Abstract
Urinary proteomic profiling has potential to identify candidate biomarkers of renal injury in infants provided an adequate urine sample can be obtained. Although diapers are used to obtain urine for clinical evaluation, their use for proteomic analysis has not been investigated. We therefore performed feasibility studies on the use of diaper-extracted urine for 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE). Pediatric waste urine (2–20 mL) was applied to gel-containing, non-gel and cotton-gauze diapers and then mechanically expressed. Urine volume and total protein were measured pre- and post-extraction. Proteins were separated via 2D-PAGE following application of urine (20–40 mL) to each matrix. 2D-PAGE was also performed on clinical specimens collected using each diaper type. Differences in the adsorption and retention of urine volume and protein were noted between matrices. Non-gel and cotton-gauze diapers provided the best protein/volume recovery and the lowest interference with the Bradford assay. 2D-PAGE was also successfully completed using urine samples from both cotton fiber matrices. Conversely, samples from low-gel diapers demonstrated poor protein separation and reproducibility. Diapers containing cotton-fiber matrices appear adequate for 2D-PAGE. Qualitative and quantitative analyses of resolved proteins using replicate, high resolution gels will be required, however, before diaper-extracted urine can be applied in proteomic profiling.
Keywords: diaper, pediatric, proteomics, urine
1. Introduction
Urinary proteomic profiling has great potential to identify candidate biomarkers of renal injury in infants and young children. However, collecting a urine sample of adequate volume and protein concentration in this patient population is often difficult. In hospitalized children, indwelling catheters placed as part of the child's standard medical care may be used to collect urine and we have previously demonstrated that samples collected via this route are adequate for proteomic analysis. [1] Given the risk of infection, however, placement of indwelling catheters in newborns is relatively uncommon, occurring in less than 3% of infants. [2] Adhesive bags applied to the perineum are acceptable alternatives to indwelling catheters although they are prone to leakage and sample loss, particularly in females. The adhesive portion of the bag can also cause significant irritation to the skin, especially in preterm infants. Consequently, alternate methods for collecting urine in this patient population must be identified.
Disposable diapers are routinely used to obtain urine for clinical evaluation in infants and young children. Recovery of urine is achieved via removing the absorbent matrix from a diaper without fecal contamination, placing it into a disposable syringe and mechanically expressing the absorbed volume. Given the nature of the absorbent matrix, samples from diapers containing absorbent gelling materials (e.g., polyacrylate) are difficult to extract and frequently contaminated with gel crystals. [3,4] Using non-gel diapers, however, urine volumes adequate for biochemical and microbiologic analysis can be obtained from infants weighing as little as 1,550 grams. [5,6] Urine electrolyte (sodium, potassium, chloride), osmolality, specific gravity, pH, bacterial count and culture results obtained using urine extracted from non-gel diapers in infants [4–7] and elderly adults [8] are also comparable to those in spontaneously voided urine.
Although extracted urine samples produce reliable biochemical and microbiologic results, it is unclear whether they may be used to assess qualitative and quantitative urinary protein expression. While a previous investigation noted comparable total protein values in extracted and spontaneously voided urine, [7] a subsequent study demonstrated a 10% reduction in total protein values in extracted samples. [9] Selective adsorption of specific urine proteins (creatinine, albumin, retinol binding protein) by cotton ball fibers has also been described. [10,11] However, the impact of diaper extraction on the urinary proteomic profile has not been investigated to date. The ability to use diaper-extracted urine samples for 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE) has also not been previously explored. We therefore performed initial feasibility studies to determine whether diapers can be used as a method of collecting urine for subsequent 2D-PAGE.
2. Materials and Methods
The study was approved by the University of Louisville Human Subjects Protection Program. A complete waiver was granted for collection and analysis of urine samples given that specimens were salvaged as waste and were de-identified following collection. All study procedures were performed in accordance with ethical standards of the local Institutional Review Board and Good Clinical Practice guidelines. The study was also registered with the clinical trials registry sponsored by the United States National Library of Medicine (www.clinicaltrials.gov, study identifier NCT00308906).
i. Urine sample collection, pre-processing and storage
Waste urine samples were collected from a healthy 3-year-old male via spontaneous void into a sterile collection hat/container. Whenever possible, samples obtained using midstream, clean catch collections were used in order to minimize bacterial contamination. A diaper sample containing fecal contamination was also collected for exploratory analysis using a commercially available non-gel containing diaper (Tushies®, TenderCare, Inc., Eau Claire, WI). Given that the standard diaper types used in our hospital changed during the course of this study, collection of samples over a 3-year period (approximately 20 collections) was necessary in order to complete analyses on all diaper types. During each spontaneous void collection, urine was salvaged over 36-hours and maintained at 4°C. At the end of the collection period, samples were pooled and stored at 4°C for a maximum of 48 hours. An aliquot of the pooled sample was removed, vortexed and centrifuged at 1,500 × g for 15 minutes to remove cells and other debris. The resulting supernatant was aliquoted into sterile polypropylene storage tubes and stored at −70°C for a maximum of 12 months. The remaining pooled urine was applied to various diapers as described below.
To assess whether 2D-PAGE could be successfully performed on actual clinical specimens, urine was also collected from a hospitalized, 28 day old preterm newborn (29 weeks gestation) using commercially available low-gel (WeePee® Three, Children's Medical Ventures, Norwell, MA) and non-gel disposable diapers. Samples were also collected using diapers containing three 10 × 10 cm2 cotton gauze pads (Avant Gauze®, Medline Industries, Mundelein, IL) inserted in place of their standard absorbent matrix. [12] Collections occurred on 3 consecutive days and a different diaper type was used each day. Diapers were placed on the infant each morning and changed every 2 to 3 hours as per institutional standard of care. Upon removal, diapers were stored at the bedside in a cooler containing several ice packs until the end of the collection period (approximately 3 – 7 hours). At the end of the collection period, urine was immediately expressed from non-stool containing diapers and samples were vortexed and centrifuged at 1,500 × g for 15 minutes to remove cells and other debris. The resulting supernatants were then pooled, aliquoted into sterile polypropylene storage tubes and stored at −70°C for no more than 2 weeks.
