Abstract
Objective
This study examines the characteristics of 96 children with attention-deficit/hyperactivity disorder (ADHD) and their families who refused a recommendation for medication as part of their treatment for disruptive disorders.
Methods
The ADHD cases were taken from a sample of 139 youth (age 6–11) who were recruited for a clinical trial that compared the administration of a modular psychosocial treatment in an outpatient clinic or community settings. Medication management was an optional treatment module for children with ADHD in both conditions. Children who were (vs. were not) taking medication at intake, and children who accepted (vs. refused) medication recommendations during the study were compared on diagnostic and clinical measures related to child, school, parent, and family domains of functioning.
Results
Parents of 30% of the children refused study medication for ADHD. Parental medication acceptability and intake correlated highly with both medication history and study refusal of medication. Increased parental self-efficacy and emotional support for their youth correlated with medication refusal. No demographics and few child or school factors were associated with medication refusal. Medication use was associated with reductions in some key ADHD symptoms, but did not affect disruptive behaviors as did the psychosocial interventions.
Conclusion
Medication refusers remain poorly understood but certain correlates, such as parental self-efficacy, parental emotional support for their youth, and medication acceptability, warrant further evaluation.
Background
Attention-deficit/hyperactivity disorder (ADHD) is a common behavioral disorder estimated to affect 3%–5% of school-aged children. In treating ADHD, despite the predominant superiority of medication as a single modality to control core ADHD symptoms (MTA Cooperative Group 1999), the literature suggests that a multimodal treatment that combines the parental preferred modality of behavior therapy with medication management may be optimal for both acute outcome and satisfaction (Bukstein 2004). Unfortunately, despite well-established practice guidelines and efficacious treatments (AACAP 2007), ∼25%–50% of youth fail to initiate or maintain a trial of medication (Jensen et al. 1999; Krain et. al 2005; Monastra 2005). A recent retrospective analysis of prescription fill rates for 16,383 newly diagnosed youth with ADHD who choose to begin a medication trial indicates a mean number of only 4.8 prescriptions over a 12-month period (Grcevich et al. 2006).
Several studies have examined factors related to medication adherence in ADHD, but we found no studies that specifically examined medication refusers, defined here as those who decline a recommendation for medication treatment. It is unknown whether medication refusers are similar or different from those who had either poor or good adherence to medication treatment. Only few studies report refusal percentages based on their initial recruitment. Therefore, we conducted a secondary data analysis on youth with ADHD referred for their disruptive behaviors.
Within the adherence literature, there are few consistent findings and no consensus informing clinical practice aside from the benefit of long-acting stimulants (Gau et al. 2006). Further, most studies have been conducted in the context of brief medication trials (Kauffman et al. 1981; Sleator et al. 1982; Brown et al. 1985, 1987, 1988; Johnson and Fine 1993) or prospective reports following medication trials (Thiruchelvam et al. 2001; Charach et al. 2004), and there have been few from community-based practices (Sleator et al. 1982; Ibrahim 2002; Monastra 2005; Gau et al. 2006). Study limitations within this field include small sample sizes, not controlling for past treatment exposure, no standard measures of adherence, a predominance of Caucasian participants, and variable adjunctive treatments (parent ADHD education or parent management training [PMT]). Despite these limitations, studies have reported a few variables that are correlated with poor medication adherence. These variables include several background factors, such as low socioeconomic status (SES) (Ibrahim 2002; Brown et al. 1988), being female (Firestone 1982), older child age (Thiruchelvam et al. 2001), increased symptoms (Brown et al. 1988), oppositional defiant disorder (ODD) (Thiruchelvam et al. 2001), and low intelligence quotient (IQ) (Firestone 1982; Brown et al. 1988). Other variables related to poor medication adherence include medication factors related to dosing schedule, poor efficacy, and side effects (Thiruchelvam et al. 2001; Monastra 2005). Additional family factors include younger parental age, low maternal IQ (Johnson and Fine 1993; Swanson 2003), psychosocial adversity (Firestone 1982; Brown et al. 1988; Thiruchelvam et al. 2001; Ibrahim 2002; Gau et al. 2006), low supervision, stigma/embarrassment (Swanson 2003), and limited parental confidence in the assessment process (Monastra 2005).
