Abstract
Rationale
We previously reported that mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is necessary for oxidized phospholipids to induce monocyte chemoattractant protein-1 (MCP-1) secretion by human aortic endothelial cells. We also reported that inhibition of tyrosine phosphatases including MKP-1 ameliorated atherosclerotic lesions in mouse models of atherosclerosis.
Objective
This study was conducted to further investigate the specific role of MKP-1 in atherogenesis.
Methods and Results
We generated MKP-1−/−/apoE−/− double-knockout mice. At 24 weeks of age, the size, macrophage and dendritic cell content of atherosclerotic lesions of the aortic root were significantly lower (~-41% for lesions and macropahges, and ~-78% for dendritic cells) in MKP-1−/−/apoE−/− mice when compared with apoE−/− mice. Total cholesterol (−18.4%, p=0.045) and very low-density lipoprotein (VLDL)/ low-density lipoprotein (LDL) (-20.0%, p=0.052) cholesterol levels were decreased in MKP-1−/−/apoE−/− mice. Serum from MKP-1−/−/apoE−/− mice contained significantly lower levels of MCP-1 and possessed significantly reduced capability to induce monocyte migration in vitro. Moreover, peritoneal macrophages isolated from MKP-1−/−/apoE−/− mice produced significantly lower levels of MCP-1 when compared to peritoneal macrophages from apoE−/− mice. Furthermore, MKP-1−/−/apoE−/− mice had significantly reduced serum hydroxyeicosatetraenoic acids (HETEs) levels, which have been reported to induce MCP-1 levels.
Conclusions
Our results demonstrate that MKP-1 deficiency significantly decreases atherosclerotic lesion development in mice, in part, by affecting MCP-1 levels in the circulation and MCP-1 production by macrophages. MKP-1 may serve as a potential therapeutic target for the treatment of atherosclerotic disease.
Keywords: mitogen-activated protein kinase phosphatase-1, atherosclerosis, monocyte chemoattractant protein-1, monocytes
Atherosclerosis is characterized by the accumulation of lipids, monocytes/macrophages, injured endothelium, and proliferation of smooth muscle cells. The formation of atherosclerosis is initiated by the adherence and migration of circulating monocytes to the activated endothelial layer, followed by penetration into the subendothelial space [1]. After the migration of monocytes into the atheroma, they further differentiate into macrophages or dendritic cells. Oxidized low-density lipoprotein (ox-LDL) plays an important role in the accumulation of monocyes/macrophages/dendritic cells in the atherosclerotic plaque [2, 3]. Using suppression subtractive hybridization (SSH) procedure, we identified several genes including MAP kinase phosphatase-1 (MKP-1) whose expression is induced by oxidized-L-a-1-palmitoyl-2-arachidonyl-sn-glycero-phosphorylcholine (ox-PAPC) in human aortic endothelial cells (HAEC) [4]. Ox-PAPC, a mixture of phospholipid oxidation products, contains the major bioactive components of minimally modified LDL (mm-LDL) [5]. In a previous study, we observed that MKP-1 is necessary for ox-PAPC induced monocyte chemoattractant protein-1 (MCP-1; that is also known as Chemokine (C-C motif) ligand 2 (CCL2)) secretion and monocyte chemotactic activity by HAEC [6]. MKP-1 belongs to a member of phosphatases known as dual-specificity phosphatases [7]. Dual-specificity phophatases can dephosphorylate both serine/threonine and tyrosine residues, thereby they are responsible for deactivation of MAP kinases [7].
Recent studies have demonstrated that MKP-1 plays an essential role to regulate the macrophage proliferation and activation [8, 9]. Recently, two subsets of mouse monocytes have been identified [10–13]. One subset is CCR2+ Ly6Chigh CX3CR1low monocytes and characterized by recruitment to inflamed tissue utilizing MCP-1, and termed inflammatory monocytes. Another subset is CCR2− Ly6Clow CX3CR1high monocytes which are found in both normal and inflamed tissue, and the latter have been termed resident monocytes. Ly6Chigh monocytes are able to differentiate into cells that up-regulate the dendritic cell marker CD11c in inflammatory conditions [12]. When dendritic cells are activated in the atherosclerotic artery, they present antigen to T cells that leads to an inflammatory response [14].
Derfman et al. reported that MKP-1 KO mice were fertile and had no phenotypic abnormalities [15], probably because other phosphatase activity can compensate for MKP-1 activity. However, these mice were not subjected to oxidative stress. We have shown that MKP-1 protein is expressed in mouse atherosclerotic lesions [16]. MKP-1 expression correlated with hypercholesterolemia and lesion development in mouse models of atherosclerosis. We previously reported that inhibition of tyrosine phosphatase activity including MKP-1 with the tyrosine phosphatase inhibitor sodium orthovanadate (NaOV) reduced the development of atherosclerotic lesions in a mouse model of atherosclerosis [16]. However, NaOV is a nonspecific inhibitor and inhibits not only MKP-1 but also other phosphatase activity. As NaOV inhibits a wide range of phosphatase activity, it is possible that NaOV also inhibits other MKPs that compensate for MKP-1 activity, and therefore these studies did not prove that MKP-1 alone was responsible for the decreased lesions.
In this study, in order to elucidate the specific role of MKP-1 in atherosclerosis development and its relationship with monocytes/macrophages/dendritic cells in atherosclerosis, we intercrossed the MKP-1−/− with hyperlipidemic apoE−/− mice, which develop spontaneous atherosclerotic lesions, and we determined the role of MKP-1.
Methods
For more details, see the expanded Material and Methods section in the online data supplement.
Generation of MKP-1−/−/apoE−/− Mice
MKP-1−/− mice on a C57BL/6J background were generously provided by Lexicon Genetics Incorporated. These mice were then crossed with apoE−/− mice on a C57BL/6J background obtained from Jackson Labs (Bar Harbor, ME) to generate MKP-1−/−/apoE−/− mice. MKP-1+/+/apoE−/− mice were used as controls. All mice were maintained on a standard chow diet for 24 weeks before sacrifice. At the end of the study, mice were fasted overnight, and blood and organs were collected. The Animal Research Committee at UCLA approved all animal protocols.
Serum lipid analysis
Serum lipids were determined as described previously [17].
Genotyping
Genotyping was performed by PCR analysis using specific primers [18, 19].
Aortic root lesion analysis
Atheroma formation in the aortic sinus of mice was analyzed as previously described [19].
Immunohistochemical staining of aortic lesions
To detect macrophage, Ly6C positive cells and dendritic cells, additional cryosections from the proximal aorta were incubated with a monoclonal rat antibody against mouse CD68 (AbD Serotec), biotinylated monoclonal rat antibody against mouse Ly6C (BD Biosciences), or monoclonal hamster antibody against mouse CD11c (BD Biosciences).