ii. Determination of urine volume and protein recovery
Aliquots (2 – 20 mL) of healthy pediatric urine were applied to the inside surface of gel-containing (Pampers® Preemie Swaddlers, Procter and Gamble, Cincinnati, OH), low-gel, non-gel and gauze insert diapers. A minimum of 8 replicates per matrix were prepared for each of the 4 urine volumes evaluated (2, 5, 10 and 20 mL). Urine was allowed to absorb into the gel and/or cotton fiber matrix for approximately 2 hours and the area surrounding the site of urine application was subsequently excised and placed into an appropriately sized disposable syringe. Urine was then mechanically expressed from the excised diaper matrix by applying pressure to the plunger and the volume obtained post-extraction was recorded. Extracted urine samples were vortexed and centrifuged at 1,500 × g for 15 minutes to remove cells and other debris. Total protein concentrations pre- and post-diaper extraction were then determined via the Bradford protein assay with bovine serum albumin (BSA) standard. (Quick Start Protein Assay Kit, Bio-Rad Laboratories, Hercules, CA). The amount of total protein (µg) and percent recovery yield were calculated using protein concentration values. Percent recovery yield was calculated as follows: total protein post-diaper extraction (µg) × 100 / total protein pre-diaper extraction (µg). [13]
iii. Confirmation of protein recovery via immunoblot analysis
Western blot analysis was performed to confirm recovery of a previously identified protein (lipocalin type prostaglandin D synthase) in pre- and post-diaper extraction urine samples obtained from the healthy 3-year-old subject. Blots (n = 3 per group) were performed using rabbit anti-human lipocalin type prostaglandin D synthase IgG (1:200) as the primary antibody (Cayman Chemical Company, Ann Arbor, MI). A total of 10µg of urine protein was loaded per lane.
iv. Preparation of Pre- and Post-Diaper Extraction Samples for Protein Separation
Healthy pediatric waste urine (20 – 40 mL) was applied to the inside surface of low-gel (n=4), non-gel (n=4) and gauze insert (n=4) diapers and extracted as described above. Buffered water (20 – 40 mL) containing BSA (0.3 mg/mL) as a positive control was also applied to low-gel (n=4), non-gel (n=4) and gauze insert (n=4) diapers and extracted as urine to determine if contaminants are introduced by the diaper matrix and/or extraction process. Pre- (n=3) and post-diaper extraction water and urine (healthy pediatric and hospitalized newborn) samples were concentrated to a volume of 1–2 mL using either an Amicon stirred cell with a 10,000 dalton molecular weight cut-off regenerated cellulose ultrafiltration membrane or an Amicon Ultra-15 centrifugal filter unit (10,000 dalton molecular weight cut-off) (Millipore, Billerica, MA). Concentrated samples were desalted via overnight dialysis against water or washing 8 times with water using the Amicon filter unit. Total protein concentration was quantitated using the Bradford protein assay with bovine serum albumin standard. Approximately 75µg of protein was lyophilized and the protein pellet was subsequently re-dissolved into immobilized pH gradient (IPG) rehydration buffer at room temperature for 1 hour with shaking and vortexing. The solution was then allowed to passively rehydrate the 7cm pH 3–10 nonlinear IPG strips for at least 18 hours.
v. Separation of Proteins via 2D-PAGE
Protein separation in the first dimension was achieved via isoelectric focusing using a stepped voltage program with the Zoom® IPG runner system and standard electrode buffers. Samples were focused for 2,000 volt-hours at a maximum voltage of 2000 V, current limitations of 1mA and power limitations of 2W. After focusing, IPG strips were conditioned and transferred onto 4–12% gradient Bis-Tris NuPAGE® pre-cast slab gels (7cm). Proteins were resolved in the second dimension by mass using electrophoresis at 200V constant until migration of the bromophenolblue dye out of the gel.
vi. Gel staining, image acquisition and replicate gel analyses
Gel slabs were fixed in 10% methanol/7% acetic acid for 30 minutes and stained overnight with SYPRO Ruby. Stained gels were imaged with the Perkin Elmer ProXpress true 16-bit CCD camera-based imaging system. Progenesis SameSpots v3.0 software (Nonlinear Dynamics, Newcastle upon Tyne, UK) was used for analysis of protein spots on replicate 2D healthy pediatric urine gels. Gel images were sorted into groups based on diaper type and each group was imported into the SameSpots software as a separate experiment. SameSpots allows for multiple groups within a single experiment; however, since we were interested in determining variability in gel images within replicates from each type of matrix, only a single group was established in each of 4 separate analyses. In each group, an area of the reference gel was delineated for analysis. The analyzed areas all contained numerous distinct spots and varied by experiment. SameSpots automatically recognized, aligned, and matched spots between gels. These spots were reviewed and user-edited as deemed necessary. The SameSpots software automatically subtracted background noise and normalized spot volumes between gels by calculating a normalization factor based on the mean value of the log of the ratio of spot volumes between the reference gel and each sample gel within a given experiment. For each set of replicate urine gels, mean normalized spot volumes and standard deviations (SD) were determined and the coefficient of variation (CV) was calculated (SD/mean). Mean and median CV values were also calculated for the total group of spots from each set of gels.
vii. Large Format Gel Analysis
A set of healthy pediatric urine samples obtained pre (n=4) and post (n=4) extraction from non-gel diapers were also analyzed using large format (18cm) gels. Samples derived from non-gel matrices were selected given that they are commercially-available (and therefore more practical for clinical use) and were successfully analyzed via 2D-PAGE using a small format platform. Approximately 150µg of lyophilized protein was re-suspended in urea solubilization buffer (Proteomic Research Services, Ann Arbor, MI) and then allowed to passively rehydrate an 18cm narrow range, pH 4–7 Immobiline* DryStrip (GE Healthcare, Piscataway, NJ) for a minimum of 18 hours. Samples were focused using the pHaser Isolelectric Focusing System (Genomic Solutions Inc., Ann Arbor, MI) for 100,000 volt-hours, with current limitations of 80µA using the Genomic Solutions Investigator 5000 programmable power supply. After focusing, the strips were equilibrated and transferred onto pre-cast 10% Duracryl-Tricine chemistry gels (NextGen Sciences, Ann Arbor, MI). Proteins were resolved in the second dimension based on mass using electrophoresis at 400V until migration of the bromophenol dye was 1cm above the bottom edge of the gel. Gels were then fixed and stained as described previously.
3. Results
i. Urine volume and protein recovery
A minimum of 8 replicates were completed for each of the 4 urine volumes evaluated (2, 5, 10 and 20 mL). Given that a negligible volume of urine was recovered from the gel-containing diapers, determination of volume and protein recovery was completed only for the highest volume (20 mL). Mean urine volume and protein recovery values for each diaper type are presented in Table 1. The volume of urine recovered was dependent on the type of absorbent matrix and the amount of urine added. The mean percent volume recovery was comparable for the low-gel, non-gel and gauze-insert containing diapers and ranged from 24–66% depending on the volume added. Conversely, recovery for the 20 mL gel-containing diaper (15%) was significantly lower than that achieved with other matrices exposed to the same volume (56–66%). For all diaper types, the percent volume recovery increased with higher saturation of the matrix with the greatest recovery observed following addition of 20mL.