As most adherence studies focus on ADHD as the study entry diagnosis, their conclusions may not translate to youth referred for disruptive behaviors with comorbid ADHD. The conduct disorder (CD) literature has identified more severe disruptive behaviors, low SES, and parental psychosocial stress as correlates of poor treatment adherence (Kazdin and Crowley 1997). In ADHD adherence studies, the number of refusers cited range from 9% to 32% of subjects refusing medication management (Brown et al. 1987; Bennett et al. 1996; Corkum et al. 1999; MTA Cooperative Group 1999). In a prospective study of ADHD treatment-seeking families at a community clinic, Krain et al. (2005) found that 33% did not pursue medications when they were recommended. This broad range of refuser prevalence reflects variations in population, methodology, and the types and settings of treatment. Overall, however, the number of refusers among youth and their families appears as large as those with poor adherence and amounts to an alarming majority population of untreated youth with ADHD.
Given the lack of empirical information on ADHD medication refusal and acceptance, one may find useful information from a small body of literature that examines treatment acceptability and ADHD, which may be a construct relevant to medication refusers. Medications are frequently cited to be the least acceptable form of treatment for ADHD by parents (Summers and Caplan 1987; Wilson and Jennings 1996; Krain et al. 2005). Several factors have been examined to understand the characteristics of parents who report limited acceptability of medications. For example, Bennett et al. (1996) found that parents who had higher levels of knowledge and information about ADHD viewed medication use more favorably. Parental medication acceptability appears to increase with ADHD education, medication exposure, and the use of combined behavioral treatments, but whether this leads to pursuit of medication remains debatable (Bennett et al. 1996; Corkum et al. 1999; Gage and Wilson 2000; Krain et al. 2005). Improving upon several limitations from prior research, including the use of a treatment naive community population, a study by Krain et al. (2005) found that medication acceptability does predict pursuit of medication treatment and that only one demographic factor (Caucasian background) predicted both increased acceptability and use of ADHD medication. Overall, the literature reports conflicting results regarding the role of demographics, clinical variables, treatment feasibility, and parental knowledge in medication acceptability and adherence for youth with ADHD.
As part of a larger study comparing psychosocial interventions for youth with disruptive behaviors that were applied in either the clinic or the community (Kolko et al. 2009), we describe the subpopulation of youth who were comorbid for ADHD with the primary aim of assessing variables associated with refusal versus acceptance of a recommendation for ADHD medication management. We also describe the characteristics of children with ADHD who came into the study already on stimulants as well as the clinical differences at posttreatment for medication refusers. Several clinical and service measures were administered to evaluate the relationship among various background factors and medication refusal in children with ADHD. Based on ADHD medication adherence literature, we hypothesized that refusers would be older, female, and minority, have decreased ADHD symptom severity, and live in families with greater psychosocial adversity. Based on the adherence literature for youth with CD symptoms, medication refusers would more likely have older age, lower SES, minority status, single parents, and increased symptom severity, parental psychosocial stress, and parental psychopathology (Nock and Kazdin 2001).
Methods
Participants
A total of 139 children clinically referred for disruptive behaviors, who were recruited for randomization to one of two experimental treatment protocols, were administered in either the community or an outpatient clinic. These cases were recruited through newspaper and radio advertisements and brochures sent to schools and local mental health centers and from program sites affiliated with the University of Pittsburgh Medical Center. All children met the following inclusion criteria: (1) ODD or CD, (2) boys or girls aged 6–11 years, (3) residence with at least one parent/guardian, (4) intellectual level no more than 2 standard deviations (SDs) below age norms on the Kaufman Brief Intelligence Test (Kaufman and Kaufman 1990), and (5) parent consent and child assent for participation as approved by the University's Institutional Review Board. Cases were excluded if they met any of the following criteria: (1) concurrent individual or family participation in a treatment program for disruptive disorders; (2) current psychosis, bipolar disorder, MDD marked by significant vegetative signs, substance abuse, or an eating disorder; or (3) suicidality with a plan or homicidality.
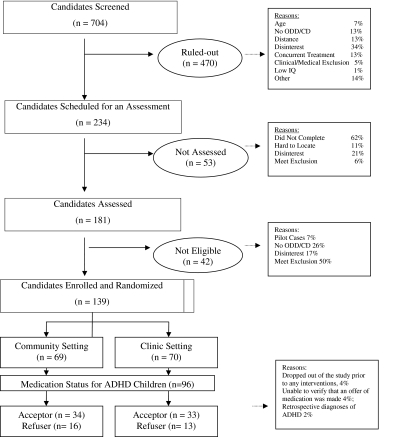
Children were randomly assigned to either the community or clinic specialty treatment conditions to compose groups that were balanced for gender and primary diagnosis (ODD vs. CD). Diagnoses were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994), Present and Lifetime (Kiddie-SADS-PL; Kaufman et al. 1996). A consort chart is provided in Figure 1.