En face lesion analysis
En face analyses of lesions in the entire aorta were performed according to procedures described by Tangirala et al [20].
Measurement of MCP-1, TNF-α, IL-1β, and IL-10
Serum MCP-1 (R&D Systems), TNF-α (Assay Designs), IL-1β (Assay Designs), and IL-10 (BioLegend) were measured by ELISA kit according to the manufacturer’s instructions.
Sample preparation for LC/MS/MS
A 100uL volume of serum sample was transferred to a 2uL polypropylene tube, hydrolyzed with 1mol/L potassium hydroxide for analysis of total hydroxyeicosatetraenoic acids (HETEs) (free + esterified), and spiked with 100uL of internal standards (15(S)-HETE-d8, 10ng/ml) (Cayman Chemicals) in methanol. HETEs were extracted using Oasis HLB solid-phase extraction (SPE) cartridge (Waters).
LC/MS/MS analysis
Liquid chromatography-tandem mass spectrometry (LC/MS/MS) was performed using a quadruple mass spectrometer (4000 QTRAP; Applied Biosystems, Foster City, CA) equipped with electrospray ionization (ESI) source. Chromatography was performed using a Luna C-18(2) column (3um particle, 150 × 3.0mm; Phenomenex) with a security guard cartridge (C-18; Phenomenex) at 40°C. Detection was accomplished by using the multiple reaction monitoring (MRM) mode with negative ion detection.
Flow Cytometry
After red blood cells (RBC) were lysed, peripheral blood and bone marrow cells were filtered and incubated for 30 min on ice with the following purified PE-, APC-, APC-Cy7, FITC- and PerCP-Cy5.5- conjugated antibodies: CD115, CD11b, CD11c, MHC-II and Ly6C. Cells were washed twice prior to analysis on the BD LSR-II Flow Cytometer or prior to sorting on the BD FACSAriaII High-Speed Cell Sorter (BD Bioscience, San Jose, CA). Data were analyzed with FlowJo (TreeStar, Ashland, OR) and BD FACSDiva (BD Bioscience, San Jose, CA) software.
Monocyte chemotaxis bioassay
MKP-1−/−/apoE−/− and control apoE−/− mouse serum induced monocyte chemotactic activity were assayed as previously described [21] with some modifications. Chemotactic activity of mouse monocytes was assayed as follows. Monocytes were isolated by flow cytometry as CD11b+ CD115+ cells. 10ng/ml of MCP-1 was added to a standard Neuroprobe chamber (Neuro Probe, Inc.), with isolated mouse peripheral blood monocytes added to the top chamber. The chamber was incubated for 4 hours at 37°C and monocyte migration was determined as described for human monocytes in Materials and Methods section in the online data supplement.
Quantitative RT-PCR Analysis
Total RNA from the aortic arch of both apoE−/− and MKP-1−/−/apoE−/− mice was isolated by RNeasy mini kit (Qiagen) and 0.1 ug RNA was used for reverse transcription with a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). 2ul of the cDNA was used for PCR reaction with gene specific primers and iQ SYBR Green Supermix (BIO-RAD) in a MyiQ Single-Color Real-Time PCR Detection System (BIO-RAD). Quantification of mRNA included normalization to mouse cyclophilin levels.
Statistical analysis
All the data were expressed as means ± SD. Differences between groups were statistically analyzed by T-test. Significance was determined as p<0.05.
Results
MKP-1−/−/apoE−/− mice
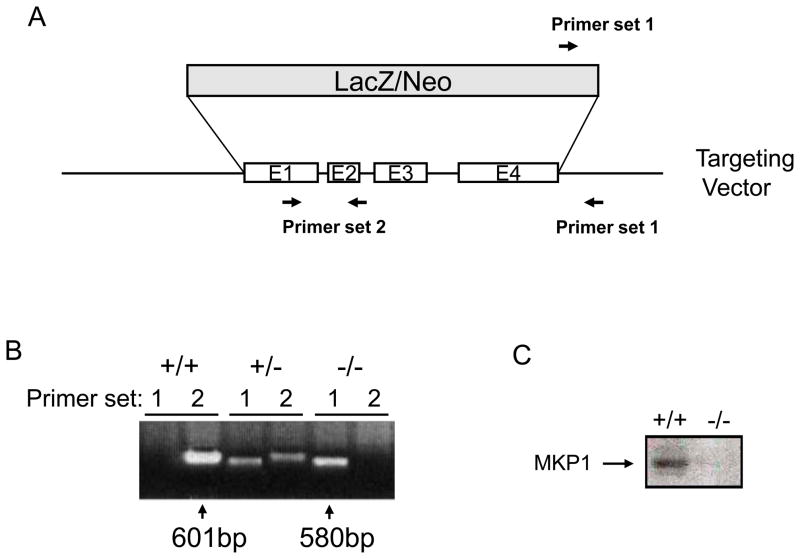
MKP-1−/− mice were produced by targeted disruption of exon 1 to 4 of the MKP-1 gene (Figure 1A). PCR analysis confirmed a deletion of the MKP-1 gene in MKP-1−/−/apoE−/− mice but not in control apoE−/− mice (Figure 1B). We detected no MKP-1 protein in peritoneal macrophages from MKP-1−/−/apoE−/− mice by Western blotting (Figure 1C).
Figure 1.
MKP-1−/−/apoE−/− mice are deficient in MKP-1. (A) For gene targeting of the MKP-1 locus, exon 1–4 was disrupted by insertion of a LacZ/Neo gene. (B) PCR analysis of DNA isolated from apoE−/− and MKP-1−/−/apoE−/− mice using primer set 1, 2 as indicated in (A). (C) Immunoblotting of MKP-1 showed the lack of immunoreactive MKP-1 protein in the peritoneal macrophage of MKP-1−/−/apoE−/− mice.
Lipid parameters
There was no significant difference in fasting levels of HDL cholesterol, triglyceride, fatty acids, glucose, and unesterified cholesterol between apoE−/− mice and MKP-1−/−/apoE−/− mice. MKP-1−/−/apoE−/− mice exhibited lower levels of total cholesterol (538.1±80.0 versus 438.9±145.8, −18.4%, p=0.045) and VLDL/LDL cholesterol (509.6±68.4 versus 407.9±12-.0,−20.0%, p=0.052) compared with their counterparts (Table 1).
Table 1.
