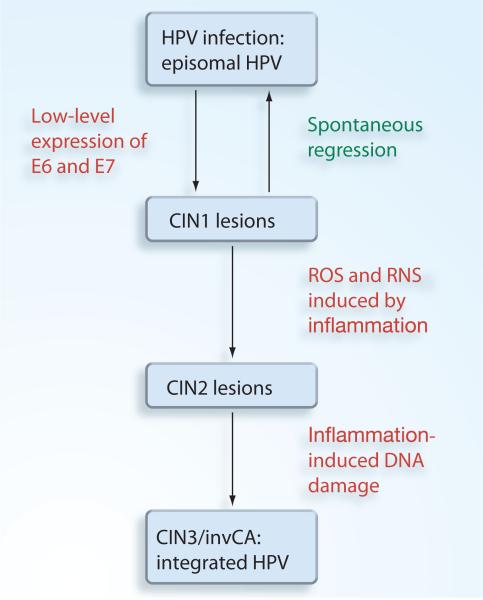
Based in the evidence presented, we propose a scheme for the involvement of inflammation in HPV carcinogenesis in Figure 1. Approximately 80% of women will be exposed to HPV within their lifetime. In the majority of these cases, the infection will spontaneously clear [33,131]. On occasion, CIN1 lesions will develop, but most of these lesions will also spontaneously clear [132]. However, in some cases, HPV-infected women will develop cervical inflammation caused by co-infection with either a viral or bacterial agent [133]. Alternatively, inflammation could be caused by other cofactors such as smoking. This inflammation will facilitate the progression of CIN1 lesions to CIN2 due to the cell proliferative and antiapoptotic effects of inflammation combined with the low-level expression of the E6 and E7 viral oncogenes from episomal HPV. At the CIN2 stage, the inflammation-induced generation of ROS and RNS induces DSBs in both the viral and host DNA. This allows HPV integration to occur. HPV integration then leads to deregulated expression of the viral oncogenes E6 and E7, (CIN3), and eventually to invasive carcinoma.
Figure 1. Inflammation-induced HPV integration.
CIN: Cervical intraepithelial neoplasia; HPV: Human papillomavirus; RNS: Reactive nitrogen species; ROS: Reactive oxygen species.
HPV carcinogenesis as a consequence of HPV integration
Research points to the fact that infection with HPV leads to expression of viral oncogenes, which modify numerous cellular pathways, cause chromosomal instability and facilitate cancer development. The HPV oncogenes E6 and E7, as expressed from episomal HPV genomes, together inactivate cellular genes involved in cell cycle regulation, the DNA damage response and apoptosis, and thus enable the virus to complete its lifecycle [134–137]. However, expression of these two oncogenes is tightly regulated by the virus, and occurs only at low levels. After integration of HR-HPV genomes into the host genome, however, expression of the viral oncogenes E6 and E7 increases significantly, usually due to loss of the E2 gene, which normally regulates viral gene expression in a negative fashion [138]. These increased levels of E6 and E7 now have the potential to affect cellular processes in ways that the lower levels of episomally expressed E6 and E7 did not. For example, E6 and E7 expressed together at high levels can cause polyploidy by deregulating cellular genes that normally control the G2M phase transition and progression through mitosis, such as the genes controlling centrosome homeostasis [139]. E6 itself exhibits a number of oncogenic activities, of which perhaps the best known is its ability to stimulate the degradation of several important proteins, including p53, by forming a ternary complex with the E6AP ubiquitin protein ligase and the target. However, E6 also binds to and inactivates or degrades other proteins in an E6AP-independent manner, and can act at the mRNA level to affect activities of proteins such as human telomerase reverse transcriptase [140–146]. Meanwhile, the E7 protein binds to, inactivates and accelerates the degradation of several members of the retinoblastoma protein family, thus removing some of the ‘brakes’ on replication that would otherwise be present [147–149]. The acute loss of the retinoblastoma protein family members by E7 induces centrosome amplification and aneuploidy, and there has been some question of whether the chromosomal instability induced by E7 facilitates integration, or whether integration is necessary before the increased levels of E7 can create chromosomal instability. A 2004 paper investigated this question and found that HPV16 E7-induced chromosomal instability was only observed after integration of HPV16 into the host genome. This observation, combined with the evidence presented earlier, strengthens the idea that HR-HPV integration is a significant event in cervical carcinogenesis that precedes the development of the chromosomal and genetic abnormalities that will ultimately drive malignant transformation [150].
Conclusion & future perspective
This article has presented several key points in support of the idea that inflammation can induce HPV integration, and subsequently, carcino genesis. Inflammation is a known cofactor in cervical carcinogenesis. It is likely to act by inducing ROS production, which causes DNA damage; this DNA damage in turn can facilitate HPV integration and high levels of oncoprotein expression. These oncoproteins then act on cellular pathways in ways that promote cellular transformation and tumor formation. Consistent with this sequence, chromosomal instability induced by HPV is only observed after HPV integration. Therefore, it is likely that inflammation provides the DNA damage needed for HPV integration, which then leads to chromosomal instability and cancer.