FIG. 1.
Overall study status of cases at the screening, enrollment, treatment, and follow-up. ADHD = attention-deficit/hyperactivity disorder; ODD/CD = oppositional defiant disorder/conduct disorder; IQ = intelligent quotient.
Overview of modular treatment protocol
Trained master's level staff administered the two modular treatment protocols focused on teaching children and parents the cognitive-behavioral skills and providing exposure to evidence-based interventions that address individualized problems. A more detailed description of the treatment protocols can be found in our initial outcome study (Kolko et al. 2009), which includes evidence-based practices for child cognitive behavioral therapy (CBT) training, PMT, parent–child treatment, school/educational intervention, peer/social network intervention, community liaison work, and case/crisis management or monitoring (Kolko 1994, 1995; Kolko and Swenson 2002).
Medication algorithm: management and monitoring procedures
As a majority of participants were diagnosed with ADHD, depriving them of an effective treatment (i.e., stimulant medication) would not have been ethical. However, medication treatment was not part of the primary study, so we proposed a naturalistic plan for those participants with an ADHD diagnosis. As a number of participants (n = 28) met study inclusion criteria despite being on a psychotropic (predominantly stimulant) medication, we left these participants on their current medication, if parents felt that medication was helping, and followed all participants through the first 4 weeks of psychosocial treatment.
Medication referral
If significant ADHD symptoms persisted on the IOWA Conners' Scale (Loney and Milich 1982; Pelham et al. 1989) (<30% improvement from baseline), therapists would then provide parents with psychoeducational material about ADHD and recommend that the parents and participant discuss medication management with the study psychiatrist (O.G.B.). The parents were not obligated to accept this recommendation; many did not (i.e., “medication refusers”) and several pursued medication management later in the treatment protocol. If the subjects/families accepted the recommendation to consider medication treatment, they met with the psychiatrist who provided additional medication information, obtaining, in all but one case, informed consent for a medication trial.
Medication definitions
Medication “acceptors” were youth who took a medication at any time during treatment as indicated by either the posttreatment K-SADS, Service Assessment for Children and Adolescents, research Treatment Termination Form, clinical Discharge Summary Form or Medication Visit Form by the study psychiatrist. Medication “acceptor” indicates consent and onset of a medication trial but provides no indication regarding adherence. Clinical response, adherence, and side-effect data were collected but are not reported as this was not the primary aim of the study and the adherence studies cited in the Introduction section are more informative in this regard. Medication “refusers” connotes that, within our procedures, medications were not used as a treatment option and does not necessarily represent stable characteristics of the youth or family. The term “refuser” is representative of those youth with documented referral to study psychiatrist and they either declined or never showed for an appointment, refused a medication trial when offered by the study psychiatrist, or accepted prescription but never used any medications.
Medication management
Borrowing from the medication treatment algorithm of the MTA Cooperative Group (1999), we proposed open, sequential trials of methylphenidate (MPH), dextroamphetamine (DEX), or mixed amphetamine salts (MAS), followed by nonstimulant medications (clonidine or bupropion), as needed. As this study took place before the marketing of long-acting stimulant and nonstimulant medications, all stimulants were of immediate release or “short acting.” The medication management procedures mirror current guidelines for ADHD treatment (AACAP 2002, 2007). The participant's past medication experience (e.g., lack of efficacy or adverse effects) or parent preference occasionally dictated deviation from the algorithm. Those already on medication at baseline were likewise managed according to the algorithm. The psychiatrist generally increased medication doses until there was no room for improvement on the IOWA/Conners' (IOWA/C), which were administered monthly to parent(s) and teacher or until the emergence of unacceptable adverse effects. Similar to routine clinical practice, parents would occasionally express a desire to remain at a given dose, despite room for improvement.
For all medicated patients, monthly psychiatrist appointments included completion of a Medication Visit Form that contained current medications, interval history, reported level of compliance, vital signs, Pittsburgh Side Effects Rating Scale (Pelham 1993), and the presence of any other medications taken.
Assessment procedures and measures
Each case was evaluated on several dimensions of functioning related to disruptive behavior disorders (DBDs), based on child, parent, and teacher reports. Additionally, demographic, parental, family, insurance, and medication variables associated with the ADHD and CD adherence literature are described below. The child and parent assessments were completed by blinded research assistants and each informant was paid $10. Although the assessments were administered at several time points, we are reporting data at intake and posttreatment only for youth with ADHD in the two intervention groups.