Lipid profile
| apoE−/− | MKP-1−/−/apoE−/− | p value | |
|---|---|---|---|
| Total cholesterol | 538.1 ± 80.0 | 438.9 ± 145.8 | 0.045* |
| VLDL/LDL cholesterol | 509.6 ± 68.4 | 407.9 ± 120.0 | 0.052 |
| HDL cholesterol | 21.6 ± 6.0 | 24.0 ± 19.6 | 0.682 |
| Triglyceride | 34.5 ± 27.9 | 34.6 ± 30.5 | 0.989 |
| Free fatty acids | 33.2 ± 10.2 | 30.2 ± 8.0 | 0.425 |
| Glucose | 182.9 ± 56.7 | 172.2 ± 38.7 | 0.583 |
| Unesterified cholesterol | 154.2 ± 22.0 | 130.1 ± 41.0 | 0.078 |
| Weight (g) | 23.3 ± 3.8 | 21.0 ± 2.0 | 0.072 |
Lipid and glucose measurements in mg/dl for apoE−/− (n=13) and MKP-1−/−/apoE−/− (n=13) female mice.
Reduced atherosclerotic lesion area
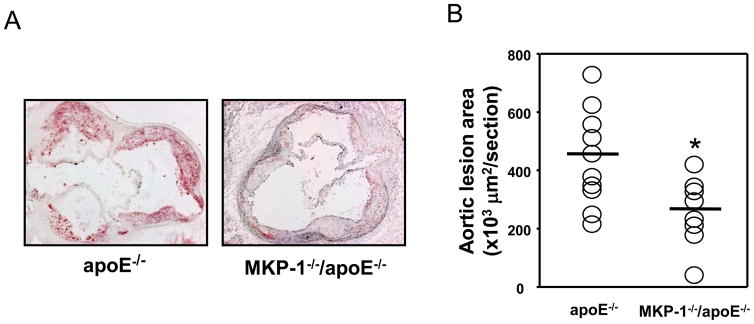
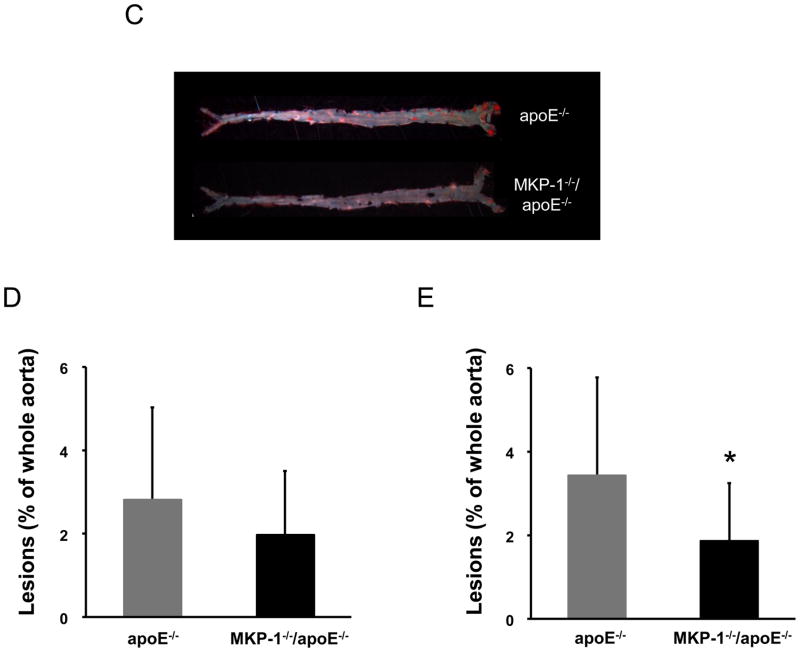
After 24 weeks on a regular chow diet, MKP-1−/−/apoE−/− mice showed significantly smaller atherosclerotic lesion area (453.911 ± 165.258 versus 269.505 ± 117.198, −40.6%, p=0.0017) (Figure 2A, 2B). Although female MKP-1−/−/apoE−/− mice showed 30.6% less en face lesions along the whole aorta compared to apoE−/− mice, this difference was not statistically significant (Figure 2C, 2D). However, when we increased the number of mice examined by including both male and female mice, MKP-1−/−/apoE−/− mice showed significantly less en face lesions along the whole aorta compared to apoE−/− mice (Figure 2E).
Figure 2.
MKP-1−/−/apoE−/− mice develop significantly smaller atherosclerotic lesions compared to apoE−/− mice. apoE−/− and MKP-1−/−/apoE−/− mice were placed on chow diet for 24 weeks, euthanized, and sections for mouse aorta were analyzed by Oil red O staining for the lesion area (as described in Materials and methods). En face staining of lesions was also performed. (A) Representative sections of mouse aortic roots, stained with Oil red O. (B) Quantitative analysis of lesion area. (female; n=10 for apoE−/− mice, n=8 for MKP-1−/−/apoE−/− mice) (C) Representative images of the whole aorta by en face method. (D). Quantitative analysis of en face assay in MKP-1−/−/apoE−/− female mice (n=10 for apoE−/− mice, n=8 for MKP-1−/−/apoE−/− mice). (E) Quantitative analysis of en face assay in MKP-1−/−/apoE−/− male+female mice. (n=18 for apoE−/− mice, n=14 for MKP-1−/−/apoE−/− mice) *p<0.05
Reduced macrophage content in the aortic root
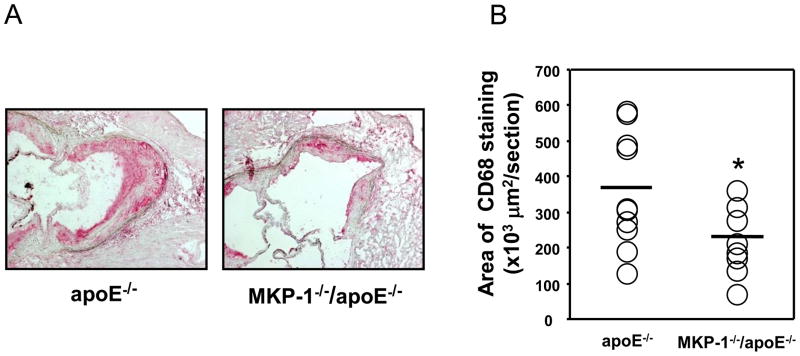
Quantitative analysis of the proximal aorta macrophage content after 24 weeks on a regular chow diet, as determined by immunohistochemistry using the monoclonal antibody against mouse macrophage CD68, revealed that the amount of macrophage staining in MKP-1−/−/apoE−/− mice was significantly reduced by 38.1% compared with control apoE−/− mice (Figure 3A, 3B).
Figure 3.
