Abstract
We studied the effects of combination treatment with an anti-inflammatory agent, interferon(IFN)-β, and a putative neuroprotective agent, an estrogen receptor(ER)-β ligand, during EAE. Combination treatment significantly attenuated EAE disease severity, preserved axonal densities in spinal cord, and reduced CNS inflammation. Combining ERβ treatment with IFNβ reduced IL-17, while it abrogated IFNβ-mediated increases in Th1 and Th2 cytokines from splenocytes. Additionally, combination treatment reduced VLA-4 expression on CD4+ T cells, while it abrogated IFNβ-mediated decreases in MMP-9. Our data demonstrate that combination treatments can result in complex effects that could not have been predicted based on monotherapy data alone.
Keywords: multiple sclerosis, experimental autoimmune encephalomyelitis, estrogen, interferon-beta, cytokine
1. Introduction
MS is a putative autoimmune demyelinating disease with a neurodegenerative component. Currently all of the approved MS treatments are targeted at the inflammation, and while this may confer some indirect neuroprotection, there are no directly neuroprotective drugs available. Often patients have progressive disability accumulation despite robust immunosuppression, thereby suggesting the need for treatment approaches which involve using an anti-inflammatory agent in combination with a neuroprotective agent.
Type I interferons (IFN-α and -β) have been widely established as effective anti-inflammatory agents in modifying the course of several inflammatory diseases, including collagen-induced arthritis (CIA), viral inflammation, and EAE (Axtell and Steinman, 2008; Billiau, 2006). IFNβ is one of several widely approved treatment choices for relapse remitting MS patients. Despite its widespread use, some patients are not responsive to therapy (Arnason, 1999). Further, IFNβ treatment often has only modest effects in slowing permanent disability accumulation even in those who initially are considered responsive based on a reduction in relapses. While relatively higher doses of IFNβ therapy may be more effective, such higher doses may be less well tolerated (Clanet et al., 2004; Clanet et al., 2002).
IFNβ receptors are endogenously expressed in a variety of tissue and cell types; therefore, it has the potential to exert influence on many aspects of MS pathogenesis. For example, IFNβ therapy in MS patients has been shown to reduce pro-inflammatory Th1 and Th17 cytokines, increase T regulatory cells, downregulate VLA-4 expression on effector T cells, and decrease MMP-9 to limit immune cell trafficking to the CNS (Comabella et al., 2009; Martin-Saavedra et al., 2007; Martin-Saavedra et al., 2008b; Muraro et al., 2000; Shinohara et al., 2008). Though the IFNβ receptor is expressed in neurons and glia, there is currently no evidence for a direct effect of IFNβ on cells outside of the immune system (Prinz et al., 2008). Thus, it is possible that IFNβ treatment could be combined with a directly neuroprotective treatment to increase efficacy.
Estrogens are good candidates to combine with IFNβ therapy since there is abundant evidence using in vitro systems and non-inflammatory neurodegenerative in vivo models that estrogen treatment can be directly neuroprotective (Honda et al., 2000; Jansson et al., 1994; Morale et al., 2003; Wu et al., 2005). Estrogens act primarily through nuclear ER subtypes, ERα and ERβ (Garidou et al., 2004; Liu et al., 2003; Morales et al., 2006; Polanczyk et al., 2003; Tiwari-Woodruff et al., 2007), while more rapid membrane effects have also been described (Wang et al., 2009b). In EAE and other inflammatory diseases, the role of ERα ligand treatment has been shown to be anti-inflammatory by decreasing Th1 cytokines, altering chemokines, and increasing T regulatory cells (Douin-Echinard et al., 2008; Elloso et al., 2005; Jansson and Holmdahl, 2001; Liu et al., 2003; Offner, 2004; Polanczyk et al., 2003). Together this results in less CNS inflammation. While ERα ligand treatment also preserves myelin and prevents axonal loss, it is unknown whether this is due to merely blocking CNS inflammation as opposed to providing direct neuroprotection to neurons, oligodendrocytes, and astrocytes since these CNS cell types all express ERα and ERβ (Maret et al., 2003; Mitra, 2003). In contrast, recent evidence suggests that ERβ ligand treatment is directly neuroprotective in EAE since it preserves myelin and prevents axonal loss without altering peripheral cytokine production or reducing CNS inflammation (Tiwari-Woodruff et al., 2007). Clinically, ERα ligand treatment is effective early during EAE (Liu et al., 2003), while ERβ ligand treatment has significant effects only in the chronic stage of disease (Tiwari-Woodruff et al., 2007). Regarding combination treatment in MS, ERβ ligand treatment provides intriguing possibilities since side effects of high dose estrogen treatment, including breast and uterine cancer, are mediated by ERα, not ERβ (Ali and Coombes, 2000).
Here, we examined combination treatment of chronic EAE using IFNβ, a primarily anti-inflammatory agent, with an ERβ ligand as the neuroprotective agent. While additive effects on clinical and neuropathologic outcomes were found, both additive and antagonistic immune interactions were observed, thereby underscoring the complexity of such combination treatments.
2. Material and Methods
Animals
Female B6.Cg-Tg (Thy1-YFP) 16Jrs/J (Thy1-YFP) mice 8-10 weeks old were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were maintained under environmentally controlled conditions in a 12 hour light/dark cycle with access to food and water ad libitum. All procedures involving animals were carried out in accordance to the NIH guidelines for the care and use of laboratory animals and approved by the UCLA Chancellor's Animal Research Committee and Division of Laboratory Animals Medicine.
Reagents
The ERβ ligand Diarylproprionitrile (DPN) was purchased from Tocris Biosciences (Ellisville, MO) and dissolved with molecular grade ethanol purchased from EM Sciences (Hatfield, PA). Miglylol 812N liquid oil was Sasol North America (Houston, TX). Recombinant mouse Interferon-beta (IFNβ) was purchased from PBL InterferonSource (Piscataway, NJ). All reagents were prepared and stored according to manufacturer's instructions.
EAE induction and treatments
Animals were injected subcutaneously with Myelin Oligodendrocyte Glycoprotein (MOG), amino acids 35-55 (200 μg/animal, American Peptides), emulsified in complete Freund's adjuvant (CFA) and supplemented with Mycobacterium TuberculosisH37ra (50 μg/animal, Difco Laboratories), over four draining inguinal and axillary lymph node sites in a volume of 0.1 ml/mouse. Seven days prior to immunization, animals received treatment that continued to the endpoint of the experiment with DPN (8 mg/kg/day, s.c. injections) dissolved in 10% molecular-grade ethanol and diluted with 90% Miglylol 812N liquid oil, rmIFNβ(20 KU, i.p. injections) diluted with injection grade PBS and 0.1% FBS carrier protein, vehicle consisting of 1:9 molecular grade ethanol/Miglylol 812N, or a combination of DPN and IFNβ. Animals were monitored daily for EAE signs based on a standard EAE 0-5 scale scoring system: 0-healthy, 1-complete loss of tail tonicity, 2-loss of righting reflex, 3-partial paralysis, 4-complete paralysis of one or both hind limbs, and 5-moribund.