Table 1.
Urine volume and protein recovery yield from diaper-derived urine samples
| Diaper type | n | Urine volume added (mL) |
Urine volume recovered* (mL) |
Volume recovery yield (%) |
Total protein added (µg) |
Total protein recovered* (µg) |
Protein recovery yield (%) |
|---|---|---|---|---|---|---|---|
| Gel | 12 | 20 | 3.0 ± 0.19 (2.8 – 3.2) |
15.1 | 1467.5 | 397.5 ± 12.2 (376.6 – 411.5) |
27.1 |
| Low gel | 9 | 2 | 0.47 ± 0.33 (0.20 – 0.90 |
23.5 | 112.1 | 36.1 ± 25.8 (15.3 – 70.5) |
32.2 |
| 9 | 5 | 2.2 ± 0.17 (2.0 – 2.4) |
44.0 | 280.2 | 162.2 ± 15.8 (144.0 – 181.7) |
57.9 | |
| 9 | 10 | 4.7 ± 0.5 (4.4 – 5.4) |
47.0 | 560.3 | 325.6 ± 33.8 (299.2 – 373.7) |
58.1 | |
| 9 | 20 | 12.8 ± 0.17 (12.6 – 13.0) |
64.0 | 1120.7 | 946.7 ± 29.5 (911.4 – 993.2) |
84.5 | |
| No gel | 9 | 2 | 0.57 ± 0.13 (0.40 – 0.70) |
28.3 | 167.7 | 37.2 ± 8.5 (25.6 – 46.7) |
22.2 |
| 9 | 5 | 1.9 ± 0.18 (1.8 – 2.2) |
38.0 | 419.2 | 130.4 ± 12.1 (116.1 – 146.1) |
31.1 | |
| 9 | 10 | 4.7 ± 0.05 (4.6 – 4.7) |
47.0 | 838.3 | 307 ± 22.8 (256.2 – 336.5) |
36.7 | |
| 9 | 20 | 11.3 ± 0.13 (11.2 – 11.5) |
56.5 | 1676.7 | 761.3 ± 45.8 (711.2 – 823.4) |
45.4 | |
| Gauze inserts | 8 | 2 | 0.55 ± 0.09 (0.40 – 0.60) |
27.5 | 89.3 | 21.8 ± 4.4 (14.2 – 27.8) |
24.5 |
| 12 | 5 | 2.2 ± 0.29 (1.9 – 2.5) |
43.5 | 223.2 | 79.9 ± 18.7 (59.1 – 106.3) |
35.5 | |
| 12 | 10 | 5.1 ± 0.55 (4.7 – 6.0) |
51.0 | 446.3 | 175.5 ± 17.7 (146.4 – 203.4) |
39.3 | |
| 12 | 20 | 13.2 ± 0.75 (12.0 – 13.9) |
65.8 | 1668.0 | 854.8 ± 51.5 (780.0 – 920.2) |
51.2 |
Values presented as mean ± SD (range)
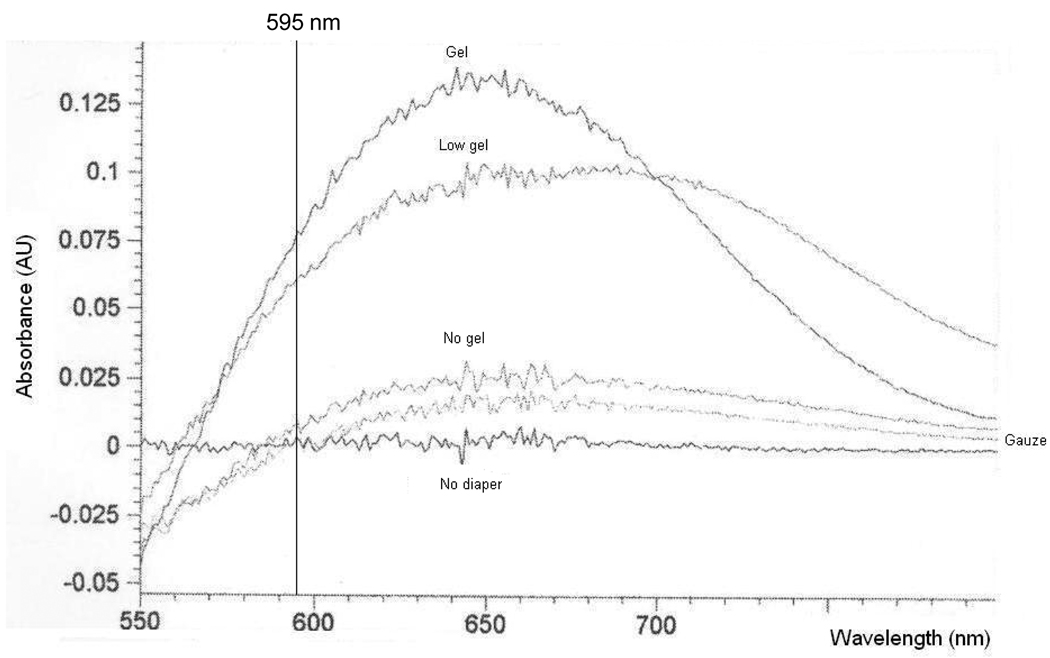
Percent recovery of total urine protein also varied with the type and saturation of the matrix. For diapers containing no gel, protein recovery was consistently lower than volume recovery suggesting that proteins may be lost during the collection and/or extraction process. In contrast, recovery of protein was significantly greater than volume for the gel-based diapers suggesting that gel-based matrices may either concentrate samples or introduce substances (such as the absorbent gel matrix) that interfere with the protein assay. We therefore repeated “protein” recovery experiments using water extracted from each diaper type. The resulting absorbance spectra for each matrix are presented in Figure 1. For all matrices, some interference was noted at 595 nm, the absorbance of the standard protein dye reagent used in the Bradford protein assay. However, the interference was minimal in samples extracted from non-gel based matrices whereas it was highly pronounced in samples from gel-based matrices. Percent “protein” recovery values in the extracted water samples were 85%, 354%, 130% and 158% for the gel, low-gel, no-gel and gauze-insert matrices, respectively.
Figure 1.