MKP-1−/−/apoE−/− mice accumulate significantly lower number of macrophage in atherosclerotic lesions compared to apoE−/− mice. apoE−/− and MKP-1−/−/apoE−/− mice were placed on chow diet for 24 weeks, euthanized, and sections for mouse aorta were analyzed by immunohistochemistry for the presence of macrophages using CD-68 antibody (as described in Materials and methods). (A) Representative image of macrophage content in atherosclerotic plaques. (B) Quantitative analysis of macrophage content. (female; n=10 for apoE−/− mice, n=8 for MKP-1−/−/apoE−/− mice) *p<0.05
Cytokines
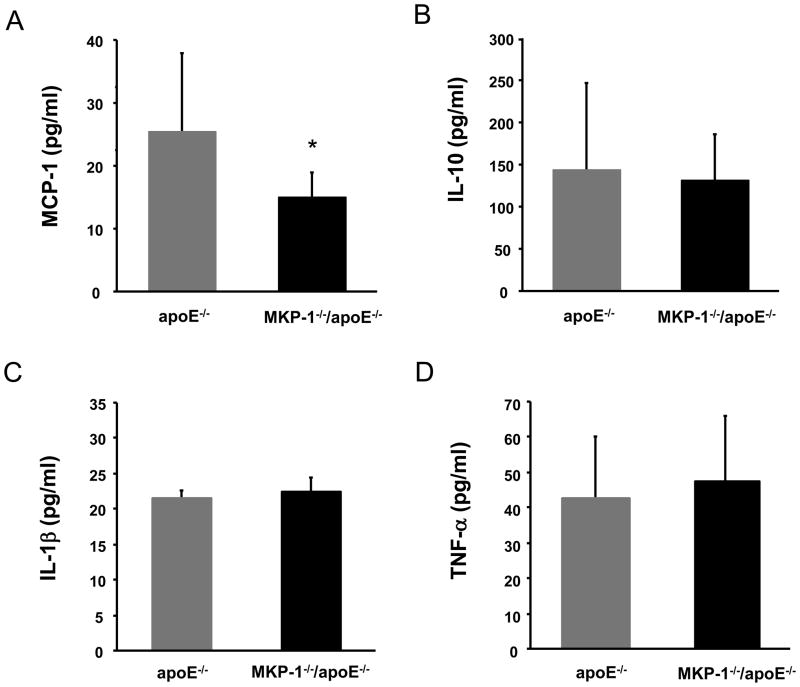
It is known that MAP kinases play pivotal roles in the regulation of the host inflammatory response, and MKP-1 has crucial role in the regulation of cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-10 in vivo [22]. We previously reported that MKP-1 is necessary for HAEC to produce MCP-1 in response to oxidized phospholipids. Inhibition of MKP-1 using either the phosphatase inhibitor NaOV or antisense oligonucleotides prevented the accumulation of MCP-1 protein in the supernatants of ox-PAPC treated HAEC [6]. To elucidate the reason of atherosclerosis suppression in MKP-1−/−/apoE−/− mice, we measured the cytokines that are related to the inflammatory process. We found that baseline TNF-α, IL-1β, IL-10 levels did not significantly differ from control apoE−/− mice. However, the baseline MCP-1 level was significantly lower in MKP-1−/−/apoE−/− mice than control mice (Figure 4).
Figure 4.
Cytokine levels of apoE−/− and MKP-1−/−/apoE−/− mice. Serum cytokine levels were determined by ELISA after 24 weeks on chow diet. (A) Serum MCP-1 levels. (B) Serum IL-10 levels. (C) Serum IL-1β levels. (D) Serum TNF-α levels. (n=9-15 for apoE−/− mice, n=6-7 for MKP-1−/−/apoE−/− mice) *p<0.05
Reduced Ly6C positive area and dendritic cells in MKP-1−/−/apoE−/− mice
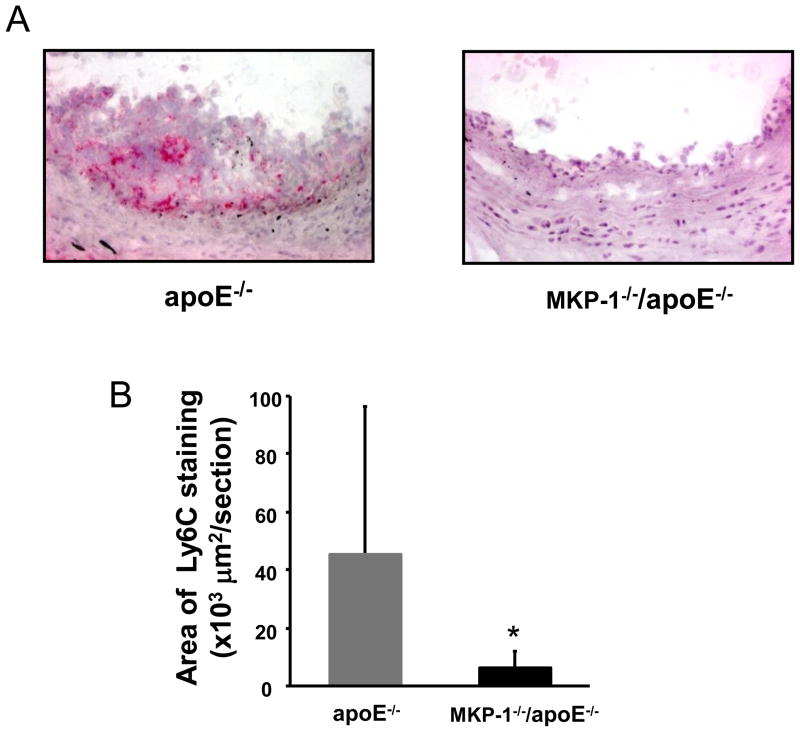
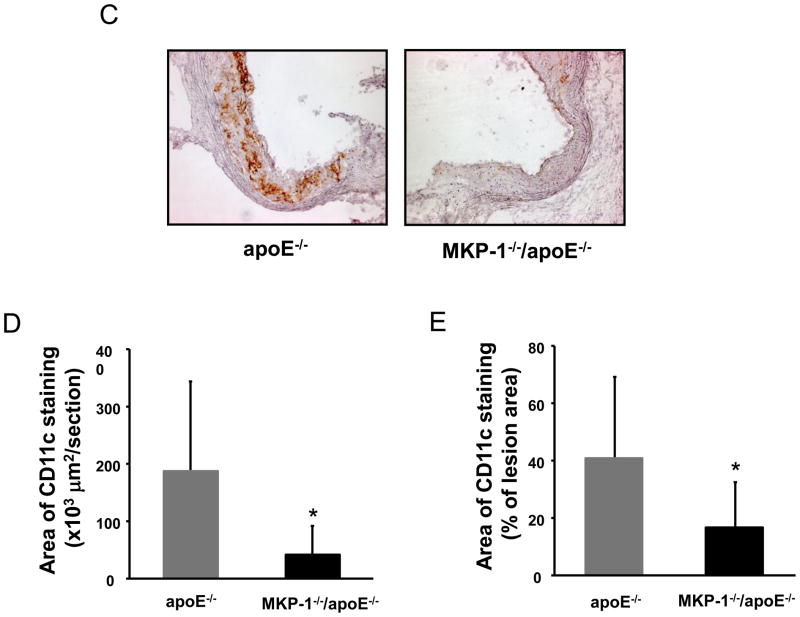
Circulating monocytes migrate into atherosclerotic lesions, where they differentiate into either macrophages or dendritic cells. Ly6Chigh monocytes which are very sensitive to MCP-1, become lesion macrophages significantly more frequently than Ly6Clow monocytes [23]. As the serum MCP-1 was decreased in MKP-1−/−/apoE−/− mice compared to apoE−/− mice, we determined the Ly6C positive area of atherosclerotic lesions by immunostaining. Ly6C positive area in aortic roots was significantly (−86.0%) lower in MKP-1−/−/apoE−/− mice compared with apoE−/− mice (Figure 5A, 5B). Recent studies indicate that Ly6Chigh monocytes are able to differentiate into cells that upregulate the dendritic cell marker CD11c in inflammatory conditions. When we stained the aortic root for CD11c, there was a significant reduction (−77.7%) of CD11c positive area in MKP-1−/−/apoE−/− mice compared with apoE−/− mice (Figure 5C, 5D). CD11c positive area as a percent of plaque area in aortic roots was also significantly lower in MKP-1−/−/apoE−/− mice compared with apoE−/− mice (Figure 5E).