Histological preparation
Mice were deeply anesthetized in isoflurane and perfused transcardially with ice-cold 1X PBS for 20-30 minutes, followed by 10% formalin. Spinal cords were dissected and submerged in 10% formalin overnight at 4°C, followed by 30% sucrose in PBS for 24 hours. Spinal cords were cut in thirds and embedded in 75% gelatin/15% sucrose solution. 40 μm thick free-floating spinal cord cross-sections were obtained with a microtome cryostat (model HM505E) at −20°C. Tissues were collected serially and stored in 1X PBS with 1% sodium azide in 4°C until immunohistochemistry.
Immunohistochemistry
40 μm thick free-floating sections were thoroughly washed with 1X PBS to dilute residual sodium azide. In the case of anti-MBP labeling, tissue sections undergo an additional 2 hour incubation with 5% glacial acetic acid in 100-proof ethanol at room temperature (RT), followed by 30 minutes incubation in 3% hydrogen peroxide in PBS. All tissue sections were permeabilized with 0.3% Triton X-100 in 1X PBS and 2% normal goat serum (NGS) for 30 minutes RT, and blocked with 10% NGS in 1X PBS, except in the case of MBP labeling, which was blocked with 10% normal sheep serum (NSS), for 2 hours or overnight at 4°C. The following primary antibodies (Abs) were used: anti-sheep MBP (1:1000), anti-CD45 (1:500), anti-CD3 (1:500), anti-Mac3 (1:500) (Chemicon), and anti-neurofilament-NF200 (1:750, Sigma). Tissues labeled with anti-sheep MBP continue with second Ab labeling step consisting of 1 hour incubation with biotinylated anti-sheep IgG Ab (1:1000, Vector Labs), followed by 1½ hour incubation with strepavidin Ab conjugated to Alexa 647 fluorochrome (Chemicon). All other tissues followed with second Abs conjugated to TRITC (1:1000) or Cy5 (1:750) (Vector labs and Chemicon) for 1½ hours. To assess the number of cells, a nuclear stain DAPI (2 ng/ml, Molecular Probes) was added 10 minutes prior to final washes after secondary Ab incubation. Sections were mounted on slides, allowed to semi-dry, and cover slipped in fluoromount G (Fisher Scientific). IgG-control experiments were performed for all primary Abs, and only non-immunoreactive tissues under these conditions were qualified for analysis.
Microscopy
Stained sections were examined and photographed using a confocal microscope (Leica TCS-SP, Mannheim, Germany) or a fluorescence microscope (BX51WI; Olympus, Tokyo, Japan) equipped with Plan Fluor objectives connected to a camera (DP70, Olympus). Digital images were collected and analyzed using Leica confocal and DP70 camera software. Images were assembled using Adobe Photoshop (Adobe Systems, San Jose, CA).
Quantification
To quantify immunohistochemical staining results, three consecutive spinal cord cross-sections at the T1-T5 level from four mice per group for a total of twelve sections per group were captured under microscope at 10X magnification for YFP/CD45 labeled sections, or 40X magnification for YFP/MBP labeled sections using the DP70 Image software and a DP70 camera (both from Olympus). All images in each experimental set were captured under the same light intensity and exposure limits. Analysis was performed on images using ImageJ Software v1.30, downloaded from the NIH website: http://rsb.info.nih.gov/ij. Inflammatory infiltrates were quantified by measuring the intensity of CD45 staining in the lateral funiculus and posterior column in captured 10X images. Axons were identified by YFP expression in the lateral funiculus and posterior column in captured 40X images and quantified with the measure function in the ImageJ software. All quantifications were performed by a blinded observer.
Splenocyte culture
Splenocytes were cultured in 24-well plates at the concentration of 4×106 cells/ml of complete RPMI medium containing 5% heat-inactivated fetal calf serum (FCS), 1 mM sodium pyruvate, L-glutamine, 2ME, NEAA, Pen-strep, and 25 mM Hepes Buffer. Cells were stimulated with 25 μg/ml MOG, amino acids 35-55, and 20 ng/ml IL-12 (BD Biosciences) for 72 hours at 37°C, 5% CO2. After 72 hours of culture, supernatants were collected and centrifuged to eliminate cellular debris prior to flash freezing in isopropanol and dry ice and stored in −80°C until ready for analysis. Cytokine analyses were performed by Searchlight Array (Thermo Fisher Scientific).
Flow cytometry
Splenocytes were collected on a 96 v-shaped plate (Titertek Co.) for flow cytometric analysis. Single cell suspensions in FACs buffer (2% FCS in PBS) were incubated with anti-CD16/32 at 1:100 dilution for 20 minutes at 4°C to block Fc receptors, centrifuged, and resuspended in FACs buffer with the following Abs added at 1:100 dilution for 30 minutes at 4°C: anti-CD11b, anti-CD11c, anti-CD19, anti-CD4, anti-CD49d (VLA-4), Rat-IgG1, -IgG2a, and -IgG2b isotype controls (Biolegend). Cells were subsequently washed twice in FACs buffer, acquired on FACSCalibur (BD Biosciences) and analyzed using Flowjo Software (Treestar). Ten thousand live events were collected for each sample and gated on either CD4 or CD8 for T cells, CD11b for macrophages, or CD19 for B cells based on isotype controls. Then, VLA-4 expression on these gated cell populations was represented by histograms. Finally, the mean fluorescence intensity (MFI) was determined for portions of the histograms that did not overlap those of respective isotype controls.
Statistical analysis
EAE severity significance was determined by one-way repeated measure Analysis of Variance (ANOVA). Statistical analysis of the data is represented as Mean ± Standard Error of pooled EAE scores. In the case where the scatter plot of immunohistochemical or flow cytometry data satisfied assumptions of normal distribution and equal variances among all groups, the data were analyzed by bootstrap one-way ANOVA and student's t-test, respectively. For these analyses, the mean or median was used as the comparator, and F-stat equation was modified such that absolute values replaced the squaring of values. For bootstrap one-way ANOVA, post-hoc analysis was performed on F-stat values at 95% confidence interval.