As summarized above, chronic inflammation, by causing oxidative DNA damage, may indeed serve as a mechanism for facilitating HPV integration, and subsequently, carcinogenesis. However, any other mechanism that induces chronic oxidative DNA damage in cells could also facilitate HPV integration. In the case of HBV infection, the virus itself is capable of increasing ROS, thereby leading to an increase in viral integration [151,152]. In fact, unpublished work from our own laboratory has demonstrated that the small isoform of HPV16 E6, E6*, can increase oxidative stress and DNA damage in both noncancerous immortalized cells and cervical cancer cell lines. This suggests that in at least some cases, HPV itself can cause the conditions needed for its integration, and this will be a topic of future research.
The possibility also exists that the ROS produced as a consequence of inflammation affects HPV integration in a less direct manner. ROS is known to act as a signaling molecule and has been demonstrated to increase viral DNA replication in liver cancer [153]. Therefore, it is possible that ROS increases the number of copies of the HPV genome present in cells, thereby increasing the mathematical probability that HPV would integrate into the host genome by some mechanism not explored in this review. Also, it is possible that inflammation works by increasing the number of CIN2 lesions by mechanisms not discussed in this review; this would also increase the mathematical probability of integration. In support of this idea, Matsumoto and coworkers found that smoking inhibits regression of low-grade CIN, implying that the inflammatory conditions caused by cofactors such as smoking could indeed increase the number of precancer lesions [154].
The potential ability of E6* to increase ROS may play a role in the viral lifecycle, as it has been shown that the differentiation of keratinocytes is favored under high oxygen concentrations (21%) [155]. Since HPV infects the basal cells where oxygen concentration is low, it is possible that the virus utilizes this function to drive differentiation, since the virus is spread in terminally differentiated cells. One side consequence of this function could be that over time and in some situations, this increase in oxygen would result in an increase in the levels of ROS and subsequent DNA damage, leading to HPV integration and cancer formation.
Two vaccines are currently on the market to prevent HPV-related cervical cancer, Cervarix®, developed by GlaxoSmithKline, and Gardasil®, developed by Merck & Co. However, these are both prophylactic and provide no benefit to individuals already infected with the virus or against strains not included in the vaccine. Therefore, there is a need to develop therapies that can benefit persons already infected with the virus who have not yet developed advanced neoplasia. Anti-inflammatory agents provide a possible mode of treatment. These agents have the potential to prevent the oxidative DNA damage induced by inflammation, thereby preventing HPV integration and progression to cancer. This approach has already been used in the treatment of other cancers, as therapies targeted against inflammatory mediators have shown promising results in the treatment of lung, colon and breast cancer [156–158]. For instance, TNF-α antagonists in patients with advanced cancer, such as renal and ovarian cancers, have resulted in disease stabilization [159–161], and COX-2 inhibitors can prevent recurrence of sporadic adenomatous polyps and adenomas in people genetically predisposed to developing them [162,163]. Such therapies could be particularly effective in preventing HPV-associated tumors, as they would be expected to work both by their general mechanisms of reducing DNA damage, and by the very specific mechanism of reducing HPV integration. This possibility is an exciting focus for future work.
Executive summary.
Human papillomaviruses
■ Human papillomaviruses (HPVs) are double-stranded viruses that infect epithelial cells.
■ The high-risk strains are associated with almost all cases of cervical cancer.
Immune response to HPV
■ The host immune response is limited mainly because the virus infects basal epithelial cells, which are shielded from circulating immune cells.
■ The HPV virus is capable of downregulating the host immune response.
■ Despite these challenges, most HPV infections are cleared due to a strong localized cell-mediated immune response by the host.
HPV-related cancer
■ HPVs are associated with development of a number of anogenital and oropharyngeal cancers.
HPV integration
■ HPV integration is usually considered a necessary event in the progression of cervical cancer. The frequency of integration for high-risk HPV genotypes in cervical cancer is consistently high.
■ Experimental data suggest that HPV integration takes place during the transition from low-grade to advanced lesions.
■ HPV integration results in the increased expression of the E6 and E7 viral oncogenes responsible for cell transformation.
■ Although little is known of how this process occurs, studies suggest that DNA damage and agents that induce DNA damage may play a role in HPV integration.
Cofactors for HPV oncogenesis
■ There are several factors associated with HPV oncogenesis, such as smoking, long-term use of oral contraceptives, high parity and co-infection with other sexually transmitted diseases.
■ Co-infection with certain sexually transmitted diseases is known to cause inflammation, which increases reactive oxygen species (ROS) levels and can lead to DNA damage.
Inflammation in cancer
■ Chronic inflammation is a known factor in the pathogenesis of several cancers.