Demographics
Caregivers provided age, gender, race, SES based on Hollingshead (Hollingshead and Redlich 1958), insurance type (health maintenance organization, private, or medical assistance), and whether prescription benefits were included in their plan.
Child measures
The IOWA/C rating scale was completed by parents and teachers to help confirm diagnoses and provide a dimensional assessment of inattention/overactivity and oppositional behaviors (Pelham et al. 1989). Behavioral problems were further qualified on completion of the Child Behavior Checklist and Teacher Report Form by caretaker and school teacher, respectively, which provides a dimensional assessment across an array of behavioral and emotional problems and social competencies (Achenbach 1991a, 1991b). The Child Behavior Checklist raw scores have been converted to t-scores, which have been standardized based on a mean of 50 and an SD of 10. The score represents how many SDs a score is either above or below the mean for the population.
Parent measures
The Credibility of Treatment Scale (COTS) was developed for this study to evaluate pretreatment parental preferences for 28 intervention components related to the method, content, participants, and/or settings that could be targeted in treatment (Kolko et al. 2009). The item content and rating scale were based on the short form of Kazdin's Treatment Evaluation Inventory, which evaluates parental ratings of treatment acceptability (Kelley et al. 1989; Johnston et al. 2008). Parents were asked to provide ratings as to the level of importance of each treatment component that the family could receive at this time. The level of importance of each item was rated on a 5-point Likert scale (1 = not at all; 5 = very much). Items were aggregated to form 11 different treatment variables that reflected four multiple-item scales (i.e., child CBT; PMT; family therapy; school intervention; α's = 0.56–0.78), two two-item scales (medication; peer help; r's = 0.58 and 0.59), and five individual items (e.g., home, school, peer, and community visits; case management). Parental medication acceptability was ascertained from two medication questions: (1) How important is “using medication for my child's hyperactivity” and (2) How important is “helping my child receive adequate medication follow-up”? We report on their summation as there was a significant correlation between these two questions (r = 0.59, p < 0.001).
Parents completed the Patient Health Questionnaire, which is the self-report version of the PRIME-MD designed to assess for the most common mental health disorders found in primary care patients (based on the Structured Clinical Interview for DSM Disorders (SCID); Spitzer et al. 1990). The Brief Symptom Inventory was included to evaluate overall self-reported parental psychopathology (Derogatis et al. 1976) in several domains (e.g., anxiety, hostility) examining current distress. The Parental Self-Efficacy Scale was completed by parents to document their perceived ability to help their children in several domains (e.g., behavior management, provider issues, school issues, advocacy, and emotional support) (Evans et al. 1997). The Alabama Parenting Questionnaire (Shelton et al. 1996) evaluates common dimensions of parenting practices and activities (e.g., involvement, positive parenting, poor monitoring, and inconsistent discipline) related to antisocial behavior. The Parent Perception Inventory was administered to children (Hazzard at al. 1983) to evaluate their perceptions of primary caretaker interactions and behavioral management. Mothers completed the Beck Depression Inventory (Beck et al. 1961), which assessed dimensional ratings of depression. The University of Rhode Island Change Assessment (URICA) was used to capture the participating caregiver's level of motivation in accord with the stages of change model (McConnaughy et al. 1983).
Family measures
The Parent–Child Conflict Tactics Scales (Straus et al. 1998) assesses parental discipline during child conflicts and we report the summary score for psychological aggression and the minor/severe/very severe assault scores. The Family Environment Scale-A (Moos et al. 1974) reflects relational aspects and we report on the positive summary score for the cohesion and control subscales. The Family Adaptability and Cohesion Scale (Olson et al. 1982) provides additional information regarding family interactions and communication.
Insurance codes
Parents reported at intake whether they had Medicaid, Medical Assistance, or an HMO and if the child's plan covered part of their medication costs.
Medication codes
Determination of medication history, including current medications at time of intake, was made on either the intake KSADS or the Service Assessment for Children and Adolescents (Hoagwood et al. 2000).
Data analysis
Descriptive statistics include a description of the sample, the medication use of the sample upon study intake, and the number of medication management visits, including medication type and doses used during the psychosocial treatment study. Children who were versus were not taking medication at intake and those who accepted versus refused study medication recommendations were compared on demographic variables. Significant differences were then used to identify relevant covariates for subsequent comparisons. Each set of the two groups were then compared on several assessment measures of child, parent, and family functioning using chi-square tests and analysis of variance or covariance.