3. Results
Combination treatment with IFNβand ERβ ligand significantly reduced EAE disease severity
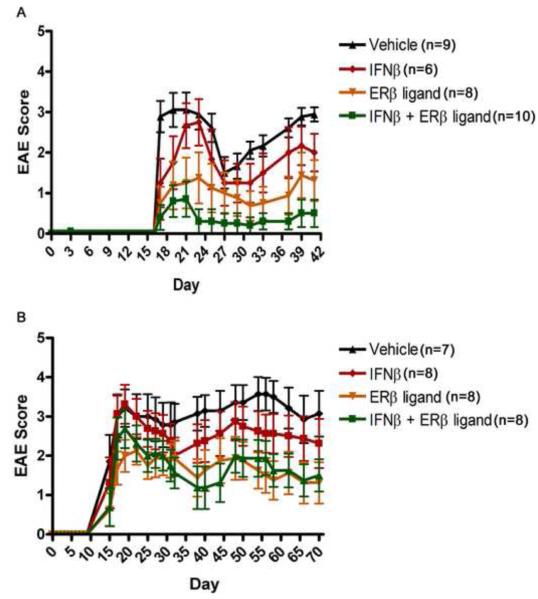
To pursue possible additive effects between two therapeutic agents in EAE, we first examined various doses of IFNβ treatment in EAE. It had previously been shown that 10 KU of IFNβ was effective in reducing mean clinical disease scores in EAE in the SJL/J strain (Jaini et al., 2006), therefore we included this dose as well as three other doses: 5 KU, 15 KU, and 20 KU. The two lower doses (5 KU and 10 KU) failed to reduce EAE scores in C57BL/6 mice, but the two higher doses (15 KU and 20 KU) worked comparably in reducing mean clinical scores as compared to vehicle treated. Notably, the highest dose of 20 KU resulted in only mild reductions in EAE scores, consistent with observations by others (Schaefer et al., 2006). Thus, 20 KU was chosen for subsequent experiments using combination treatment. The dose of the ERβ ligand which could reduce EAE scores was previously established in our lab (Tiwari-Woodruff et al., 2007). We then determined whether combination treatment using an ERβ ligand with IFNβ might be additive in reducing EAE clinical scores. As shown in Figure 1A, there was a trend for IFNβ treatment alone to reduce the severity of EAE when compared to vehicle treated groups, but this did not reach significance. Similar to our previous publication (Tiwari-Woodruff et al., 2007), ERβ ligand treatment alone did not have significant therapeutic effect until the chronic phase of EAE, although there was a trend for a treatment effect early in disease (p=0.01, days 31 to endpoint, repeated measures one-way ANOVA). In contrast, combination treatment using IFNβ with the ERβ ligand resulted in lower mean clinical scores compared with vehicle or IFNβ treatment alone. Indeed, mice in the combination treatment group showed near complete clinical recovery from day 23 to endpoint at day 40 (Figure 1A, p<0.001, repeated measures one-way ANOVA). The clinical benefit of combination treatment was sustained, as demonstrated in another experiment in which animals were treated to a later time point, day 70 (Figure 1B, p=0.001, repeated measures one-way ANOVA).
Figure 1. Combination treatment using IFNβ with ERβ ligand was additive in reducing EAE.
Mean clinical scores ± SD of EAE mice treated with vehicle (black), IFNβ (red), ERβ ligand (gray), or the combination (green). A) In mice treated with IFNβ alone, there was a trend for reduced disease as compared to vehicle treated, but this did not reach significance. In contrast, combination treatment of IFNβ and the ERβ ligand significantly reduced EAE from the onset of disease to the endpoint of the experiment at day 40 (p<0.0001, repeated measures one-way ANOVA). B) In a separate experiment, followed to day 70, combination treatment had a sustained effect of the decrease in EAE severity.
Combination treatment with IFNβand ERβ ligand preserved axon densities in spinal cords of EAE mice
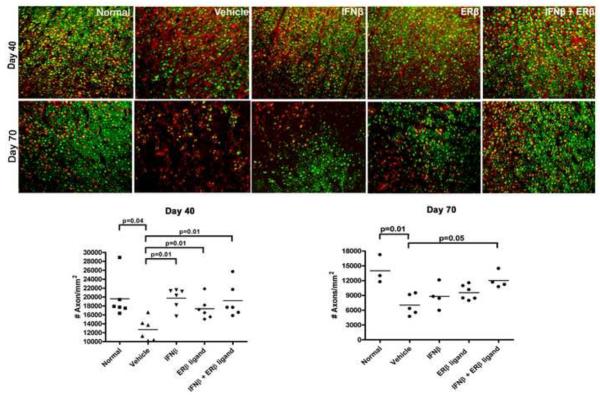
Axonal loss has been proposed as a neuropathologic substrate for clinical disease severity in EAE (Trapp et al., 1999; Wujek et al., 2002). We had previously shown that ERβ ligand treatment preserved axon densities in spinal cords of EAE mice (Tiwari-Woodruff et al., 2007). To determine the effect of combination treatment on axonal loss, we examined thoracic spinal cords of treated EAE mice. Since the mice used in our experiments were transgenic for yellow fluorescent protein (YFP) which is driven by the neuronal-specific thy1 promoter, YFP served as an axonal marker. Indeed, staining in spinal cord sections with the neuronal marker NF200 completely co-localized with YFP expression (not shown). Hence, spinal cord cross sections were directly examined for YFP+ axons. As shown in Figure 2, combination treatment preserved axonal densities in the lateral funiculus of the spinal cord at day 40 of EAE as compared to vehicle treated (p=0.01, one-way ANOVA). Also, as previously reported, treatment with ERβ ligand alone preserved axon densities (Tiwari-Woodruff et al., 2007). Surprisingly, IFNβ treatment alone preserved axonal densities despite the lack of a significant effect of IFNβ treatment on clinical EAE severity (Figure 2, p=0.01, one-way ANOVA). It was possible that the anti-inflammatory properties of IFNβ merely delayed, but did not prevent axonal loss. Thus, we next examined spinal cord sections in another set of mice which were sacrificed at a later time point, day 70. Similar to the results at day 40, combination treatment continued to preserve axonal densities in the lateral funiculus up to day 70 as compared to vehicle treatment (Figure 2, p=0.05, one-way ANOVA). However, at this later time, neither IFNβ nor ERβ ligand treatment alone significantly prevented axonal loss. Similar results were obtained upon examination of the posterior columns (Supplemental Figure 1, p=0.001, one-way ANOVA). These results show that ERβ ligand treatment in combination with IFNβ treatment is additive with respect to preserving axon densities in spinal cords of mice at relatively late stages of EAE.