Figure 5.
MKP-1−/−/apoE−/− mice contain significantly decreased Ly6C positive area and reduced dendritic cell content in atherosclerotic lesions. apoE−/− and MKP-1−/−/apoE−/− mice were placed on chow diet for 24 weeks, euthanized, and sections for mouse aorta were analyzed by immunohistochemistry for the presence of Ly6C positive cells and dendritic cells using Ly6C and CD11c antibody (as described in Materials and methods). (A) Representative sections of mouse aortic roots, stained with Ly6C antibody. (B) Quantitative analysis of Ly6C positive area. (C) Representative sections of mouse aortic roots, stained with CD11c. (D) Quantitative analysis of CD11c positive area. (E) The aortic root CD11c stained area was express as a percentage of the area of Oil red O in each mouse. (female; n=10 for apoE−/− mice, n=8 for MKP-1−/−/apoE−/− mice) *p<0.05
FACS analysis
MKP-1 expression is rapidly induced in bone-marrow-derived macrophages by macrophage colony-stimulating factor (M-CSF) [9]. M-CSF contributes to the monocyte differentiation, proliferation, and mobilization. We hypothesized that a change in monocyte/macrophage/dendritic cell number, differentiation, or activation may be key factors in the suppression of atherosclerotic lesions in MKP-1−/−/apoE−/− mice. We analyzed the types and numbers of these cells in peripheral blood and bone marrow from MKP-1−/−/apoE−/− mice and control apoE−/− mice with the aim of identifying MKP-1−/− induced changes using flow cytometric analyses. Similar amounts of Ly6Chigh inflammatory monocytes and total monocytes (including Ly6Chigh and Ly6Clow monocytes) were quantified in the blood (Supplemental Figure 1A and 1B). We also analyzed bone marrow Ly6Chigh monocytes which are the source for peripheral blood monocytes [11]. However, there was no statistical difference in the proportion of Ly6Chigh monocytes in the blood of MKP-1−/−/apoE−/− and apoE−/− mice (Supplemental Figure 1C). The total number of dendritic cells represented by CD11c+ cells [24] (Supplemental Figure 1D), mature dendritic cells represented by CD11c+ MHCII+ cells [24] (Supplemental Figure 1E) and polymorphonuclear neutrophils (PMN) (data not shown) also were not different in the peripheral blood of MKP-1−/−/apoE−/− and apoE−/− mice.
Serum HETEs
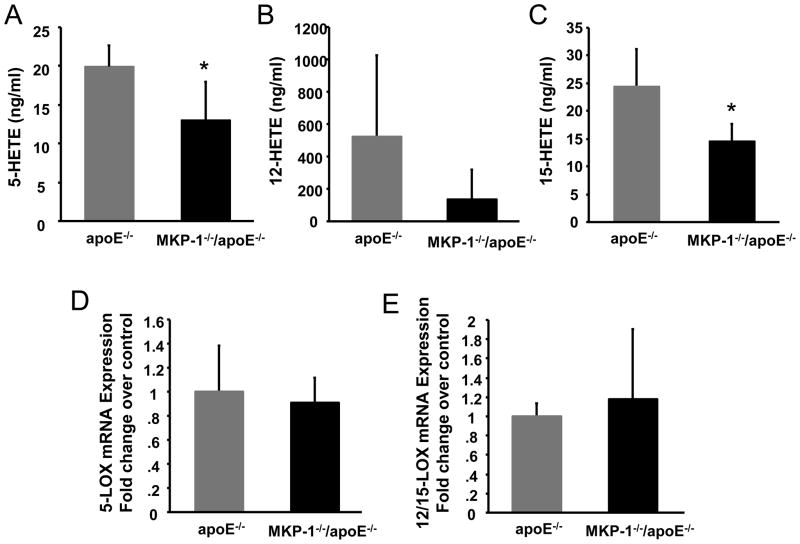
Monocytes, macrophages and dendritic cells are a major source of 12/15-lipoxygnease (LOX) and 5-LOX. 12/15-LOX is implicated in the oxidative modification of LDL. The 5-LOX pathway is responsible for the biosythesis of leukotrienes (LTs), inflammatory mediators implicated in a number of inflammatory diseases including atherosclerosis. Both 12/15-LOX-deficient apoE−/− mice and 5-LO-deficient Ldlr−/− mice showed reduced atherosclerotic lesions, thus the 12/15-LOX pathway and 5-LOX pathway have been implicated in atherogenesis. 12-HETE and 15-HETE, which are major metabolites of the 12/15-LOX pathway increased MCP-1 mRNA and protein expression in a mouse macrophage cell line [25]. On the other hand, recent evidence indicated that MCP-1 receptor CCR2 deficient mice have reduced ox-LDL induced lipid body assembly and LT release [26]. We therefore measured serum HETEs including 12-HETE, 15-HETE, and 5-HETE. Serum 15-HETE, and 5-HETE levels were significantly decreased in MKP-1−/−/apoE−/− compared to apoE−/− mice (Figure 6A, 6C). Although the serum 12-HETE level was 73.4% reduced in MKP-1−/−/apoE−/− mice, it was not statistically significant because of the large variation in apoE−/− mice (Figure 6B). Levels of plasma 9-HETE, 20-HETE, 9-HODE, oxidized lipids produced by different pathways from12/15-LOX and 5-LOX, were not different between MKP-1−/−/apoE−/− mice and controls (data not shown). Next, we measured the 12/15-LOX and 5-LOX mRNA expression in the aortic arch of MKP-1−/−/apoE−/− and apoE−/− mice. However, there was no difference of mRNA expression between the two groups (Figure 6D, 6E). We also examined the mRNA expression level of VCAM-1 and ICAM-1 in the aortic arch, and there was no difference between the groups (Supplemental Figure 2).