Results
A comparison of the youth and parent background characteristics of those youth who were on or off medication for ADHD at intake is presented in Tables 1 and 2, respectively. The entire sample for the intervention study consisted of 139 participants with either ODD or CD (ODD = 112 [81%], CD = 27 [19%]). Not included in the analyses below are four participants who dropped out of the study prior to any interventions, four participants for whom we could not verify that an offer of medication for ADHD was made, and two participants who were included as ADHD after the intervention by retrospective diagnoses.
Table 1.
Correlates of Youth Already on Medicine at Intake: Background and Child Clinical Variables
| Variable (SD or %) | Total sample or mean (SD) | On medication | No medication | Significance (p) |
|---|---|---|---|---|
| Background variables | ||||
| ADHD | 96 | 28 | 68 | |
| Age | 8.6 (1.6) | 8.8 (1.6) | 8.5 (1.6) | NS, 0.438 |
| Male | 87 (91%) | 25 (89) | 62 (91%) | NS, 0.773 |
| Female | 9 (9%) | 3 (11%) | 6 (9%) | |
| White | 44 (46%) | 19 (68%) | 25 (37%) | p = 0.005 |
| Minority | 52 (54%) | 9 (32%) | 43 (63%) | |
| Socioeconomic status | 37.4 (11.3) | 41.3 (12.8) | 35.8 (10.3) | p = 0.029 |
| Married | 38 (40%) | 14 (50%) | 24 (35%) | NS, 0.180 |
| Not married | 58 (60%) | 14 (50%) | 44 (65%) | |
| Two adults | 45 (47%) | 17 (61%) | 28 (41%) | NS, 0.081 |
| One adult | 51 (53%) | 11 (39%) | 40 (59%) | |
| Oppositional defiant disorder | 74 (77%) | 22 (79%) | 52 (76%) | NS, 0.824 |
| Conduct disorder | 22 (23%) | 6 (21%) | 16 (24%) | |
| Medicaid/MA | 47 (48%) | 11 (42%) | 36 (50%) | NS, 0.290 |
| No Medicaid/MA | 51 (52%) | 15 (58%) | 36 (50%) | |
| Health maintenance organization | 46 (53%) | 15 (65%) | 31 (48%) | NS, 0.167 |
| Medicaid/MA | 41 (47%) | 8 (35%) | 33 (52%) | |
| Prescription benefits | 86 (97%) | 23 (96%) | 63 (97%) | NS, 0.800 |
| No benefits | 3 (3%) | 1 (4%) | 2 (3%) | |
| Child clinical variables | ||||
| CBCL externalizing | 74.0 (5.9) | 72.4 (6.3) | NS, 0.268 | |
| CBCL attention | 70.4 (6.9) | 70.0 (7.7) | NS, 0.818 | |
| CBCL internalizing | 64.2 (11.6) | 62.5 (9.6) | NS, 0.487 | |
| TRF externalizing | 65.5 (12.7) | 72.0 (9.2) | p = 0.008 | |
| TRF attention | 62.8 (10.2) | 68.3 (9.0) | p = 0.015 | |
| IOWA/C parent inattention/overactivity | 11.4 (2.1) | 10.4 (3.0) | NS, 0.146 | |
| IOWA/C parent oppositional defiant | 12.7 (2.4) | 11.4 (2.7) | p = 0.033 | |
| IOWA/C teacher inattention/overactivity | 8.9 (4.5) | 11.3 (3.4) | p = 0.005 | |
| IOWA/C teacher oppositional defiant | 6.7 (5.3) | 9.7 (4.4) | p = 0.005 | |
SD = standard deviation; ADHD = attention-deficit/hyperactivity disorder; CBCL = Child Behavior Checklist, TRF = Teacher Report Form; IOWA/C = IOWA/Conners' Rating Scale; NS = not statistically significant; MA = medical assistance.
Table 2.