Absorbance spectra for buffered water samples extracted from various diaper matrices. The absorbance of the standard protein dye reagent used in the Bradford protein assay (595 nm) is denoted in the Figure.
ii. Confirmation of protein recovery via Western blot
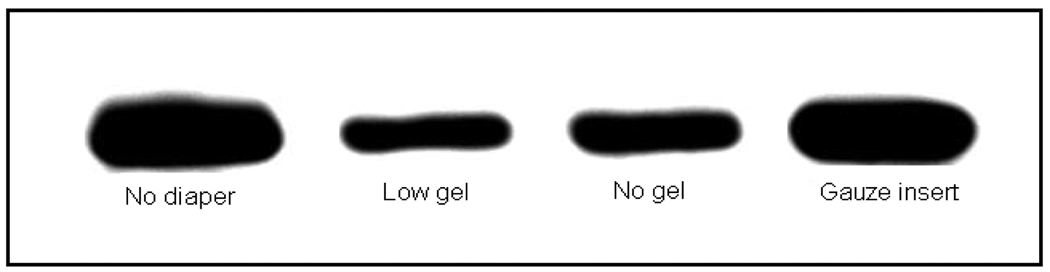
Representative lipocalin type prostaglandin D synthase immunoblots are presented in Figure 2. Band intensities, expressed as a percentage of the pre-diaper sample, differed among diaper matrices and were greatest for the gauze inserts (96.4%) followed by non-gel (69%) and low-gel (50%) containing diapers.
Figure 2.
Representative lipocalin type prostaglandin D synthase immunoblots for various diaper matrices. Blots were performed using rabbit anti-human lipocalcin type prostaglandin D synthase IgG (1:200) as the primary antibody. A total of 10 µg of urine protein was loaded per lane.
iii. Protein separation via 2D-PAGE
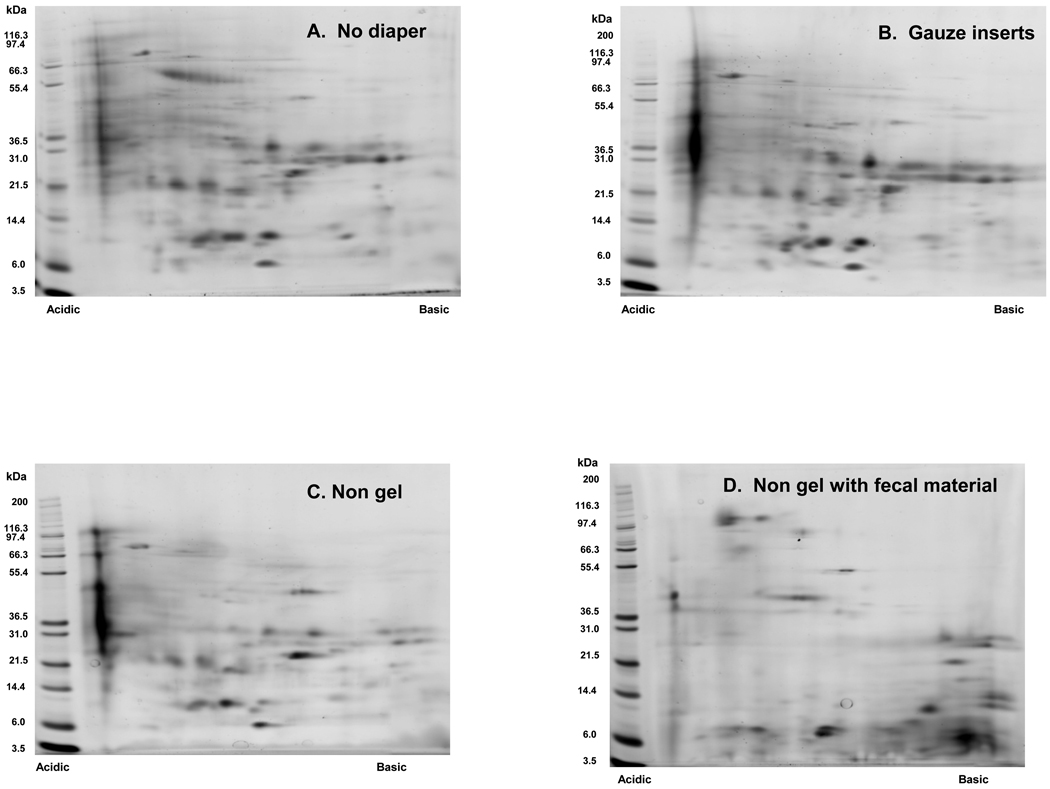
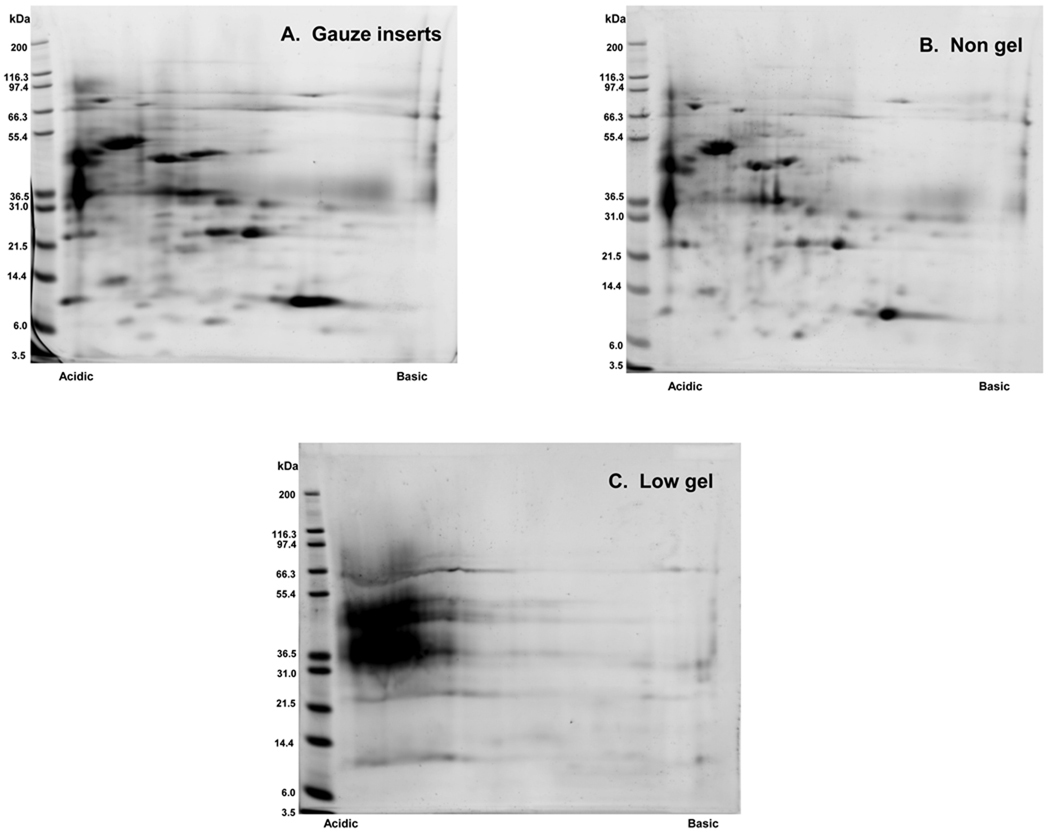
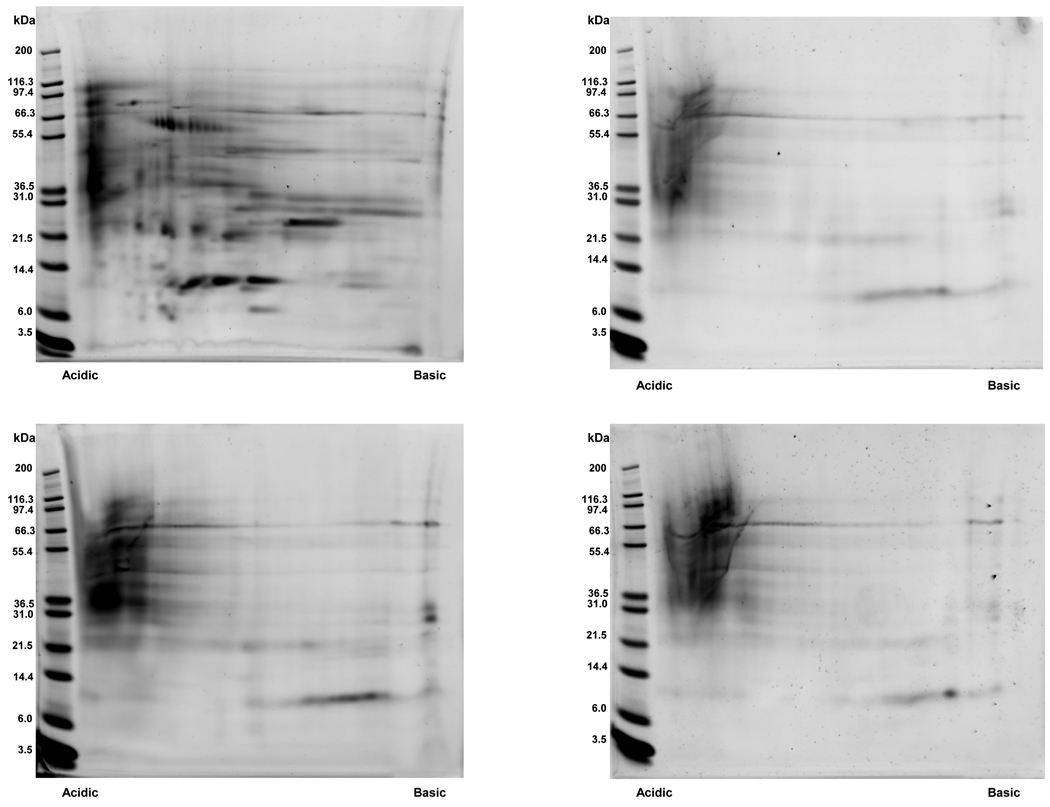
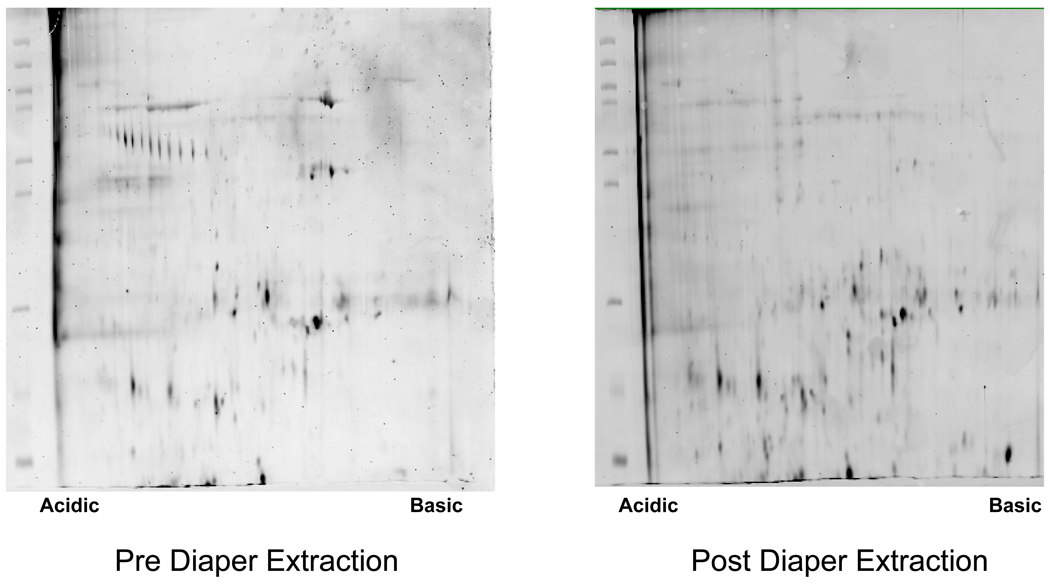
Separation of proteins via 2D-PAGE was achieved using healthy pediatric urine samples obtained pre and post extraction from non-gel (n=4) and gauze insert (n=4) diapers. Representative 2D gel images for each of these matrices are presented in Figure 3. 2D-PAGE was also successfully performed on clinical specimens obtained from a hospitalized newborn infant using non-gel and gauze insert diapers. (Figure 4) In contrast, protein separation was successful in only 1 of 4 healthy pediatric samples extracted from low-gel diapers. (Figure 5) Inadequate protein separation also occurred in the single clinical specimen obtained using a gel-based diaper matrix. (Figure 4) Although separation of proteins was accomplished using urine extracted from a diaper with fecal contamination, an apparent increase in the number of low-molecular weight proteins and/or protein fragments (compared to samples without fecal contamination) was noted on visual inspection of the 2D gel image. (Figure 3) There also appears to be a shift in the isoelectric point of separated proteins as evidenced by an increase in the number of basic species visible on the 2D gel.
Figure 3.