Figure 2. Combination treatment using IFNβ with ERβ ligand preserved axonal densities in the spinal cord of EAE mice.
40x images of the lateral funiculus of the thoracic spinal cord of day 40 and day 70 EAE mice were stained for myelin with MBP. Yellow-fluorescent-protein (YFP) expression identified axons. At day 40, IFNβ treatment alone, ERβ ligand treatment alone, and combination treatment significantly preserved axonal densities compared to vehicle treated (p=0.01, one-way ANOVA). By day 70, only combination treatment continued to significantly preserve axonal densities compared to vehicle treated (p=0.05, one-way ANOVA). In these experiments, three consecutive spinal cord cross-sections at the T1-T5 level from each of six mice per group (for a total of eighteen sections per group) were used for analysis. Images are representative of three repeated experiments.
Combination treatment with IFNβ and ERβ ligand is additive in reducing infiltration of T cells and macrophages into the CNS of EAE mice
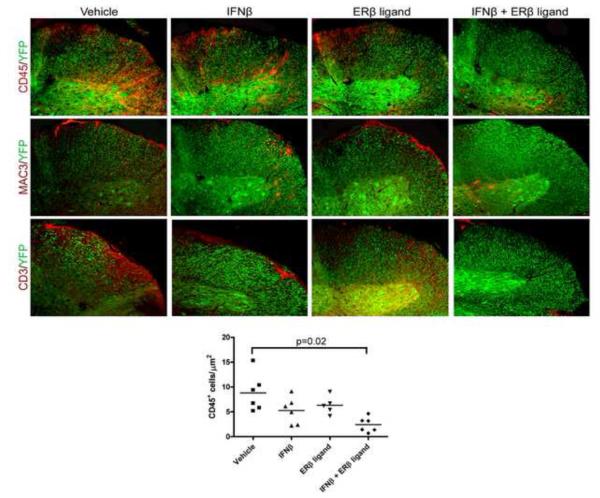
One of the primary actions of IFNβ is to reduce inflammation in the CNS. To determine whether the addition of ERβ ligand treatment influenced this effect of IFNβ, we assessed the degree of inflammation in spinal cords of EAE mice treated with vehicle, IFNβ alone, ERβ ligand alone, or the combination. At the endpoint of disease (day 40), thoracic spinal cord sections were examined for CD45+ cells, a pan-immune cell marker, by immunohistochemistry. While a trend existed, IFNβ treatment alone did not significantly reduce the infiltration of CD45+ cells into the CNS of EAE mice, as compared to vehicle treated (Figure 3). Similar to our previous experiments in active EAE [18], ERβ ligand treatment alone in EAE mice did not decrease inflammation as compared to vehicle treatment. In contrast, combination treatment with IFNβ and ERβ ligand significantly reduced CD45+ staining in the CNS of EAE mice in both the lateral funiculus (Figure 3, p=0.02, one-way ANOVA) and posterior column (not shown, p=0.01, one-way ANOVA).
Figure 3. Combination treatment using IFNβ with ERβ ligand reduced inflammatory cell infiltration in the spinal cord of EAE mice.
10x images of the lateral funiculus of the thoracic spinal cord of day 40 EAE mice were stained for the pan-immune cell marker CD45 (top), Mac3 (middle), and CD3 (bottom). YFP expression identified neurons and axons. Vehicle treated mice exhibited high levels of inflammation in the CNS. Mac3 and CD3 staining revealed that inflammatory infiltrates consisted of macrophages and T cells, respectively. There was a trend for IFNβ treatment alone and ERβ ligand treatment alone to decrease CD45 staining as compared to vehicle treated, but this did not reach significance. In contrast, combination treatment using both IFNβ and ERβ ligand significantly reduced CD45 staining as compared to vehicle treated (p=0.02, one-way ANOVA). In these experiments, three consecutive spinal cord cross-sections at the T1-T5 level from each of six mice per group (for a total of eighteen sections per group) were used for analysis. Images are representative of three repeated experiments.
To determine which immune cell types were affected by treatment, these thoracic spinal cord sections were also examined for CD3R+ T cells and Mac3+ macrophages. Combination treatment reduced staining for both T cells and macrophages in the CNS (Figure 3).
ERβ ligand antagonizes IFNβ treatment effects on Th1 and Th2 cytokine levels
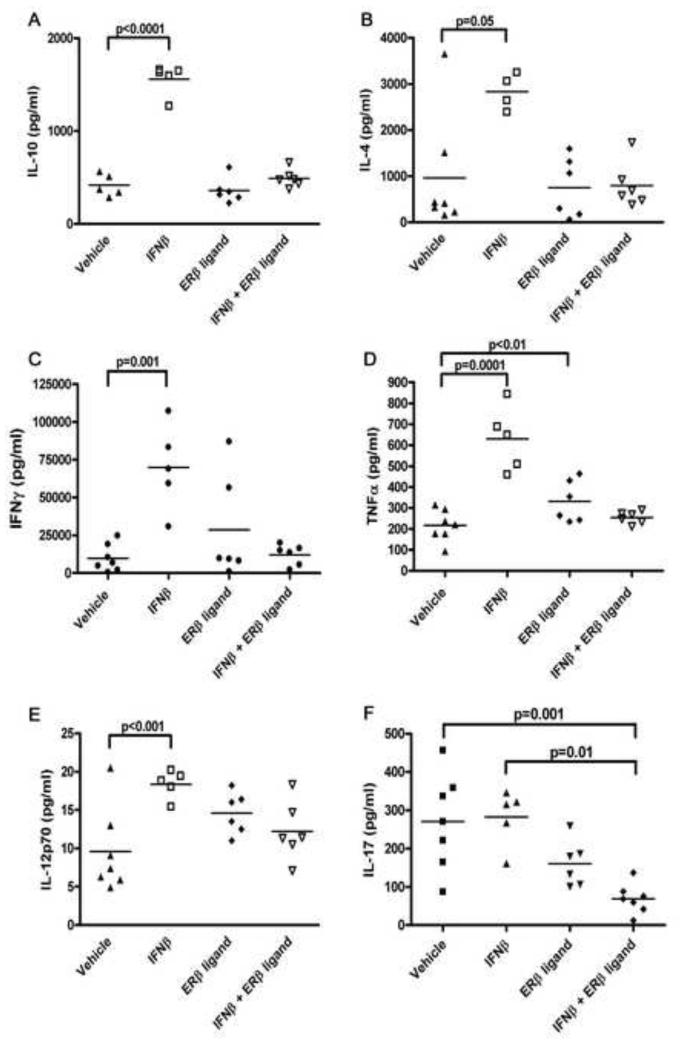
It had previously been shown that IFNβ treatment alone affected cytokine production of peripheral immune responses (Martin-Saavedra et al., 2008b), while ERβ ligand treatment alone did not (Tiwari-Woodruff et al., 2007). Thus, we next assessed cytokine levels (IL-10, IL-4, TNFα, IFNγ, IL-12p70, and TGFβ) upon ex vivo stimulation of splenocytes with autoantigen at day 40 of EAE, thereby focusing on a time point where there was a clinical difference. Treatment with IFNβ alone significantly increased levels of the Th2 cytokines IL-10 (Figure 4A, p<0.0001) and IL-4 (Figure 4B, p=0.001). Interestingly, the Th1 cytokines IFNγ (Figure 4C, p<0.0001), TNFα (Figure 4D, p=0.0001), and IL-12p70 (Figure 4E, p=0.001) were also increased with IFNβ treatment (p-values by one-way ANOVA, Figure 4). Consistent with previous literature, ERβ ligand treatment did not significantly alter cytokine levels as compared to vehicle (Tiwari-Woodruff et al., 2007). Surprisingly, the addition of ERβ ligand treatment to IFNβ in the combination treatment arm resulted in abrogation of the immunostimulatory effects of IFNβ treatment on cytokines. We repeated these analyses in another experiment in which the animals were sacrificed at day 70 and achieved similar results (data not shown). Thus, while IFNβ and ERβ ligand treatments were additive with respect to clinical and neuropathologic outcomes, ERβ ligand treatment in combination with IFNβ abrogated IFNβ mediated effects on Th1 and Th2 cytokines by peripheral immune cells in both the early and later time points of disease.