Correlates of Youth Already on Medicine at Intake: Parent and Family Variables
| Variable | On medication Score (SD) | No medication Score (SD) | Significance (p) |
|---|---|---|---|
| Parental variables | |||
| BSI | 39.9 (6.6) | 39.9 (9.2) | NS, 0.986 |
| BDI | 8.9 (7.0) | 8.1 (7.5) | NS, 0.644 |
| COTS sum med-acceptability | 7.8 (2.5) | 5.5 (2.8) | p = 0.00 |
| APQ involvement | 38.0 (5.0) | 37.1 (5.9) | NS, 0.471 |
| APQ positive parenting | 26.4 (3.3) | 25.2 (4.1) | NS, 0.186 |
| APQ poor monitoring | 16.3 (5.0) | 15.0 (5.7) | NS, 0.331 |
| APQ inconsistent discipline | 16.5 (4.0) | 16.2 (4.0) | NS, 0.739 |
| PPI (net positive) | 10.7 (12.2) | 11.9 (10.1) | NS, 0.629 |
| PHQ (total) | 5.3 (6.2) | 6.6 (6.3) | NS, 0.351 |
| PSES behavior management | 17.7 (4.0) | 18.7 (3.0) | NS, 0.188 |
| PSES provider issues | 23.2 (1.1) | 23.3 (1.6) | NS, 0.877 |
| PSES school issues | 13.8 (1.6) | 13.6 (1.9) | NS, 0.751 |
| PSES advocacy | 13.0 (2.8) | 13.2 (2.0) | NS, 0.698 |
| PSES emotional support | 13.2 (3.3) | 14.6 (2.3) | p = 0.041 |
| URICA | 11.1 (1.6) | 10.8 (1.6) | NS, 0.448 |
| Family variables | |||
| FACES cohesion | 62.8 (7.2) | 62.3 (11.2) | NS, 0.696 |
| FACES adaptability | 50.1 (7.8) | 48.9 (7.7) | NS, 0.882 |
| CTSPC total | 74.1 (42.5) | 65.3 (31.8) | NS, 0.265 |
| FES positive | 12.7 (3.4) | 12.9 (2.7) | NS, 0.692 |
APQ = Alabama Parenting Questionnaire; BDI = Beck Depression Inventory; BSI = Brief Symptom Inventory; CTSPC = Parent-Child Conflict Tactics Scales; COTS = Credibility of Treatment Services; FACES = Family Adaptability and Cohesion Scale; FES = Family Environment Scale; PHQ = Patient Health Questionnaire; PPI = Parent Perception Inventory; PSES = Parental Self-Efficacy Scale; URICA = University of Rhode Island Change Assessment; SD = standard deviation.
Youth comorbid for ADHD who were offered medication included 96 children (69%) of this ODD/CD sample. These youth represented 87 boys (91%) and 9 girls (9%), with a mean age of 8.6 years (SD = 1.6). Approximately 69% of the cases were between 6 and 9 years. Caucasian youth comprised 46% (n = 44) and minorities 54% (n = 52) of the ADHD sample that was offered medication. Household composition reflected that 40% (n = 38) of the participants were living with married adults and 47% (n = 45) with two adults and had a mean income of 236,230 (SD = 231,024).
Intake medication status
At intake, 28 (29%) participants were on ADHD medications. Of the 28 youth on medications, 18 were on MPH, 5 on MAS, 2 on DEX, 1 on clonidine and MPH, 1 on Wellbutrin, and 1 on Wellbutrin and MPH. We found that two demographic factors, minority status and lower SES, were significantly associated with a lower likelihood of being medicated at intake (83% of minorities and 57% of Caucasians were not on medications at intake: X2[1] = 7.72, p = 0.005; lower SES were less likely to be medicated: F [1, 94] = 4.89, p = 0.029). Among the clinical factors associated with no medications at intake were teacher ratings of increased symptoms for externalizing, oppositional defiant, attention, inattention/overactivity, and adaptability and lower parental ratings for oppositional defiant symptoms. Parental factors associated with no medications at intake were decreased medication acceptability and increased emotional support.
Relationship between intake and study medication status
Children who were on medication at intake were more likely than those not on medication at intake to accept medication during the study (93% vs. 60%; χ2 = 9.98, degrees of freedom = 1, p = 0.003) regardless of treatment assignment. Because of this relationship, we conducted analyses either controlling for prior intake medication status or including intake medication status as another independent variable in order to examine interactions between the two variables.
Study medication management status
All of the 28 youth already on ADHD medication had documented persistence of symptoms and were referred to the study psychiatrist (O.G.B.). A total of 96 of 98 participants with ADHD had documentation that they were offered a medication evaluation with the study psychiatrist. Of this group, 67 (70%) participants began medication management by the study psychiatrist and took at least one pill. A total of 29 (30%) participants declined medication as a treatment option. Of all 67 patients who chose to receive medications, participants had an average of 3.05 monthly visits per participant during the course of the 4–6-month treatment (range: 1–5). Of these children (minus one participant with missing data), 43 (66.2%) participants received MPH, 18 (27.7%) MAS, 4 (6.2%) DEX, 3 (4.6%) clonidine, 2 bupropion, and 1 fluoxetine at some point during the course of the study, with some participants taking multiple medications.