■ Inflammation can increase ROS, thus leading to DNA damage.
Inflammation & ROS
■ Chronic inflammation induces the expression of proinflammatory cytokines, which in turn cause ROS overproduction.
■ Free radicals react with all components of the cell to cause damage.
Model for inflammation-induced HPV integration
■ HPV infections can cause cervical intraepithelial neoplasia 1 lesions. Inflammation or other cofactors may then facilitate the progression to cervical intraepithelial neoplasia 2 lesions.
■ ROS and reactive nitrogen species generated by inflammation induce double strand breaks in both the viral and host DNA, allowing integration to occur.
HPV carcinogenesis as a consequence of HPV integration
■ HPV integration leads to high-level expression of the viral oncogenes, E6 and E7, which cause cell transformation and eventually cancer.
Conclusion & future perspective
■ Inflammation induced by co-infection provides a plausible mechanism for HPV integration to occur.
■ Other agents capable of inducing DNA damage in both the viral and host genome can also play a role in HPV integration.
■ As a result, the use of anti-inflammatory agents as a therapy for individuals already infected with the virus is an area deserving of future attention.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Flores E, Lambert P. Evidence for a switch in the mode of human papillomavirus type 16 DNA replication during the viral life cycle. J. Virol. 1997;71(10):7167–7179. doi: 10.1128/jvi.71.10.7167-7179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calleja-Macias IE, Kalantari M, Allan B, et al. Papillomavirus subtypes are natural and old taxa: phylogeny of human papillomavirus types 44 and 55 and 68a and -b. J. Virol. 2005;79(10):6565–6569. doi: 10.1128/JVI.79.10.6565-6569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ault KA. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol. Oncol. 2007;107(2 Suppl. 1):S31–S33. doi: 10.1016/j.ygyno.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 4.Guccione E, Pim D, Banks L. HPV-18 E6*I modulates HPV-18 full-length E6 functions in a cell cycle dependent manner. Int. J. Cancer. 2004;110(6):928–933. doi: 10.1002/ijc.20184. [DOI] [PubMed] [Google Scholar]
- 5.Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J. Am. Osteopath. Assoc. 2006;106(Suppl. 1):S2–S8. [PubMed] [Google Scholar]
- 6.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 7 ■.Wei L, Gravitt PE, Song H, Maldonado AM, Ozbun MA. Nitric oxide induces early viral transcription coincident with increased DNA damage and mutation rates in human papillomavirus-infected cells. Cancer Res. 2009;69(11):4878–4884. doi: 10.1158/0008-5472.CAN-08-4695. [Nitric oxide induces DNA double-strand breaks in human papillomavirus (HPV)-infected cells along with a higher frequency of mutation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 9.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: Comparison by geographic region and with cervical cancer. Cancer Epidemiol. Biomarkers Pre. 2005;14(5):1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Tergaonkar V, Krishna S, Androphy EJ. Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor α correlates with increased accumulation of reactive oxygen species. J. Biol. Chem. 1999;274(35):24819–24827. doi: 10.1074/jbc.274.35.24819. [DOI] [PubMed] [Google Scholar]
- 11.Lowy D, Kirnbauer R, Schiller J. Genital human papillomavirus infection. PNAS. 1994;91(7):2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedell MA, Hudson JB, Golub TR, et al. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 1991;65(5):2254–2260. doi: 10.1128/jvi.65.5.2254-2260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley MA, Browne HM, Appleby M, Minson AC. Properties of a non-tumorigenic human cervical keratinocyte cell line. Int. J. Cancer. 1989;43(4):672–676. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- 14.Pett M, Coleman N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J. Pathol. 2007;212(4):356–367. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9(19):2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 16.Higgins GD, Uzelin DM, Phillips GE, Mcevoy P, Marin R, Burrell CJ. Transcription patterns of human papillomavirus type 16 in genital intraepithelial neoplasia: evidence for promoter usage within the E7 open reading frame during epithelial differentiation. J. Gen. Virol. 1992;73(8):2047–2057. doi: 10.1099/0022-1317-73-8-2047. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 18.Hasan UA, Bates E, Takeshita F, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007;178(5):3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 19.Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes. J. Virol. 2001;75(9):4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl. 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am. J. Clin. Pathol. 1994;102(6):768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 22.Boccardo E, Lepique AP, Villa LL. The role of inflammation in HPV carcinogenesis. Carcinogenesis. 2010;31(11):1905–1912. doi: 10.1093/carcin/bgq176. [DOI] [PubMed] [Google Scholar]