Figure 4. Treatment with ERβ ligand in combination with IFNβ antagonized the stimulatory effect of IFNβ on Th1 and Th2 cytokines, while it reduced Th17 cytokine levels.
Th1, Th2 and Th17 cytokine levels measured from supernatants of splenocytes stimulated with MOG 35-55 revealed that IFNβ treatment alone increased IL-10 (4A, p<0.0001), IL-4 (4B, p=0.001), IFNγ (4C, p<0.0001), TNFα (4D, p=0.0001), and IL-12p70 (4E, p=0.001), as compared to vehicle treatment. In contrast, IFNβ treatment alone did not affect levels of IL-17 (4F). ERβ ligand treatment alone had no effect on Th1 and Th2 cytokine levels, but when combined with IFNβ, it negated the changes seen with IFNβ treatment alone. ERβ ligand treatment alone tended to decrease levels of the Th17 cytokine IL-17, but this did not reach significance. However, when ERβ ligand was combined with IFNβ, it reduced the levels of IL-17 as compared to IFNβ treatment alone (4F, p=0.01) and vehicle treatment (4F, p=0.001, one-way ANOVA).
Combination treatment with IFNβ and ERβ ligand are additive in reducing IL-17 levels
Th17 cells have been shown to play an important role in EAE, particularly during the later, more chronic phase of disease (Steinman, 2007). Since we had observed significant axonal sparing relatively late in disease with combination treatment, we next determined the levels of Th17 cytokine production during treatment with IFNβ alone, ERβ ligand alone, or the combination. Supernatants from autoantigen stimulated splenocytes from EAE mice were analyzed for IL-17 and IL-23. Interestingly, IFNβ treatment alone did not affect the levels of IL-17, whereas ERβ ligand treatment alone showed a trend towards decreased IL-17 production, but this did not reach significance (Figure 4F). In contrast, combination treatment with IFNβ and ERβ ligand significantly reduced levels of IL-17 as compared to vehicle treatment (Figure 4F, n=7, p=0.02, one-way ANOVA). Since IL-23 is a key cytokine to maintaining Th17 activity (Stritesky et al., 2008), we also examined levels of IL-23 and found that they were no different between any treatment groups (not shown). Thus, in contrast to antagonistic effects of combination treatment on Th1 and Th2 cytokines, combination treatment significantly reduced IL-17 levels from autoantigen stimulated splenocytes.
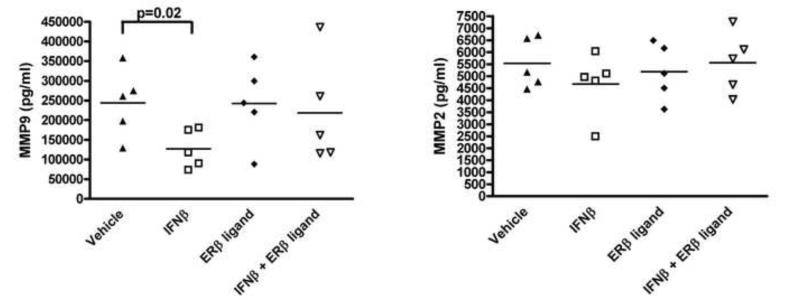
ERβ ligand treatment antagonizes IFNβ effects on MMP-9
In light of the additive effect of combination treatment on CNS inflammation (Figure 3), we next focused on molecules involved in immune cell trafficking to the CNS. In MS and EAE, MMP-9 and MMP-2 can be involved in mediating inflammation in the CNS. We therefore assessed the effect of IFNβ, ERβ ligand, or combination treatment on MMP-9 and MMP-2 expression by autoantigen stimulated splenocytes from mice with EAE. Consistent with work in MS, treatment with IFNβ alone significantly reduced MMP-9 in EAE (n=5, p=0.002, one-way ANOVA), while MMP-2 was unchanged, as compared to vehicle treated (Figure 5). ERβ ligand treatment alone had no effect on MMP expression. Surprisingly, the addition of ERβ ligand treatment to IFNβ treatment antagonized the IFNβ-mediated decrease in MMP-9. Therefore, with respect to both Th1 and Th2 cytokine production and MMP-9 expression, ERβ ligand treatment in combination with IFNβ abrogated the immunomodulatory effects of IFNβ treatment.
Figure 5. Treatment with ERβ ligand in combination with IFNβ antagonized the effect of IFNβ on reducing MMP-9.
IFNβ treatment alone significantly decreased MMP-9 levels in supernatants as compared to vehicle treated, while ERβ ligand treatment alone did not affect production of MMPs (p=0.02, one-way ANOVA). The addition of ERβ ligand treatment to IFNβ during combination treatment abrogated the effect of IFNβ on MMP-9. There were no differences in MMP-2 between any of the treatment groups. Data shown are representative of three repeated experiments.