Tables 3 and 4 show a comparison of youth and parental variables of those cases who accepted or refused medication for ADHD during the study, respectively. In a comparison of medication acceptors and refusers, we found that increased teacher scores for externalizing symptoms and decreased parental scores for inattention/overactivity were significantly associated with medication refusal. Parentally, medication acceptability also was lower for medication refusers than acceptors. Parental emotional support (p = 0.011) and the total self-efficacy scale (p = 0.039) were higher for medication refusers. There were no significant differences on any other demographic or family variables.
Table 3.
Predictors of Medication Refusal: Background and Child Clinical Variables
| Variable | Total/mean (SD) | Medication acceptor | Medication refuser | Significance (p) |
|---|---|---|---|---|
| Background variable | ||||
| Medication status | 96 | 67 | 29 | |
| Age | 8.61 (1.6) | 8.5 (1.6) | 8.8 (1.6) | NS, 0.337 |
| Male | 87 (91%) | 63 (94%) | 24 (83%) | NS, 0.124 |
| Female | 9 (9%) | 4 (6%) | 5 (17%) | |
| White | 44 (46%) | 35 (52%) | 9 (31%) | NS, 0.075 |
| Minority | 52 (54%) | 32 (48%) | 20 (69%) | |
| Socioeconomic status | 37.4 (11.3) | 37.4 (11.6) | 37.3 (10.7) | NS, 0.977 |
| Oppositional defiant disorder | 74 (77%) | 54 (81%) | 20 (69%) | NS, 0.290 |
| Conflict disorder | 22 (23%) | 13 (19%) | 9 (31%) | |
| Medicaid/MA | 47 (51%) | 34 (52%) | 13 (48%) | NS, 0.820 |
| No Medicaid/MA | 45 (49%) | 31 (48%) | 14 (52%) | |
| Health maintenance organization | 46 (53%) | 31 (52%) | 15 (55%) | NS, 0.818 |
| Medical assistance | 41 (47%) | 29 (48%) | 12 (45%) | |
| Prescription benefits | 86 (97%) | 60 (97%) | 26 (96%) | NS, 1.000 |
| No benefits | 3 (3%) | 2 (3%) | 1 (4%) | |
| Community | 50 (52%) | 34 (51%) | 16 (55%) | NS, 0.824 |
| Clinic | 46 (48%) | 33 (49%) | 13 (45%) | |
| Child clinical variables | ||||
| CBCL externalizing | 72.8 (6.2) | 72.658 (6.1) | 73.4 (6.5) | NS, 0.579 |
| CBCL attention | 70.1 (7.4) | 70.5 (6.9) | 69.3 (8.6) | NS, 0.474 |
| CBCL internalizing | 63.0 (10.2) | 64.0 (10.6) | 60.4 (8.8) | NS, 0.120 |
| TRF externalizing | 70.2 (10.6) | 68.6 (10.8) | 73.9 (9.1) | p = 0.025 |
| TRF attention | 66.8 (9.6) | 66.4 (9.9) | 67.7 (9.1) | NS, 0.553 |
| IOWA/C parent inattention/overactivity | 10.7 (2.8) | 11.1 (2.6) | 9.7 (3.0) | p = 0.023 |
| IOWA/C parent oppositional defiant | 11.8 (2.7) | 12.1 (2.6) | 11.2 (2.8) | NS, 0.124 |
| IOWA/C teacher inattention/overactivity | 10.6 (3.9) | 10.3 (4.1) | 11.2 (3.5) | NS, 0.313 |
| IOWA/C teacher oppositional defiant | 8.8 (4.8) | 8.3 (5.1) | 9.9 (3.9) | NS, 0.149 |
NS = not statistically significant; SD = standard deviation; CBCL = Child Behavior Checklist; TRF = Teacher Report Form; IOWA-C/IOWA/Conners' Rating Scale.
Table 4.