- 23.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the united states. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl. 3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 25.Klozar J, Tachezy R, Rotnáglová E, Košlabová E, Saláková M, Hamšíková E. Human papillomavirus in head and neck tumors: epidemiological, molecular and clinical aspects. WMW Wiener Medizinische Wochenschrift. 2010;160(11):305–309. doi: 10.1007/s10354-010-0782-5. [DOI] [PubMed] [Google Scholar]
- 26.Watson M, Saraiya M, Wu X. Update of HPV-associated female genital cancers in the united states, 1999–2004. J. Women's Health (Larchmt) 2009;18(11):1731–1738. doi: 10.1089/jwh.2009.1570. [DOI] [PubMed] [Google Scholar]
- 27.Frisch M, Fenger C, Van Den Brule AJC, et al. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999;59(3):753–757. [PubMed] [Google Scholar]
- 28.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2):270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 29.Hopman A, Theelen W, Hommelberg P, et al. Genomic integration of oncogenic HPV and gain of the human telomerase gene TERC at 3q26 are strongly associated events in the progression of uterine cervical dysplasia to invasive cancer. J. Pathol. 2006;210(4):412–419. doi: 10.1002/path.2070. [DOI] [PubMed] [Google Scholar]
- 30.Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J. Pathol. 2006;208(2):152–164. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 31.Pirami L, Giachè V, Becciolini A. Analysis of HPV16, 18, 31, and 35 DNA in pre-invasive and invasive lesions of the uterine cervix. J. Clin. Pathol. 1997;50:600–604. doi: 10.1136/jcp.50.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullen AP, Reid R, Campion M, Lorincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J. Virol. 1991;65(2):606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinokurova S, Wentzensen N, Kraus I, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68(1):307–313. doi: 10.1158/0008-5472.CAN-07-2754. [DOI] [PubMed] [Google Scholar]
- 34.Gray E, Pett MR, Ward D, et al. In vitro progression of human papillomavirus 16 episome-associated cervical neoplasia displays fundamental similarities to integrant-associated carcinogenesis. Cancer Res. 2010;70(10):4081–4091. doi: 10.1158/0008-5472.CAN-09-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J. Gen. Virol. 1997;78(5):1095–1101. doi: 10.1099/0022-1317-78-5-1095. [DOI] [PubMed] [Google Scholar]
- 36.Vinokurova S, Wentzensen N, Kraus I, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68(1):307–313. doi: 10.1158/0008-5472.CAN-07-2754. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz E, Freese UK, Gissmann L, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 38.Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J. Clin. Microbiol. 2006;44(5):1755–1762. doi: 10.1128/JCM.44.5.1755-1762.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briolat J, Dalstein V, Saunier M, et al. HPV prevalence, viral load and physical state of HPV-16 in cervical smears of patients with different grades of cin. Int. J. Cancer. 2007;121(10):2198–2204. doi: 10.1002/ijc.22959. [DOI] [PubMed] [Google Scholar]
- 40.Hudelist G, Manavi M, Pischinger KID, et al. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol. Oncol. 2004;92(3):873–880. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Peter M, Stransky N, Couturier J, et al. Frequent genomic structural alterations at HPV insertion sites in cervical carcinoma. J. Pathol. 2010;221(3):320–330. doi: 10.1002/path.2713. [DOI] [PubMed] [Google Scholar]
- 42.Wentzensen N, Vinokurova S, Doeberitz MVK. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004;64(11):3878–3884. doi: 10.1158/0008-5472.CAN-04-0009. [DOI] [PubMed] [Google Scholar]
- 43 ■.Hiraku Y. Formation of 8-nitroguanine, a nitrative DNA lesion, in inflammation-related carcinogenesis and its significance. Environ. Health Prev. Med. 2010;15(2):63–72. doi: 10.1007/s12199-009-0118-5. [8-nitroguanine is formed as a consequence of chronic inflammation. Strong 8-nitroguanine formation in tumor tissues is closely associated with a poor prognosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J. Natl Cancer Inst. 2000;92(9):690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 45.Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc. Natl Acad. Sci. USA. 1995;92(5):1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badaracco G, Venuti A, Sedati A, Marcante ML. HPV16 and HPV18 in genital tumors: significantly different levels of viral integration and correlation to tumor invasiveness. J. Med. Virol. 2002;67(4):574–582. doi: 10.1002/jmv.10141. [DOI] [PubMed] [Google Scholar]
- 48.Romanczuk H, Howley PM. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl Acad. Sci. USA. 1992;89(7):3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popescu NC, Dipaolo JA. Integration of human papillomavirus 16 DNA and genomic rearrangements in immortalized human keratinocyte lines. Cancer Res. 1990;50(4):1316–1323. [PubMed] [Google Scholar]
- 50.Ferber MJ, Montoya DP, Yu C, et al. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003;22(24):3813–3820. doi: 10.1038/sj.onc.1206528. [DOI] [PubMed] [Google Scholar]