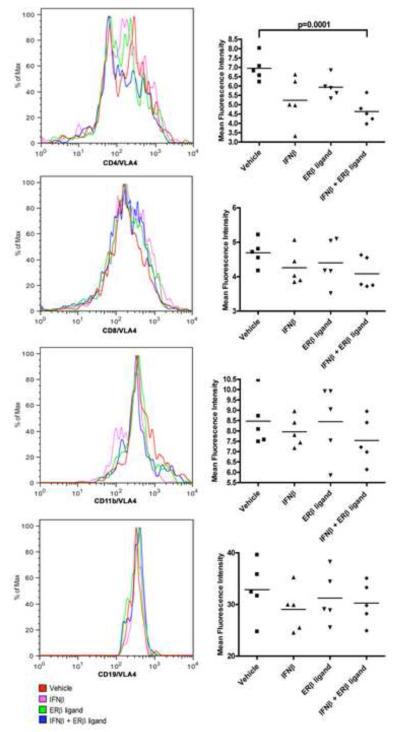
Combination treatment with IFNβ and ERβ ligand are additive in reducing VLA-4 expression on CD4+ T cells in EAE
To explore other potential transmigratory factors underlying additive clinical and neuropathologic effects, we next focused on a critical cell adhesion molecule. VLA-4 (CD49d) is known to play an important role in immune cell trafficking in both MS and EAE. Splenocytes from EAE mice treated with either IFNβ, ERβ ligand or the combination were stimulated ex vivo with autoantigen and analyzed for expression of VLA-4 on T cells, B cells, and macrophages. There was a trend towards decreased VLA-4 expression on CD4+ T cells with IFNβ treatment alone compared to vehicle treated, but this did not reach significance, and there was no effect of ERβ ligand treatment alone (Figure 6). In contrast, the expression of VLA-4 was significantly lower on CD4+ T cells of EAE mice treated with the combination (n=7, p=0.0001, one-way ANOVA). There were no differences in VLA-4 expression on CD8+, CD19+, or CD11b+ cells between any treatment groups. These results demonstrated that combining ERβ ligand treatment with IFNβ treatment was additive with respect to decreasing VLA-4 expression on CD4+ T cells in EAE, consistent with the additive effect of these two treatments on reducing inflammation in the CNS (Figure 3).
Figure 6. Treatment with ERβ ligand in combination with IFNβ reduced VLA-4 expression on CD4+ T cells of EAE mice.
Representative histograms of the level of VLA-4 expression on gated CD4 and CD8 (T cells), CD19 (B cells), and CD11b (macrophages and monocytes). There was a trend for IFNβ treatment alone and ERβ ligand treatment alone to decrease VLA-4 expression on CD4+ T cells, but this did not reach significance. In contrast, combination treatment using both IFNβ and ERβ ligand significantly reduced VLA-4 expression (blue, p=0.0001, one-way ANOVA), as compared to vehicle (red) treated mice. No differences in VLA-4 expression were observed on CD8, CD19 and CD11b cells between any treatment groups. Histograms are representative of three repeated experiments.
4. Discussion
MS is a putative autoimmune demyelinating disease with a neurodegenerative component. Currently all of the approved MS treatments are anti-inflammatory, and therefore indirectly neuroprotective through reducing immune attacks on the CNS. Often patients have progressive disability accumulation despite robust immunosuppression, suggesting the need for treatment approaches which involve using an anti-inflammatory agent in combination with a neuroprotective agent. Here, we explored this approach by combining a purely anti-inflammatory agent, IFNβ, with a putative neuroprotective agent, an ERβ ligand, in the MS model EAE. Combination treatment resulted in an additive effect at the clinical level, as shown by decreased EAE disease severity. This additive effect was also observed at the neuropathologic level, as shown by reduced inflammation and preserved axonal densities in the spinal cord. Surprisingly, combination treatment elicited additive effects in the peripheral immune system with respect to VLA-4 expression and IL-17 levels, while demonstrating antagonistic effects on MMP-9 expression and Th1 and Th2 cytokines.
It is interesting that IFNβ treatment alone preserved axonal densities of EAE mice at day 40 despite overt clinical disability in this treatment group at this time. Since mice were spared from axonal loss at this relatively earlier time point, it is likely that the clinical disability in IFNβ treated mice was due to conduction block. Importantly, despite preserving axon numbers relatively early in disease, treatment with a single agent of either IFNβ or ERβ ligand alone was not capable of preventing axonal loss later in disease. In contrast, combination treatment spared axon numbers both early and late. Thus, both IFNβ and ERβ ligand may temporarily delay axonal loss when administered alone, but when combined, they provide long-term neuroprotection.
The additive and antagonistic effects observed in the peripheral immune system upon combination treatment with IFNβ and ERβ ligand during EAE underscores the complexity of drug interactions. Our lab had previously shown that ERβ ligand treatment alone preserved axon densities and myelin staining, but had no significant effects on altering cytokine production of peripheral immune cells or reducing inflammation in the target organ, the CNS (Tiwari-Woodruff et al., 2007). This suggested “direct” neuroprotection by ERβ ligand that was independent from a reduction in CNS inflammation. With regard to immune mechanisms, we did not expect that ERβ ligand treatment would add to the effect of IFNβ treatment in reducing VLA-4 expression on T cells, nor did we expect that combination treatment would decrease IL-17 levels. IFNβ treatment had previously been shown to downregulate VLA-4 expression on T cells (Schmidt et al., 2001), and estrogens had been shown to reduce VLA-4 expression on T cells, but this latter effect was attributed to ERα, not ERβ, using ERα deficient mice (Polanczyk et al., 2003). Nevertheless, when ERβ ligand was used in combination with IFNβ, it was additive with IFNβ in reducing VLA-4 expression and reducing CNS inflammation. With respect to treatment effects on Th17 activity, IFNβ treatment in EAE has been shown to decrease IL-17 levels (Martin-Saavedra et al., 2008a). We did not replicate this finding with IFNβ treatment alone. This could be due to differences in IFNβ dose or other methodological differences. Although estrogen mediated changes in IL-17 levels have been reported (Wang et al., 2009a), neither treatment with ERα nor ERβ ligand alone were previously shown to affect IL-17 in EAE (Tiwari-Woodruff et al., 2007). Yet, when ERβ ligand was used in conjunction with IFNβ, the combination significantly reduced the levels of IL-17.