Representative 2D gel images obtained using urine samples collected via (A) Spontaneous void (B) Gauze insert diapers (C) Non-gel diapers and (D) Non-gel diaper with fecal contamination. Total urine protein (75 µg) was loaded onto pre-cast 7 cm IPG strips (pH 3–10 range nonlinear) for isoelectric focusing. Proteins were then separated in the second dimension using 4–12% gradient Bis-Tris NuPAGE® pre-cast slab gels (7 cm). Protein spots were visualized by staining the gels with SYPRO Ruby.
Figure 4.
Representative 2D gel images obtained using urine samples collected from a hospitalized, 28 day old, preterm newborn infant (29 weeks gestation) via (A) Gauze insert diapers (B) Non-gel diapers and (C) Low-gel diapers. Urine collections occurred on 3 consecutive days (over a 3–7 hour period) and a different diaper type was used each day. At the end of the collection period, urine was expressed from non-stool containing diapers and pooled for 2D-PAGE.
Figure 5.
Replicate 2D gel images obtained using urine extracted from low-gel diapers. Healthy pediatric waste urine was applied to the inside surface of low-gel containing diapers and then mechanically expressed using a disposable syringe. A total of 4 replicates were prepared and analyzed via 2D-PAGE.
Using a set of samples obtained pre (n=4) and post (n=4) extraction from non-gel diapers, we were also able to perform 2D-PAGE with large format (18cm) gels. Representative large format gel images are presented in Figure 6. Visual inspection of the pre and post extraction gel images suggests that selective loss of certain protein species, particularly those of higher molecular weights, occurs in samples collected using non-gel diapers
Figure 6.
Representative large format 2D gel images obtained using urine samples collected pre- and post-extraction from non-gel containing diapers. Total urine protein (150 µg) was loaded onto 18 cm IPG strips (pH 4–7 narrow range) for isoelectric focusing. Proteins were then separated in the second dimension using pre-cast 10% Duracryl-Tricine chemistry gels. Protein spots were visualized by staining the gels with SYPRO Ruby.
iv. Within-Matrix Variability for Each Diaper Type
Replicate 2D gels for spontaneous void (n=3), non-gel diaper (n=4) and gauze insert diaper (n=4) samples were used to estimate the extent of variability associated with each collection method. Given that only 1 replicate was completed for the low gel diapers, we were unable to estimate the variability associated with this matrix type. Values for the mean, median and range of CVs for the total group of matched protein spots from each set of replicate gels are presented in Table 2. Samples obtained from non-gel diapers demonstrated the least variability of all collection methods evaluated. Both diaper matrices demonstrated lower variability than that observed with samples collected via spontaneous void.
Table 2.
Normalized Protein Spot Volume Variability in Replicate 2D Gels from Various Urine Collection Methods
| Number of Replicates |
Number of Spot Matches |
Mean CV* (%) |
Median CV* (%) |
Minimum CV* (%) |
Maximum CV* (%) |
|
|---|---|---|---|---|---|---|
| No diaper | 3 | 104 | 41.9 | 35.1 | 1.7 | 124.3 |
| Gauze inserts | 4 | 119 | 33. | 27.4 | 4.3 | 115.3 |
| No gel | 4 | 100 | 25.2 | 20.1 | 1.2 | 123.6 |
Calculated as (SD/mean)*100
v. 2D-PAGE of Diaper-Extracted Buffered Water
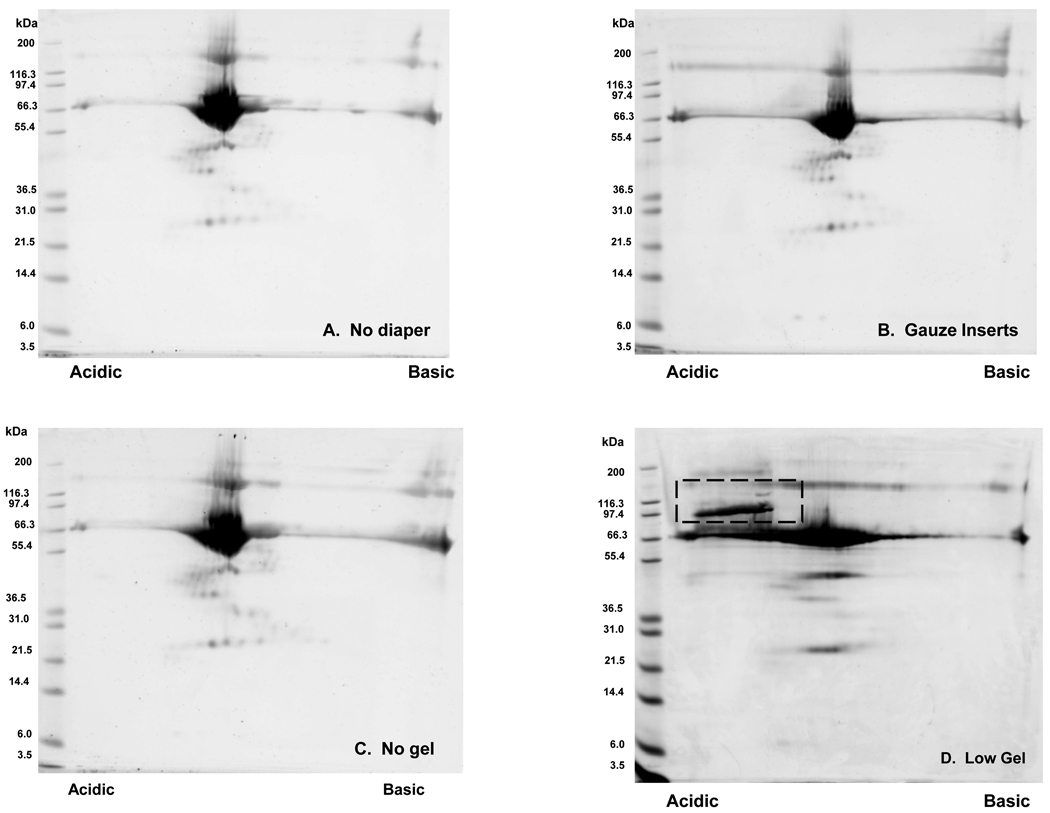
Representative gel images from 2D-PAGE analysis of buffered water (with BSA standard) obtained pre (n=4) and post extraction from low-gel (n=4), non-gel (n=4) and gauze insert (n=4) diapers are presented in Figure 7. Qualitative expression patterns observed in gels from samples obtained post extraction from non-gel and gauze insert diapers are comparable (on visual inspection) to those achieved with non-extracted samples. This suggests that the cotton fiber based matrices do not introduce contaminants that interfere with or are detectable via 2D-PAGE. In contrast, images for the low gel diapers demonstrate an area of intensity in the upper left corner of the gel image that is not present in those images from the other collection methods. Given that these “contaminant” spot(s) appear to be acidic, high molecular weight species, it is likely that they are gel or matrix fiber particles rather than protein degradation products.