- 51 ■■.Winder DM, Pett MR, Foster N, et al. An increase in DNA double-strand breaks, induced by KU70 depletion, is associated with human papillomavirus 16 episome loss and de novo viral integration events. J. Pathol. 2007;213(1):27–34. doi: 10.1002/path.2206. [Increased DNA double-strand breaks are associated with HPV16 episomal loss and integration in cervical keratinocytes. High-level chromosomal instability was not observed until cells containing the new integrant were almost fully selected.] [DOI] [PubMed] [Google Scholar]
- 52.Someya M, Sakata K-I, Matsumoto Y, et al. The association of DNA-dependent protein kinase activity with chromosomal instability and risk of cancer. Carcinogenesis. 2006;27(1):117–122. doi: 10.1093/carcin/bgi175. [DOI] [PubMed] [Google Scholar]
- 53.Winder D, Pett M, Foster N, et al. An increase in DNA double-strand breaks, induced by KU70 depletion, is associated with human papillomavirus 16 episome loss and de novo viral integration events. J. Pathol. 2007;213(1):27–34. doi: 10.1002/path.2206. [DOI] [PubMed] [Google Scholar]
- 54.Dandri M, Burda MR, Bürkle A, et al. Increase in de novo HBV DNA integrations in response to oxidative DNA damage or inhibition of poly(ADP-ribosyl)ation. Hepatology. 2002;35(1):217–223. doi: 10.1053/jhep.2002.30203. [DOI] [PubMed] [Google Scholar]
- 55.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387(4):365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 56.Almonte M, Albero G, Molano M, Carcamo C, García PJ, Pérez G. Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine. 2008;26(Suppl. 11):L16–L36. doi: 10.1016/j.vaccine.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Munoz N, Franceschi S, Bosetti C, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case–control study. Lancet. 2002;359(9312):1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 58.Plummer M, Herrero R, Franceschi S, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case–control study. Cancer Causes Control. 2003;14(9):805–814. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 59.Deacon JM, Evans CD, Yule R, et al. Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case–control study nested within the manchester cohort. Br. J. Cancer. 2000;83(11):1565–1572. doi: 10.1054/bjoc.2000.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tollefson AK, Oberley-Deegan RE, Butterfield KT, et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic. Biol. Med. 2010 doi: 10.1016/j.freeradbiomed.2010.09.022. DOI: 10.1016/j.freeradbiomed.2010.09.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellsague X, Munoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis – role of parity, oral contraceptives, and tobacco smoking. J. Natl Cancer Inst. Monogr. 2003;(31):20–28. [PubMed] [Google Scholar]
- 62.Vaccarella S, Herrero R, Dai M, et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol. Biomarkers Prev. 2006;15(11):2148–2153. doi: 10.1158/1055-9965.EPI-06-0556. [DOI] [PubMed] [Google Scholar]
- 63.Moreno V, Bosch FX, Muñoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case–control study. Lancet. 2002;359(9312):1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 64.Appleby P, Beral V, Berrington de González A, International Collaboration of Epidemiological Studies of Cervical Cancer Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 65.Smith JS, Green J, De Gonzalez AB, et al. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361(9364):1159–1167. doi: 10.1016/s0140-6736(03)12949-2. [DOI] [PubMed] [Google Scholar]
- 66.Castle PE, Giuliano AR. Chapter 4: genital tract infections, cervical inflammation, and antioxidant nutrients – assessing their roles as human papillomavirus cofactors. J. Natl Cancer Inst. Monogr. 2003;2003(31):29–34. doi: 10.1093/oxfordjournals.jncimonographs.a003478. [DOI] [PubMed] [Google Scholar]
- 67 ■■.Castle PE, Hillier SL, Rabe LK, et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol. Biomarkers Prev. 2001;10(10):1021–1027. [Inflammation may be associated with the progression to high-grade lesions in women infected with HPV.] [PubMed] [Google Scholar]
- 68.Kulkarni S, Rader JS, Zhang F, et al. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin. Cancer Res. 2001;7(2):429–434. [PubMed] [Google Scholar]
- 69.Kim GE, Kim YB, Cho NH, et al. Synchronous coexpression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix. Clin. Cancer Res. 2004;10(4):1366–1374. doi: 10.1158/1078-0432.ccr-0497-03. [DOI] [PubMed] [Google Scholar]