Figure 6.
MKP-1−/−/apoE−/− mice have decreased levels of serum 12/15-Lipoxygenase and 5- Lipoxygenase products, 15-HETE and 5-HETE, in vivo. (A.B.C.) Serum from apoE−/− and MKP-1−/−/apoE−/− mice were collected at the end of the study, and lipids were extracted from the serum using solid phase extraction column. HETEs were measured by LC/MS/MS as described in Materials and Methods. (female; n=5 for apoE−/− mice, n=5 for MKP-1−/−/apoE−/− mice) *p<0.05 (D.E.) Quantitative RT-PCR data from aortic arch RNA of these mice, performed with primer specific for the gene encoding mouse 12/15-LOX and 5-LOX. mRNA was quantified using SYBR green and real-time PCR. The specific mRNA product was normalized to cyclophilin. (n=3 for apoE−/− mice, n=4 for MKP-1−/−/apoE−/− mice).
Monocyte chemotactic activity
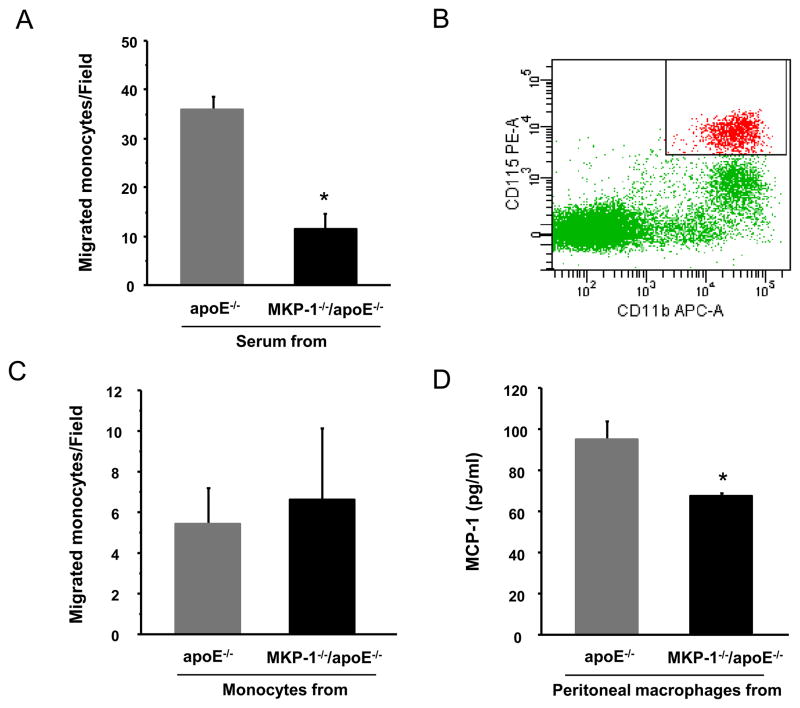
We assessed monocyte chemotactic activity of mouse serum from MKP-1−/−/apoE−/− and control apoE−/− mice. The monocyte chemotactic activity induced by serum from MKP-1−/−/apoE−/− was significantly (-68.3%, p=0.001, Figure 7A) less than that induced by serum from apoE−/− mice. In addition, we compared the response of mouse monocytes from both groups (separated from their blood by flow cytometry) to purified MCP-1 protein. Mouse monocytes from each group were separated as CD11b+ CD115+ cells[12] (Figure 7B) and stimulated with MCP-1. There was no significant difference in the response between the two groups (Figure 7C). Finally, MCP-1 synthesis was significantly lower in peritoneal macrophages obtained from MKP-1−/−/apoE−/− mice when compared to those obtained from apoE−/− mice (Figure 7D).
Figure 7.
Serum from MKP-1−/−/apoE−/− mice induces less monocyte chemotactic activity compared to serum from apoE−/− mice. (A) Monocytes migration caused by mouse serum. Human monocytes migration in response to serum from apoE−/− and MKP-1−/−/apoE−/− mice were studied as described in MATERIALS and METHODS. (n=3 for apoE−/− mice, n=4 for MKP-1−/−/apoE−/− mice) *p=0.0001 (B, C) Comparison of mouse monocytes migration obtained from apoE−/− and MKP-1−/−/apoE−/− mice. Mouse monocytes from each group were separated as CD11b+ CD115+ cells (B) and stimulated by MCP-1 as described in MATERIALS and METHODS. (n=3 for apoE−/− mice, n=4 for MKP-1−/−/apoE−/− mice) (D) Comparison of cytokine synthesis by peritoneal macrophages obtained from apoE−/− and MKP-1−/−/apoE−/− mice. Peritoneal macrophages were cultured for 24hour with 10% FBS and culture fluid were assayed for MCP-1 generation by ELISA. (n=3 for apoE−/− mice, n=2 for MKP-1−/−/apoE−/− mice)
Discussion
MAP kinases and stress-activated protein kinase pathways regulate cell growth, apoptosis, differentiation and stress responses, and are regulated by phosphorylation of tyrosine and serine/threonine residues. MAP kinases are mainly deactivated by a group of MKP family members including MKP-1. Our results are similar to those of Shen et al. [27], in that both studies demonstrate a decrease in atherosclerotic lesion size and a corresponding reduction in macrophage accumulation in the absence of the MKP-1 gene in apoE−/− mice. However, there are some significant differences in lipid and cytokine measurements between the results of the two studies which may be due to methodological differences, different ages of the mice, and/or perhaps differences in passenger genes that accompanied the breeding process of the two sets of knockout mice.
MKP-1 plays crucial roles in M-CSF-mediated signal transduction [8, 9]. Since monocytes originate from bone marrow hematopoietic precursors in a M-CSF receptor dependent manner [28] we speculated that there might be differences in the populations of monocytes in the peripheral blood or bone marrow of MKP-1−/−/apoE−/− mice. Monocytes enter the circulation from bone marrow as Ly6Chigh monocytes, and they become Ly6Clow monocytes in the circulation [11]. Bone marrow monocytes are almost exclusively Ly6Chigh monocytes [11]. However, there was no difference of the distribution of monocytes (Ly6Chigh and total monocytes), or dendritic cells in the blood and bone marrow between two groups (Supplemental Figure 1).