Figure 7.
Comparison of 2D gel images obtained using buffered water samples obtained pre- and post-extraction from various diaper matrices. BSA (0.3 mg/mL) was added to all samples prior to extraction as a positive control. Representative gel images from samples obtained (A) pre-extraction and post-extraction from (B) gauze insert, (C) non-gel and (D) low-gel diapers are presented. Qualitative expression patterns observed in gels from samples obtained post-extraction from non-gel and gauze insert diapers appear comparable (on visual inspection) to those achieved with non-extracted samples. For samples extracted from a low-gel containing matrix, however, there is an area of intensity in the upper left corner of the image that is not present images from the other collection methods. This area is denoted with a dashed, rectangular box on the gel image.
4. Discussion
Urinary proteomic profiling has emerged as a potentially useful tool to identify candidate biomarkers of acute and chronic renal disease with several studies to date having successfully applied these techniques in both adult [16,17] and pediatric [18,19] populations. Given the difficulties in obtaining adequate urine samples, however, application of these discovery methods in newborns and infants has been limited. In this proof-of-concept investigation, we demonstrated that small and large platform 2D-PAGE can be successfully performed using urine samples extracted from non-gel containing diaper matrices. The ability to use this routine urine collection method to obtain samples for subsequent proteomic analysis should greatly facilitate use of these techniques in studies of newborns and young children. Collection via disposable diapers will also better enable urinary profiling in elderly adults and other individuals with urinary incontinence.
For all diapers evaluated, it was possible to extract adequate urine volumes and total protein for 2D-PAGE analysis. The extent of volume and total protein recovered was dependent on the amount of urine added and increased with higher degrees of matrix saturation. This is consistent with data from a previous investigation that demonstrated a positive correlation between protein recovery and volume of urine applied per unit weight of absorbent material. [10] In order to optimize sample recovery, the urine collection period should therefore be adjusted to achieve the greatest matrix saturation possible. This is particularly important in newborns and young children who produce relatively small urine volumes within a given time period. It is also an important consideration in patients with renal insufficiency or other etiologies of reduced urine output.
Given differences in the physical and chemical properties of the absorbent matrices, it is not surprising that there were variations in the extent of volume recovery between diaper types. Gel-containing or ultra-absorbent diapers yielded the lowest volume recovery (15%) following addition of the largest urine volume (20 mL). This is consistent with previous investigations that have consistently demonstrated limited ability to extract urine from ultra-absorbent gel diapers. [4–6] It is also anticipated since these diapers contain highly absorbent polyacrylate crystals and were specifically developed to absorb and retain urine. Using salt solutions (e.g., calcium chloride) to collapse the polyacrylate polymer matrix, it may be possible to improve recovery of both urine volume and protein from gel-based diapers. [20] However, altering the salt content of the sample may detrimentally affect the ability to separate urine proteins particularly in the first dimension.
The absorbent gel matrix also appears to introduce substances that interfere with commonly used methods for protein quantitation. Using refractometry, higher specific gravity values have been demonstrated following extraction of urine from diapers containing polyacrylate gel. [3] We observed a similar phenomenon using the Bradford protein assay that determines protein concentrations in a given sample via measurement of absorbance values. Given that both refractometry and spectroscopy results may be affected significantly by the number, mass and chemical structure of dissolved particles it is possible that gel or other matrix particles concomitantly extracted with the urine may be interfering with the analytical assay. This is supported by the absorbance spectra from water extracted samples which demonstrated significant interference in samples from both types of gel-containing diapers at the desired wavelength. In samples extracted from gel-based diapers, estimates of total protein concentrations determined via absorbance-based methods should therefore be interpreted cautiously.
Concomitantly extracted polyacrylate gel particles, which are typically sodium or potassium salts, may also compromise the ability to perform 2D-PAGE since they can substantially increase the salt content of the extracted sample. Given that the amount of co-extracted gel is highly variable, however, it is likely that changes in the salt concentration will be inconsistent among extracted samples making it difficult to optimize/adapt methods for sample processing. Although we were able to successfully perform replicate 2D-PAGE analysis of all buffered water samples (n=4) extracted from low-gel matrices, visual inspection of these gel images indicates the presence of high molecular weight, acidic species that may be gel particles. In contrast, we were able to achieve adequate focusing and protein separation in only 1 of 5 urine samples despite desalting of our samples prior to analysis 2D-PAGE. These biologic samples, unlike buffered water, inherently contain salts that may have been partially retained after sample processing. The salt content of these samples could have, in turn, been further increased by addition of gel particles during the extraction process. Differences in salt content among the extracted urine may therefore have affected our ability to separate proteins in samples obtained from gel-containing diaper matrices.
The process of collecting and extracting urine from diapers containing cotton fiber matrices results in loss of total urine protein in excess of that expected from unrecovered volume. It is likely that similar losses of total protein also occur with the gel-based diapers although they are not readily apparent due to the interference of gel/matrix contaminants with the protein quantitation assay. Visual inspection of large format gel images also suggests that selective loss of certain protein species occurs during the process of urine absorption into and extraction from non-gel containing diaper matrices. Protein loss likely occurs as a consequence of adsorption of the proteins to the fiber matrix and, as evidenced by both protein recovery and immunoblot data, appears to be greater for the non-gel diapers, which contain a bulky cotton matrix, than for the lower bulk cotton gauze inserts. This is consistent with prior data in which differential effects on urine creatinine concentrations were noted following extraction from cotton matrices of varying thicknesses and from rayon fibers. [10] The various absorbent matrices therefore appear to adsorb and retain proteins differently depending on the physical and/or chemical properties of their component materials. For a given matrix, proteins also appear to interact differently with the fiber contents. Consequently, consideration should be given to the proteins of interest and their potential interactions with the diaper matrix prior to collection of samples for proteomic profiling. Investigators should also be aware that when using diapers, low abundant protein species may be completely lost during the urine collection and extraction process.