- 70.Saldivar JS, Lopez D, Feldman RA, et al. COX-2 overexpression as a biomarker of early cervical carcinogenesis: a pilot study. Gynecol. Oncol. 2007;107(1 Suppl. 1):S155–S162. doi: 10.1016/j.ygyno.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 71.Hammes LS, Tekmal RR, Naud P, et al. Up-regulation of VEGF, c-FMS and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol. Oncol. 2008;110(3):445–451. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 72.Pikarsky E, Porat RM, Stein I, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 73.Ravi R, Bedi A. NF-κB in cancer – a friend turned foe. Drug Resist. Updat. 2004;7(1):53–67. doi: 10.1016/j.drup.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen SJ, Eckmann L, Quayle AJ, et al. Secretion of proinflammatory cytokines by epithelial cells in response to chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 1997;99(1):77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finan RR, Musharrafieh U, Almawi WY. Detection of chlamydia trachomatis and herpes simplex virus type 1 or 2 in cervical samples in human papilloma virus (HPV)-positive and HPV-negative women. Clin. Microbiol. Infect. 2006;12(9):927–930. doi: 10.1111/j.1469-0691.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- 76.Hawes SE, Kiviat NB. Are genital infections and inflammation cofactors in the pathogenesis of invasive cervical cancer? J. Natl Cancer Inst. 2002;94(21):1592–1593. doi: 10.1093/jnci/94.21.1592. [DOI] [PubMed] [Google Scholar]
- 77.Szostek S, Zawilinska B, Kopec J, Kosz-Vnenchak M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 2009;56(2):337–342. [PubMed] [Google Scholar]
- 78.Paba P, Bonifacio D, Di Bonito L, et al. Co-expression of HSV2 and chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia lesions is associated with aberrations in key intracellular pathways. Intervirology. 2008;51(4):230–234. doi: 10.1159/000156481. [DOI] [PubMed] [Google Scholar]
- 79.Schwebke JR, Zajackowski ME. Effect of concurrent lower genital tract infections on cervical cancer screening. Genitourin. Med. 1997;73(5):383–386. doi: 10.1136/sti.73.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nasca MR, Innocenzi D, Micali G. Association of penile lichen sclerosus and oncogenic human papillomavirus infection. Int. J. Dermatol. 2006;45(6):681–683. doi: 10.1111/j.1365-4632.2005.02608.x. [DOI] [PubMed] [Google Scholar]
- 81.Barbagli G, Palminteri E, Mirri F, Guazzoni G, Turini D, Lazzeri M. Penile carcinoma in patients with genital lichen sclerosus: a multicenter survey. J. Urol. 2006;175(4):1359–1363. doi: 10.1016/S0022-5347(05)00735-4. [DOI] [PubMed] [Google Scholar]
- 82.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbeck's Arch. Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 83.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 84.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 85.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum. Pathol. 1998;29(9):932–948. doi: 10.1016/s0046-8177(98)90198-8. [DOI] [PubMed] [Google Scholar]
- 87.Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26(S3):34S–38S. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 88.Brechot C, Kremsdorf D, Soussan P, et al. Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC): molecular mechanisms and novel paradigms. Pathol. Biol. 2010;58(4):278–287. doi: 10.1016/j.patbio.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Khan G. Epstein–Barr virus, cytokines, and inflammation: a cocktail for the pathogenesis of Hodgkin's lymphoma? Exp. Hematol. 2006;34(4):399–406. doi: 10.1016/j.exphem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Moore MM, Chua W, Charles KA, Clarke SJ. Inflammation and cancer: causes and consequences. Clin. Pharmacol. Ther. 2010;87(4):504–508. doi: 10.1038/clpt.2009.254. [DOI] [PubMed] [Google Scholar]
- 91 ■.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [Discusses the link between inflammation and cancer. Also deals with the use of nonsteriodal anti-inflammatory drugs in the treatment of malignant disease.] [DOI] [PubMed] [Google Scholar]
- 92.Peebles KA, Lee JM, Mao JT, et al. Inflammation and lung carcinogenesis: applying findings in prevention and treatment. Expert Rev. Anticancer Ther. 2007;7(10):1405–1421. doi: 10.1586/14737140.7.10.1405. [DOI] [PubMed] [Google Scholar]
- 93.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005;37(12):2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 2003;111(6):769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krüncke KD. Nitrosative stress and transcription. Biol. Chem. 2005;384(10–11):1365–1377. doi: 10.1515/BC.2003.153. [DOI] [PubMed] [Google Scholar]
- 96.Chang M-L, Chen J-C, Chang M-Y, et al. Acute expression of hepatitis C core protein in adult mouse liver: mitochondrial stress and apoptosis. Scand. J. Gastroenterol. 2008;43(6):747–755. doi: 10.1080/00365520701875987. [DOI] [PubMed] [Google Scholar]
- 97.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 98.Tamir S, Tannenbaum SR. The role of nitric oxide (NO·) in the carcinogenic process. Biochim. Biophys. Acta. 1996;1288(2):F31–F36. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 99.Baron JA, Sandler RS. Nonsteroidal anti-inflammatory drugs and cancer prevention. Ann. Rev. Med. 2003;51(1):511–523. doi: 10.1146/annurev.med.51.1.511. [DOI] [PubMed] [Google Scholar]
- 100.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta. 2000;1470(2):M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 101.Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ. Mol. Mutagen. 2010;51(5):380–390. doi: 10.1002/em.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Disilvestro RA. Influence of copper intake and inflammation on rat serum superoxide dismutase activity levels. J. Nutr. 1988;118(4):474–479. doi: 10.1093/jn/118.4.474. [DOI] [PubMed] [Google Scholar]