Monocytes accumulate continuously during atheroma progression, and monocyte accumulation in mouse atherosclerotic lesions is proportional to the extent of disease [29]. Using apoE-deficient mice, Swirski et al. reported that Ly6high monocytes were increased in the circulation and become lesional macrophages [23]. They also showed Ly6Chigh monocytes preferentially accumulated in aortas more than Ly6Clow monocytes suggesting that circulating Ly6Chigh monocytes are direct precursors of lesional macrophages. Both Ly6Chigh and Ly6Clow monocytes can differentiate into dendritic cells [10, 12, 30]. We found that the area of Ly6C positive staining in MKP-1−/−/apoE−/− mice was significantly less than in apoE−/− mice (Figure 5A, 5B). However, Ly6C antigen is a glycosylphosphatidylinositol-anchored molecule, receptor/ligand [13], and not only monocytes but also endothelial cells, granulocytes, dendritic cells, T-cells, and NK cells can express the Ly6C antigen [28, 31-33]. Since control apoE−/− mice had increased Ly6C positive cell area in the lesions and Ly6Chigh monocytes can differentiate into dendritic cells, and dendritic cells can also express Ly6C antigen, we hypothesized that there might be a difference in the content of lesion CD11c cells between MKP-1−/−/apoE−/− mice and apoE−/− mice. Indeed, immunostaining of atherosclerotic lesions revealed significantly reduced CD11c positive dendritic cell area in the MKP-1−/−/apoE−/− mice (Figure 5C-E) consistent with this hypothesis. Both Ly6Chigh monocytes and dendritic cells express the CC-chemokine receptor 2 (CCR2) receptor, and circulating dendritic cells may utilize MCP-1 for entry into the atherosclerotic plaque [34]. Although dendritic cells have been described in both healthy and lesion prone arterial walls, their number is highly expanded in atherosclerotic lesions and they have been considered to have an important role in the progression of atherosclerosis.
We previously reported that MKP-1 protein is highly expressed in atherosclerotic plaque co-localizing with both Oil-Red-O and CD68 positive staining areas [16]. We hypothesized that that the decreased macrophage content in MKP-1−/−/apoE−/− mice was a direct effect of MKP-1 on macrophage apoptosis and/or recruitment. However, apoptosis (using the TUNEL assay) in the atherosclerotic lesions of apoE−/− and MKP-1−/−/apoE−/− mice (data not shown) were similar suggesting that, MKP-1 may be involved in macrophage recruitment.
MCP-1 plays a crucial role in initiating atherosclerosis lesion by recruiting monocytes to the vessel wall. To elucidate the impact of reduced MCP-1 on monocyte migration, we conducted a bioassay of using human monocytes and serum from both groups of mice. These studies confirmed that MKP-1−/−/apoE−/− mouse serum has less chemotactic activity. Monocytes isolated from both groups of mice showed similar migration capabilities when stimulated by MCP-1 suggesting that the monocytes were not defective. Interestingly, Shen et al. found that thioglycollate induced peritoneal macrophages from MKP-1−/−/apoE−/− mice had significant impairments in chemotaxis in response to 10% fetal bovine serum. Cushing and Fogelman previously reported that migration of monocytes/macrophages into inflammatory lesions may be amplified by their own production of MCP-1 [35]. Our in vitro experiment suggested that MCP-1 synthesis by peritoneal macrophages from MKP-1−/−/apoE−/− under conditions similar to those used by Shen et al. was reduced when compared to control macrophages. Reduced MCP-1 production from MKP-1−/−/apoE−/− macrophage may be the mechanism behind reduced atherosclerotic lesions and lesional macrophage content observed both in our studies and those reported by Shen et al. [27].
There is accumulating evidence that 5, 12/15-LOX and their metabolites 5, 12, 15-HETE induce MCP-1 secretion [25, 36-41]. Potula et al. reported that 5(S), 12(S), 15(S)-HETE stimulated vascular smooth muscle cell migration and 15(S)-HETE induced MCP-1 secretion via Src-STAT-3 signaling [25, 37]. 12(S)-HETE increased MCP-1 mRNA and protein expression in mouse macrophages. The expression and secretion of MCP-1 was attenuated in mouse peritoneal macrophages isolated from 12/15-LO knockout mice [25, 39]. Murine fibroblasts expressing human 15-LOX secret significantly higher levels of MCP-1 than LacZ-expressing control cells [38]. MCP-1 and its receptor CCR2 regulate lipid body biogenesis in macrophage. Lipid bodies were shown to be intracellular sites of LT synthesis in macrophage and CCR2 deficient mice showed reduced LT release that is one of metabolites of the 5-LOX pathway [26, 42]. Our results did not show any differences in mRNA levels of 5-LOX and 12/15-LOX between the two groups of mice (Figure 6D-E). However, there were significant differences in the amount of metabolites produced by 5-LOX and 12/15-LOX pathways (Figure 6A-C). Several possible mechanisms are postulated in the literature for the reduced production of HETEs, including the amount of free arachidonic acid available as a substrate or its accessibility [26, 42-46]. Further studies are required to elucidate whether, i) reduction of HETEs is directly related to reduction of MCP-1 secretion, or vice versa, and ii) the mechanism behind the reduced serum levels of HETEs and MCP-1 in MKP-1−/−/apoE−/− mice.
In conclusion, our results demonstrate that the absence of MKP-1 in apoE−/− mice results in decreased atherosclerosis by a mechanism that involves reduced macrophage accumulation. Taken together with our previous findings [4, 6, 16] the present studies further affirm that inhibition of MKP-1 may lead to prevention of atherosclerosis. MKP-1 may thus be a potential therapeutic target for the treatment of atherosclerotic disease.
Supplementary Material
Acknowledgments
We thank Lexicon Genetics for providing the MKP-1−/− mice.
Sources of Funding
Grant Support: This work was supported in part by US Public Health Service grants HL-30568, HL 082823, and the Laubisch, Castera, and M.K. Grey Funds at UCLA.