We speculate that reduced inflammation in the spinal cords of EAE mice treated with IFNβ and ERβ ligand may be attributed to the limitation of firm adhesion and transendothelial migration of effector T cells into the CNS, as suggested by an additive decrease in VLA-4 expression on CD4+ T cells. Extensive data underscore the importance of VLA-4 in CNS inflammation in both MS and EAE (Cannella and Raine, 1993; Muraro et al., 2000). In MS, treatment with Natalizumab reduces clinical relapses by two thirds and reduces gadolinium enhancing lesions on MRI by 92% (Havrdova et al., 2009). In EAE, mice treated with anti-VLA-4 antibody had CD4+ T cells that were unable to traverse the blood brain barrier and enter into the CNS (Bauer et al., 2009). In support of our theory, IFNβ and ERβ ligand treatment alone each only had a modest effect on VLA-4 expression, and correspondingly, a modest effect on reducing inflammation in the CNS. In contrast, combination treatment led to a significant reduction of VLA-4 expression on CD4+ T cells, and inflammation was also significantly reduced in the CNS of these mice.
In addition to effects on VLA-4 expression, the additive effect of combination treatment on Th17 likely plays a pivotal role in reducing EAE disease severity and sparing axons in the CNS later in disease. Since the discovery of the Th17 lineage of T cells, this IL-17-producing subset has been largely attributed to the sustenance of inflammation in chronic inflammatory diseases including RA and MS, as well as in their respective animal models (Domingues et al., 2008; Fletcher et al., 2006; Komiyama et al., 2006; Stromnes et al., 2007). In our study, lower levels of IL-17 during combination treatment were associated with decreased disease severity, reduced CNS inflammation, and greater axonal sparing late in disease.
Combination treatment had very different effects on Th1 and Th2 cytokines, as compared to Th17. The antagonistic effects of ERβ ligand on IFNβ mediated stimulation of Th1 and Th2 cytokines was unexpected, as ERβ ligand treatment alone did not affect levels of those cytokines. The differential effects of ERβ ligand on IFNβ mediated changes in Th1, Th2 and Th17 cytokines may reflect differential regulation of gene transcription at a level downstream of NFκB, a general proinflammatory transcription factor. Interestingly, the antagonistic effects of ERβ ligand treatment on IFNβ mediated cytokine changes did not adversely affect the beneficial clinical outcome of combination treatment.
This paper focused on the potential additive effects of an anti-inflammatory agent, IFNβ, and a putative neuroprotective agent, an ERβ ligand, in the treatment of chronic EAE. We have shown that these two agents are additive at the clinical and neuropathological levels. However, our data showing both additive and antagonistic effects in the peripheral immune system during combination treatment underscore the complexity of drug interactions since the outcome of combination treatment could not have been predicted based on data from use of each single agent alone. Notably, immunomodulatory effects of ERβ ligand treatment are not mutually exclusive with more “direct” neuroprotective effects of ERβ ligand on neurons, oligodendrocytes or astrocytes in this model. Thus, additive effects of combination treatment with IFNβ and ERβ ligand on clinical scores and axon densities may be due to additive immunomodulatory properties as well as the addition of protective effects of ERβ ligand at the level of the target organ, the CNS. Our results have important translational implications with regards to current treatment trials in MS exploring combination treatments with two disease-modifying agents. These include the current trial of Rebif (IFN-1a) in combination with estradiol in Europe and of Copaxone in combination with estriol in the U.S (http://www.clinicaltrials.gov/ct2/show/NCT00451204?term=multiple+sclerosis+estriol &rank=1). Combination treatment could result in surprising effects on various immune mechanisms, even in a setting of overall clinical improvement. Because of the rather modest effect of currently available monotherapies in MS, approaches using combination treatments should be explored. However, complex interactions similar to those repeated herein should be considered in these trial designs.
Supplementary Material
Combination treatment using IFNβ with ERβ ligand preserved axonal densities in the posterior column of EAE mice. (Top) 40x images of the posterior column of the thoracic spinal cord of day 70 EAE mice were stained for myelin with MBP (red). YFP (green) expression identified neurons. (Bottom) DAPI (blue) field was added to reveal the distribution of cell bodies, clustered in areas of low axon densities in vehicle treated, IFNβ treatment alone, and ERβ treatment alone. Combination treatment significantly preserved axonal densities when compared with vehicle treatment alone (p=0.001, one-way ANOVA). In these experiments, three consecutive spinal cord cross-sections at the T1-T5 level from each of six mice per group (for a total of eighteen sections per group) were used for analysis.
Acknowledgements
The authors acknowledge Elizabeth Umeda, B.S. for technical laboratory assistance and Stefan Gold, Ph.D. for helpful suggestions and discussions. Support for this work was provided by National Institutes of Health grant K24NS062117, and National Multiple Sclerosis Society grants RG 3593, 4033 and CA1028 to R.R.V., as well as by the Skirball Foundation and the Sherak Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: Occurrence and significance. Journal of Mammary Gland Biology and Neoplasia. 2000;5:271–281. doi: 10.1023/a:1009594727358. [DOI] [PubMed] [Google Scholar]
- Arnason BGW. Immunologic therapy of multiple sclerosis. Annual Review of Medicine. 1999;50:291–302. doi: 10.1146/annurev.med.50.1.291. [DOI] [PubMed] [Google Scholar]
- Axtell RC, Steinman L. Type 1 interferons cool the inflamed brain. Immunity. 2008;28:600–602. doi: 10.1016/j.immuni.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Bauer M, Brakebusch C, Coisne C, Sixt M, Wekerle H, Engelhardt B, Fassler R. beta(1) integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1920–1925. doi: 10.1073/pnas.0808909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A. Anti-inflammatory properties of Type I interferons. Antiviral Res. 2006;71:108–116. doi: 10.1016/j.antiviral.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella B, Raine CS. The Vcam-1/Vla-4 Pathway Is Involved in Chronic Lesion Expression in Multiple-Sclerosis (Ms) Journal of Neuropathology and Experimental Neurology. 1993;52:311–311. [Google Scholar]
- Clanet M, Kappos L, Hartung HP, Hohlfeld R. Interferon beta-1a in relapsing multiple sclerosis: four-year extension of the European IFNbeta-1a Dose-Comparison Study. Mult Scler. 2004;10:139–144. doi: 10.1191/1352458504ms990oa. [DOI] [PubMed] [Google Scholar]
- Clanet M, Radue EW, Kappos L, Hartung HP, Hohlfeld R, Sandberg-Wollheim M, Kooijmans-Coutinho M, Tsao EC, Sandrock AW, Invest EADS. A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology. 2002;59:1507–1517. doi: 10.1212/01.wnl.0000032256.35561.d6. [DOI] [PubMed] [Google Scholar]