- 103.Srivastava S, Natu SM, Gupta A, et al. Lipid peroxidation and antioxidants in different stages of cervical cancer: prognostic significance. Indian J. Cancer. 2009;46:297–302. doi: 10.4103/0019-509X.55549. [DOI] [PubMed] [Google Scholar]
- 104.Manju V, Kalaivani Sailaja J, Nalini N. Circulating lipid peroxidation and antioxidant status in cervical cancer patients: a case–control study. Clin. Biochem. 2002;35(8):621–625. doi: 10.1016/s0009-9120(02)00376-4. [DOI] [PubMed] [Google Scholar]
- 105.Looi ML, Mohd Dali AZ, Md Ali SA, Wan Ngah WZ, Mohd Yusof YA. Oxidative damage and antioxidant status in patients with cervical intraepithelial neoplasia and carcinoma of the cervix. Eur. J. Cancer Prev. 2008;17(6):555–560. doi: 10.1097/CEJ.0b013e328305a10b. [DOI] [PubMed] [Google Scholar]
- 106.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 107.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 108.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371(1):86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 110.Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16(9):2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 111.Akaike T, Okamoto S, Sawa T, et al. 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc. Natl Acad. Sci. USA. 2003;100(2):685–690. doi: 10.1073/pnas.0235623100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 2001;488(1):65–76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 113.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Sgambato A, Zannoni GF, Faraglia B, et al. Decreased expression of the CDK inhibitor p27KIP1 and increased oxidative DNA damage in the multistep process of cervical carcinogenesis. Gynecol. Oncol. 2004;92(3):776–783. doi: 10.1016/j.ygyno.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Sawa T, Akaike T, Ichimori K, et al. Superoxide generation mediated by 8-nitroguanosine, a highly redox-active nucleic acid derivative. Biochem. Biophys. Res. Commun. 2003;311(2):300–306. doi: 10.1016/j.bbrc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki N, Yasui M, Geacintov NE, Shafirovich V, Shibutani S. Miscoding events during DNA synthesis past the nitration-damaged base 8-nitroguanineâ. Biochemistry. 2005;44(25):9238–9245. doi: 10.1021/bi050276p. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi T, Nau M, Chiba I, et al. P53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 118.Tamura G, Nishizuka S, Maesawa C, et al. Mutations in mitochondrial control region DNA in gastric tumours of Japanese patients. Eur. J. Cancer. 1999;35(2):316–319. doi: 10.1016/s0959-8049(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 119.Horton TM, Petros JA, Heddi A, et al. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes Chromosomes Cancer. 1996;15(2):95–101. doi: 10.1002/(SICI)1098-2264(199602)15:2<95::AID-GCC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 120.Beckman KB, Ames BN. Oxidative decay of DNA. J. Biol. Chem. 1997;272(32):19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 121.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 122.Subrahmanyam VV, McGirr LG, O'Brien PJ. Glutathione oxidation during peroxidase catalysed drug metabolism. Chem. Biol Interact. 1987;61(1):45–59. doi: 10.1016/0009-2797(87)90018-4. [DOI] [PubMed] [Google Scholar]
- 123.Uchida K. 4-hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42(4):318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 124.Blair IA. Lipid hydroperoxide-mediated DNA damage. Exp. Gerontol. 2001;36(9):1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 125.Nair J, Barbin A, Velic I, Bartsch H. Etheno DNA-base adducts from endogenous reactive species. Mutat. Res. 1999;424(1–2):59–69. doi: 10.1016/s0027-5107(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 126.Ruef J, Rao GN, Li F, et al. Induction of rat aortic smooth muscle cell growth by the lipid peroxidation product 4-hydroxy-2-nonenal. Circulation. 1998;97(11):1071–1078. doi: 10.1161/01.cir.97.11.1071. [DOI] [PubMed] [Google Scholar]
- 127.Cheng J-Z, Singhal SS, Saini M, et al. Effects of MGST a4 transfection on 4-hydroxynonenal-mediated apoptosis and differentiation of K562 human erythroleukemia cells. Arch. Biochem. Biophys. 1999;372(1):29–36. doi: 10.1006/abbi.1999.1479. [DOI] [PubMed] [Google Scholar]
- 128.Hu W, Feng Z, Eveleigh J, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23(11):1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 129.Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24(8):792–799. doi: 10.1002/hed.10108. [DOI] [PubMed] [Google Scholar]
- 130.Kumagai T, Kawamoto Y, Nakamura Y, et al. 4-hydroxy-2-nonenal, the end product of lipid peroxidation, is a specific inducer of cyclooxygenase-2 gene expression. Biochem. Biophys. Res. Commun. 2000;273(2):437–441. doi: 10.1006/bbrc.2000.2967. [DOI] [PubMed] [Google Scholar]
- 131.Song SH, Lee JK, Oh MJ, Hur JY, Park YK, Saw HS. Risk factors for the progression or persistence of untreated mild dysplasia of the uterine cervix. Int. J. Gynecol. Cancer. 2006;16(4):1608–1613. doi: 10.1111/j.1525-1438.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- 132.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 1993;12(2):186–192. [PubMed] [Google Scholar]