Non-standard Abbreviations and Acronyms
- MKP-1
mitogen-activated protein kinase phosphatase-1
- MCP-1
monocyte chemoattractant protein-1
- VLDL
very low-density lipoprotein
- LDL
low-density lipoprotein
- HETE
hydroxyeicosatetraenoic acids
- ox-LDL
oxidized low-density lipoprotein
- ox-PAPC
oxidized-L-a-1-palmitoyl-2-arachidonyl-sn-glycero-phosphorylcholine
- HAEC
human aortic endothelial cells
- NaOV
sodium orthovanadate
- HDL
high-density lipoprotein
- LC/MS/MS
Liquid chromatography-tandem mass spectrometry
- TNF-α
tumor necrosis factor-α
- IL
interleukin
- M-CSF
macrophage colony-stimulating factor
- LOX
lipoxygnease
- LT
leukotriene
Footnotes
Disclosures
MN, STR, SH, and AMF are principals in Bruin Pharma and AMF is an officer is Bruin Pharma
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 3.Weis M, Schlichting CL, Engleman EG, Cooke JP. Endothelial determinants of dendritic cell adhesion and migration: new implications for vascular diseases. Arterioscler Thromb Vasc Biol. 2002;22:1817–1823. doi: 10.1161/01.atv.0000036418.04998.d5. [DOI] [PubMed] [Google Scholar]
- 4.Reddy ST, Grijalva V, Ng C, Hassan K, Hama S, Mottahedeh R, Wadleigh DJ, Navab M, Fogelman AM. Identification of genes induced by oxidized phospholipids in human aortic endothelial cells. Vascul Pharmacol. 2002;38:211–218. doi: 10.1016/s1537-1891(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 5.Navab M, Hama SY, Reddy ST, Ng CJ, Van Lenten BJ, Laks H, Fogelman AM. Oxidized lipids as mediators of coronary heart disease. Curr Opin Lipidol. 2002;13:363–372. doi: 10.1097/00041433-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Reddy S, Hama S, Grijalva V, Hassan K, Mottahedeh R, Hough G, Wadleigh DJ, Navab M, Fogelman AM. Mitogen-activated protein kinase phosphatase 1 activity is necessary for oxidized phospholipids to induce monocyte chemotactic activity in human aortic endothelial cells. J Biol Chem. 2001;276:17030–17035. doi: 10.1074/jbc.M011663200. [DOI] [PubMed] [Google Scholar]
- 7.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Tillo E, Comalada M, Farrera C, Valledor AF, Lloberas J, Celada A. Macrophage-colony-stimulating factor-induced proliferation and lipopolysaccharide-dependent activation of macrophages requires Raf-1 phosphorylation to induce mitogen kinase phosphatase-1 expression. J Immunol. 2006;176:6594–6602. doi: 10.4049/jimmunol.176.11.6594. [DOI] [PubMed] [Google Scholar]
- 9.Valledor AF, Arpa L, Sanchez-Tillo E, Comalada M, Casals C, Xaus J, Caelles C, Lloberas J, Celada A. IFN-{gamma}-mediated inhibition of MAPK phosphatase expression results in prolonged MAPK activity in response to M-CSF and inhibition of proliferation. Blood. 2008;112:3274–3282. doi: 10.1182/blood-2007-11-123604. [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 12.Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 13.Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 16.Reddy ST, Nguyen JT, Grijalva V, Hough G, Hama S, Navab M, Fogelman AM. Potential role for mitogen-activated protein kinase phosphatase-1 in the development of atherosclerotic lesions in mouse models. Arterioscler Thromb Vasc Biol. 2004;24:1676–1681. doi: 10.1161/01.ATV.0000138342.94314.64. [DOI] [PubMed] [Google Scholar]
- 17.Hedrick CC, Castellani LW, Warden CH, Puppione DL, Lusis AJ. Influence of mouse apolipoprotein A-II on plasma lipoproteins in transgenic mice. J Biol Chem. 1993;268:20676–20682. [PubMed] [Google Scholar]
- 18.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 19.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 20.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 21.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 22.Li L, Chen SF, Liu Y. MAP kinase phosphatase-1, a critical negative regulator of the innate immune response. Int J Clin Exp Med. 2009;2:48–67. [PMC free article] [PubMed] [Google Scholar]
- 23.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol. 2008;294:H1933–1938. doi: 10.1152/ajpheart.00260.2007. [DOI] [PubMed] [Google Scholar]
- 26.Silva AR, Pacheco P, Vieira-de-Abreu A, Maya-Monteiro CM, D'Alegria B, Magalhaes KG, de Assis EF, Bandeira-Melo C, Castro-Faria-Neto HC, Bozza PT. Lipid bodies in oxidized LDL-induced foam cells are leukotriene-synthesizing organelles: a MCP-1/CCL2 regulated phenomenon. Biochim Biophys Acta. 2009;1791:1066–1075. doi: 10.1016/j.bbalip.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Chandrasekharan UM, Ashraf MZ, Long E, Morton RE, Liu Y, Smith JD, DiCorleto PE. Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis. Circ Res. 106:902–910. doi: 10.1161/CIRCRESAHA.109.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Y, Latchman Y, Elkon KB. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777–2785. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 33.Hanninen A, Jaakkola I, Salmi M, Simell O, Jalkanen S. Ly-6C regulates endothelial adhesion and homing of CD8(+) T cells by activating integrin-dependent adhesion pathways. Proc Natl Acad Sci U S A. 1997;94:6898–6903. doi: 10.1073/pnas.94.13.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cushing SD, Fogelman AM. Monocytes may amplify their recruitment into inflammatory lesions by inducing monocyte chemotactic protein. Arterioscler Thromb. 1992;12:78–82. doi: 10.1161/01.atv.12.1.78. [DOI] [PubMed] [Google Scholar]
- 36.Sozzani S, Zhou D, Locati M, Bernasconi S, Luini W, Mantovani A, O'Flaherty JT. Stimulating properties of 5-oxo-eicosanoids for human monocytes: synergism with monocyte chemotactic protein-1 and -3. J Immunol. 1996;157:4664–4671. [PubMed] [Google Scholar]
- 37.Potula HS, Wang D, Quyen DV, Singh NK, Kundumani-Sridharan V, Karpurapu M, Park EA, Glasgow WC, Rao GN. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J Biol Chem. 2009;284:31142–31155. doi: 10.1074/jbc.M109.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dioszeghy V, Rosas M, Maskrey BH, Colmont C, Topley N, Chaitidis P, Kuhn H, Jones SA, Taylor PR, O'Donnell VB. 12/15-Lipoxygenase regulates the inflammatory response to bacterial products in vivo. J Immunol. 2008;181:6514–6524. doi: 10.4049/jimmunol.181.9.6514. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2009;17:1657–1663. doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res. 2005;46:220–229. doi: 10.1194/jlr.M400328-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Pacheco P, Vieira-de-Abreu A, Gomes RN, Barbosa-Lima G, Wermelinger LB, Maya-Monteiro CM, Silva AR, Bozza MT, Castro-Faria-Neto HC, Bandeira-Melo C, Bozza PT. Monocyte chemoattractant protein-1/CC chemokine ligand 2 controls microtubule-driven biogenesis and leukotriene B4-synthesizing function of macrophage lipid bodies elicited by innate immune response. J Immunol. 2007;179:8500–8508. doi: 10.4049/jimmunol.179.12.8500. [DOI] [PubMed] [Google Scholar]
- 43.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 44.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 45.Luo M, Jones SM, Peters-Golden M, Brock TG. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc Natl Acad Sci U S A. 2003;100:12165–12170. doi: 10.1073/pnas.2133253100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.