The goal of this initial pilot investigation was to determine whether 2D-PAGE could be performed on urine samples extracted from diaper matrices. We therefore chose to analyze samples using a small format platform in order to generate initial proof-of-concept regarding feasibility of this sample collection method. Given that limited information regarding qualitative and quantitative protein expression can be obtained from small format gels, we are unable to definitively comment on changes in urine proteins that may have occurred during the collection and/or extraction process. Based on visual inspection of gel images from diaper-extracted buffered water samples, however, it appears that the cotton-fiber matrices do not introduce substances that can be visualized on 2D gels. In contrast, presence of fecal material appears to significantly contribute to and alter in-gel protein abundance. It is unknown whether similar changes in protein quality (e.g., degradation) also occur as a result of the urine collection process itself and this will need to be examined in subsequent investigations using higher quality (i.e., large format) gel analyses.
We conclude that 2D-PAGE may be performed on urine samples extracted from non-gel containing, cotton fiber diapers. Certain protein species, however, may be selectively lost or concentrated by the fiber matrix. Different matrices will also adsorb and retain proteins differently depending on their physical and/or chemical properties. All samples in a given investigation should therefore be collected using the same matrix type. Selection of a non-gel diaper for sample collection should also be based on the patient population to be studied, the goal of the planned experiments (global profiling vs. investigation of specific proteins), the protein species of interest and their potential interactions with the specific diaper matrix. Before this collection method can be used for proteomic profiling, however, qualitative and quantitative analyses of resolved protein spots using replicate high resolution (large format) gel images are both necessary and warranted.
Acknowledgements
We gratefully acknowledge Elizabeth McDowell, R.N., Soumya Niranjan, B.S. and Gordon Stout, B.S. for their assistance with sample preparation and Michelle Barati, Ph.D., Danny Wilkey, B.S., and Janice Scherzer, M.S., for their assistance with sample and data analysis.
Supported in part by grant numbers #5 U10 HD045934-04 (Network of Pediatric Pharmacology Research Units) and 1R21HD050564-01A1 from the National Institute of Child Health and Human Development, Bethesda, Maryland.
List of abbreviations used
- 2D-PAGE
2-dimensional polyacrylamide gel electrophoresis
- BSA
bovine serum albumin
- IPG
immobilized pH gradient
Footnotes
Conflict of Interest Statement
The authors have no financial/commercial conflicts of interest to declare.
References
- 1.Kennedy MJ, Merchant M, Griffin A, DeVillez A, Klein J. Urinary proteomic profiling identifies differentially expressed proteins in aminoglycoside-treated children with and without nephrotoxicity. J Am Soc Nephrol. 2006;17:405A. [Google Scholar]
- 2.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grobskopf LA, Levine GL, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821–827. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 3.Kirkpatrick JM, Alexander J, Cain RM. Recovering urine from diapers: Are test results accurate? Am J Matern Child Nurs. 1997;22:92–102. doi: 10.1097/00005721-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad T, Vickers D. Urine collection from disposable nappies. Lancet. 1991;338:674–676. doi: 10.1016/0140-6736(91)91242-m. [DOI] [PubMed] [Google Scholar]
- 5.Burke N. Alternative methods for newborn urine sample collection. Pediatr Nurs. 1995;21:546–549. [PubMed] [Google Scholar]
- 6.Muratore C, Dhanireddy R. Urine collection for disposable diapers in premature infants: Biochemical analysis. Clin Pediatr. 1993;32:314–315. doi: 10.1177/000992289303200516. [DOI] [PubMed] [Google Scholar]
- 7.Beeram MR, Dhanireddy R. Urinalysis: Direct versus diaper collection. Clin Pediatr. 1991;30:278–280. doi: 10.1177/000992289103000502. [DOI] [PubMed] [Google Scholar]
- 8.Belmin J, Hervias Y, Avellano E, Oudart O, Durand I. Reliability of sampling urine from disposable diapers in elderly incontinent women. J Am Geriatr Soc. 1993;41:1182–1186. doi: 10.1111/j.1532-5415.1993.tb07300.x. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane PI, Ellis R, Hughes C, Houghton C, Lord R. Urine collection pads: are samples reliable for urine biochemistry and microscopy? Pediatr Nephrol. 2005;20:170–179. doi: 10.1007/s00467-004-1709-4. [DOI] [PubMed] [Google Scholar]
- 10.Mock DM. Rayon balls and disposable-diaper material selectively adsorb creatinine. Am J Clin Nutr. 1992;55:326–330. doi: 10.1093/ajcn/55.2.326. [DOI] [PubMed] [Google Scholar]
- 11.Smith GC, Taylor CM. Recovery of protein from urine specimens collected in cotton wool. Arch Dis Child. 1992;67:1486–1487. doi: 10.1136/adc.67.12.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Barr DB, Akland G, Melnyk L, et al. Collecting urine samples from young children using cotton gauze for pesticide studies. J Expo Anal Environ Epidemiol. 2000;10:703–709. doi: 10.1038/sj.jea.7500134. [DOI] [PubMed] [Google Scholar]
- 13.Thongboonkerd V, Chutipongtanate S, Kanlaya R. Systematic evaluation of sample preparation methods for gel-based human urinary proteomics: quantity, quality and variability. J Proteome Res. 2006;5:183–191. doi: 10.1021/pr0502525. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SM, Thomas MR, Sebastian LT, Pedersen SK, Harcourt RL, Sloane AJ, et al. Optimal replication and the importance of experimental design for gel-based quantitative proteomics. J Proteome Res. 2005;3:809–819. doi: 10.1021/pr049758y. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson JS, Ceasar R, Glasbey CA, Blomberg A, Rudemo M. Statistical exploration of variation in quantitive two-dimensional gel electrophoresis data. Proteomics. 2004;4:3791–3799. doi: 10.1002/pmic.200300824. [DOI] [PubMed] [Google Scholar]
- 16.Otu HH, Spentzos D, Nelson RG, Hanson RL, Looker HC, et al. Prediction of diabetic nephropathy using urine proteomic profiling 10 years prior to development of nephropathy. Diabetes Care. 2007;30:638–643. doi: 10.2337/dc06-1656. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khurana M, Traum AZ, Aivado M, Wells MP, Guerrero M, et al. Urine proteomic profiling of pediatric nephrotic syndrome. Pediatr Nephrol. 2006;21:1257–1265. doi: 10.1007/s00467-006-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, et al. Neutrophil gelatinase-associated lipocalcin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. 205. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Beach J, Raymer J, Gardner M. Disposable diaper to collect urine samples from young children for pyrethroid pesticide studies. J Expo Anal Environ Epidemiol. 2004;14:378–384. doi: 10.1038/sj.jea.7500334. [DOI] [PubMed] [Google Scholar]