- 133.Tamim H, Finan RR, Sharida HE, Rashid M, Almawi WY. Cervicovaginal coinfections with human papillomavirus and chlamydia trachomatis. Diagn. Microbiol. Infect. Dis. 2002;43(4):277–281. doi: 10.1016/s0732-8893(02)00403-0. [DOI] [PubMed] [Google Scholar]
- 134.Münger K, Basile JR, Duensing S, et al. Biological activities and molecular targets of the human papillomavirus e7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 135.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 136.Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62(23):7075–7082. [PubMed] [Google Scholar]
- 137.Zur Hausen H. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 1999;9(6):405–411. doi: 10.1006/scbi.1999.0144. [DOI] [PubMed] [Google Scholar]
- 138.von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur. J. Cancer. 2002;38(17):2229–2242. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 139.Patel D, Incassati A, Wang N, Mccance DJ. Human papillomavirus type 16 E6 and E7 cause polyploidy in human keratinocytes and up-regulation of G2–M-phase proteins. Cancer Res. 2004;64(4):1299–1306. doi: 10.1158/0008-5472.can-03-2917. [DOI] [PubMed] [Google Scholar]
- 140.Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl Acad. Sci. USA. 1991;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 142.Werness B, Levine A, Howley P. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 143.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus type-16 or type-18. Embo J. 1991;10(13):4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the CDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell Biol. 1993;13(2):775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tungteakkhun SS, Duerksen-Hughes PJ. Cellular binding partners of the human papillomavirus E6 protein. Arch. Virol. 2008;153(3):397–408. doi: 10.1007/s00705-007-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20(54):7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 147.Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. Embo J. 1989;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 1992;66(12):6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mclaughlin-Drubin ME, Münger K. The human papillomavirus E7 oncoprotein. Virology. 2009;384(2):335–344. doi: 10.1016/j.virol.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150 ■.Pett MR, Alazawi WOF, Roberts I, et al. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64(4):1359–1368. doi: 10.1158/0008-5472.can-03-3214. [Development of high-level chromosomal instability in W12 cells occurred only after integration of HPV16 DNA in these cells.] [DOI] [PubMed] [Google Scholar]
- 151.Tsai WL, Chung RT. Viral hepatocarcinogenesis. Oncogene. 2010;29(16):2309–2324. doi: 10.1038/onc.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dandri M, Burda MR, Bürkle A, et al. Increase in de novo HBV DNA integrations in response to oxidative DNA damage or inhibition of poly(ADP-ribosyl)ation. Hepatology. 2002;35(1):217–223. doi: 10.1053/jhep.2002.30203. [DOI] [PubMed] [Google Scholar]
- 153.Koike K. Hepatitis B virus X gene is implicated in liver carcinogenesis. Cancer Lett. 2009;286(1):60–68. doi: 10.1016/j.canlet.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 154.Matsumoto K, Oki A, Furuta R, et al. Tobacco smoking and regression of low-grade cervical abnormalities. Cancer Sci. 2010;101(9):2065–2073. doi: 10.1111/j.1349-7006.2010.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ngo MA, Sinitsyna NN, Qin Q, Rice RH. Oxygen-dependent differentiation of human keratinocytes. J. Invest. Dermatol. 2006;127(2):354–361. doi: 10.1038/sj.jid.5700522. [DOI] [PubMed] [Google Scholar]
- 156.Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin. Oncol. 2004;31(Suppl. 7):45–52. doi: 10.1053/j.seminoncol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 157.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin. Oncol. 2004;31(Suppl. 7):22–29. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 158.Reddy BS, Hirose Y, Lubet R, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60(2):293–297. [PubMed] [Google Scholar]
- 159.Madhusudan S, Muthuramalingam SR, Braybrooke JP, et al. Study of etanercept, a tumor necrosis factor-α inhibitor, in recurrent ovarian cancer. J. Clin. Oncol. 2005;23(25):5950–5959. doi: 10.1200/JCO.2005.04.127. [DOI] [PubMed] [Google Scholar]
- 160.Harrison ML, Obermueller E, Maisey NR, et al. Tumor necrosis factor {α} as a new target for renal cell carcinoma: Two sequential Phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 2007;25(29):4542–4549. doi: 10.1200/JCO.2007.11.2136. [DOI] [PubMed] [Google Scholar]
- 161.Brown ER, Charles KA, Hoare SA, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-α inhibitor, in patients with advanced cancer. Ann. Oncol. 2008;19(7):1340–1346. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- 162.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 163.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]