- Comabella M, Rio J, Espejo C, Ruiz de Villa M, Al-Zayat H, Nos C, Deisenhammer F, Baranzini SE, Nonell L, Lopez C, Julia E, Oksenberg JR, Montalban X. Changes in matrix metalloproteinases and their inhibitors during interferon-beta treatment in multiple sclerosis. Clin Immunol. 2009;130:145–150. doi: 10.1016/j.clim.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Domingues HS, Krishnamoorthy G, Lassmann H, Wekerle H. Differential roles of Th1 and Th17 CD4+T cell subsets in the pathogenesis of EAE. Journal of Neuroimmunology. 2008;203:238–239. [Google Scholar]
- Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, Chambon P, Gourdy P, Arnal JF, Guery JC. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol. 2008;180:3661–3669. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J Endocrinol. 2005;185:243–252. doi: 10.1677/joe.1.06063. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Costelloe L, O'Farrelly C, Tubridy N, Mills KHG. IL-17 producing T cells and their induction in multiple sclerosis. Multiple Sclerosis. 2006;12:S219–S219. [Google Scholar]
- Garidou L, Laffont S, Douin V, Krust A, Chambon P, Guy JC, Guery JC. Estrogen receptor alpha-signaling in inflammatory leukocytes is dispensable for 17 beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 2004;154:83–83. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, O'Connor PW, Giovannoni G, Phillips JT, Lublin FD, Pace A, Kim R, Hyde R. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurology. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. Journal of Neuroscience Research. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jaini R, Hannaman D, Johnson JM, Bernard RM, Altuntas CZ, Delasalas MM, Kesaraju P, Luxembourg A, Evans CF, Tuohy VK. Gene-based intramuscular interferon-beta therapy for experimental autoimmune encephalomyelitis. Mol Ther. 2006;14:416–422. doi: 10.1016/j.ymthe.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Enhancement of collagen-induced arthritis in female mice by estrogen receptor blockage. Arthritis and Rheumatism. 2001;44:2168–2175. doi: 10.1002/1529-0131(200109)44:9<2168::aid-art370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Jansson L, Olsson T, Holmdahl R. Estrogen Induces a Potent Suppression of Experimental Autoimmune Encephalomyelitis and Collagen-Induced Arthritis in Mice. Journal of Neuroimmunology. 1994;53:203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. Journal of Immunology. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. European Journal of Immunology. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- Martin-Saavedra FM, Flores N, Dorado B, Eguiluz C, Bravo B, Garcia-Merino A, Ballester S. Beta-interferon unbalances the peripheral T cell proinflammatory response in experimental autoimmune encephalomyelitis. Mol Immunol. 2007;44:3597–3607. doi: 10.1016/j.molimm.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Martin-Saavedra FM, Gonzalez-Garcia C, Bravo B, Ballester S. Beta interferon restricts the inflammatory potential of CD4(+) cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Molecular Immunology. 2008a;45:4008–4019. doi: 10.1016/j.molimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Martin-Saavedra FM, Gonzalez-Garcia C, Bravo B, Ballester S. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol Immunol. 2008b;45:4008–4019. doi: 10.1016/j.molimm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Mitra SW. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha (vol 144, pg 2055, 2003) Endocrinology. 2003;144:2844–2844. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra PA, Caniglia S, Testa N, Tirolo C, L'Episcopo F, Gennusol F, Desole MS, Marchetti B. Estrogen triggers astrocyte-microglia crosstalk in experimental Parkinsonism: Implications for neuroprotection. Glia. 2003:26–26. [Google Scholar]
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro PA, Leist T, Bielekova B, McFarland HF. VLA-4/CD49d downregulated on primed T lymphocytes during interferon-beta therapy in multiple sclerosis. J Neuroimmunol. 2000;111:186–194. doi: 10.1016/s0165-5728(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Offner H. Neuroimmunoprotective effects of estrogen and derivatives in experimental autoimmune encephalomyelitis: therapeutic implications for multiple sclerosis. J Neurosci Res. 2004;78:603–624. doi: 10.1002/jnr.20330. [DOI] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, Teuscher C, Vandenbark AA, Offner H. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Schmidt H, Mildner A, Knobeloch KP, Hanisch UK, Raasch J, Merkler D, Detje C, Gutcher I, Mages J, Lang R, Martin R, Gold R, Becher B, Bruck W, Kalinke U. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Schaefer C, Hidalgo TR, Cashion L, Petry H, Brooks A, Szymanski P, Qian HS, Gross C, Wang P, Liu P, Goldman C, Rubanyi GM, Harkins RN. Gene-based delivery of IFN-beta is efficacious in a murine model of experimental allergic encephalomyelitis. Journal of Interferon and Cytokine Research. 2006;26:449–454. doi: 10.1089/jir.2006.26.449. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Sturzebecher S, Toyka KV, Gold R. Interferon-beta treatment of experimental autoimmune encephalomyelitis leads to rapid nonapoptotic termination of T cell infiltration. J Neurosci Res. 2001;65:59–67. doi: 10.1002/jnr.1128. [DOI] [PubMed] [Google Scholar]
- Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Medicine. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Yeh N, Kaplan MH. IL-23 Promotes Maintenance but Not Commitment to the Th17 Lineage. Journal of Immunology. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I, Goverman J, Liggitt D. The role of IL-17 and IFN-gamma in defining pathogenic potential of T cells in EAE. Clinical Immunology. 2007;123:S61–S62. [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Current Opinion in Neurology. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009a;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li YX, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane Estrogen Receptor Regulates Experimental Autoimmune Encephalomyelitis through Up-regulation of Programmed Death 1. Journal of Immunology. 2009b;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD. 17 beta-Estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-amp response element binding protein signal pathway and Bcl-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. Journal of Neuropathology and Experimental Neurology. 2002;61:23–32. doi: 10.1093/jnen/61.1.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combination treatment using IFNβ with ERβ ligand preserved axonal densities in the posterior column of EAE mice. (Top) 40x images of the posterior column of the thoracic spinal cord of day 70 EAE mice were stained for myelin with MBP (red). YFP (green) expression identified neurons. (Bottom) DAPI (blue) field was added to reveal the distribution of cell bodies, clustered in areas of low axon densities in vehicle treated, IFNβ treatment alone, and ERβ treatment alone. Combination treatment significantly preserved axonal densities when compared with vehicle treatment alone (p=0.001, one-way ANOVA). In these experiments, three consecutive spinal cord cross-sections at the T1-T5 level from each of six mice per group (for a total of eighteen sections per group) were used for analysis.