Predictors of Medication Refusal: Parent and Family Variables
| Variable | Total/mean (SD) | Medication acceptor | Medication refuser | Significance (p) |
|---|---|---|---|---|
| Parental variables | ||||
| BSI | 39.9 (8.5) | 39.8 (8.1) | 40.2 (9.7) | NS, 0.818 |
| BDI | 8.4 (7.3) | 8.4 (7.4) | 8.4 (7.3) | NS, 0.993 |
| COTS med acceptability | 6.14 (2.9) | 7.0 (2.6) | 4.1 (2.4) | p = 0.000 |
| APQ involvement | 37.4 (5.6) | 37.1 (5.7) | 38.1 (5.4) | NS, 0.400 |
| APQ positive parenting | 25.5 (3.9) | 25.9 (3.7) | 24.6 (4.2) | NS, 0.138 |
| APQ poor monitoring | 15.4 (5.5) | 14.9 (5.1) | 16.6 (6.4) | NS, 0.192 |
| APQ inconsistent discipline | 16.3 (3.9) | 16.0 (4.3) | 16.8 (2.9) | NS, 0.395 |
| PPI (net positive) | 11.5 (10.7) | 10.9 (10.0) | 13.1 (12.2) | NS, 0.360 |
| PHQ (total) | 6.2 (6.3) | 5.6 (5.8) | 7.7 (7.3) | NS, 0.160 |
| PSES behavior management | 18.4 (3.3) | 18.1 (3.4) | 18.9 (3.1) | NS, 0.282 |
| PSES provider issues | 23.3 (1.4) | 23.1 (1.6) | 23.6 (1.0) | NS, 0.134 |
| PSES school issues | 13.7 (1.8) | 13.5 (2.0) | 13.9 (1.5) | NS, 0.340 |
| PSES advocacy | 13.1 (2.3) | 13.0 (2.4) | 13.4 (1.7) | NS, 0.378 |
| PSES emotional support | 14.2 (3.0) | 13.7 (3.1) | 15.3 (2.3) | p = 0.011 |
| URICA | 10.9 (1.6) | 10.9 (1.6) | 10.6 (1.5) | NS, 0.431 |
| Family variables | ||||
| FACES cohesion | 62.4 (9.3) | 61.5 (9.3) | 64.4 (9.0) | NS, 0.155 |
| FACES adaptability | 49.0 (7.5) | 48.3 (7.9) | 50.7 (6.3) | NS, 0.156 |
| CTSPC total | 67.9 (35.2) | 66.7 (38.4) | 70.7 (27.0) | NS, 0.612 |
| FES positive | 12.9 (2.9) | 12.7 (3.0) | 13.2 (2.9) | NS, 0.502 |
When analyses run solely on participants but not on medications at intake (N = 68), only the COTS variable remained significant.
BSI = Brief Symptom Inventory; BDI = Beck Depression Inventory; COTS = Credibility of Treatment Services; APQ = Alabama Parenting Questionnaire; PPI = Parent Perception Inventory; PHQ = Patient Health Questionnaire; PSES = Parental Self-Efficacy Scale; URICA = University of Rhode Island Change Assessment; FACES = Family Adaptability and Cohesion Scale; CTSPC = Parent-Child Conflict Tactics Scale; FES = Family Environment Scale; NS = not statistically significant; SD = standard deviation.
Course of study intervention comparisons
We compared the clinical outcomes of medication refusers and acceptors during the course of the intervention using analysis of covariances that controlled for medication status at intake. The results of these comparisons are found in Table 5. Significant group differences emerged on two-teacher ratings. Specifically, medication acceptors (vs. refusers) showed fewer symptoms of inattention and overactivity on the IOWA/C subscale (F [1, 86] = 4.17, p = 0.044) and fewer inattention problems on the Teacher Report Form (F [1, 83] = 7.30, p = 0.008).
Table 5.
Comparisons of Medication Acceptors and Refusers on Clinical Outcomes
| |
Medication acceptor mean (SD) |
Medication refuser mean (SD) |
|
|
|
||
|---|---|---|---|---|---|---|---|
| Variable | Intake | Posttreatment | Intake | Posttreatment | Test statistic | df | Significance (p) |
| CBCL externalizing | 72.6 (6.1) | 64.6 (10.0) | 73.4 (6.5) | 64.1 (10.0) | F = 0.60 | 1, 91 | NS, 0.442 |
| CBCL attention | 70.5 (6.9) | 64.0 (8.7) | 69.3 (8.6) | 62.6 (8.5) | F = 0.00 | 1, 91 | NS, 0.969 |
| TRF externalizing | 68.9 (11.0) | 62.9 (11.0) | 73.7 (9.4) | 68.4 (13.2) | F = 0.34 | 1, 83 | NS, 0.560 |
| TRF attention | 66.6 (10.0) | 59.7 (7.6) | 68.1 (9.1) | 66.8 (11.5) | F = 7.30 | 1, 83 | p = 0.008 |
| IOWA/C parent inattention/overactivity | 11.1 (2.6) | 7.4 (3.6) | 9.7 (3.0) | 7.5 (3.8) | F = 3.56 | 1, 92 | NS, 0.063 |
| IOWA/C parent oppositional defiant | 12.1 (2.6) | 8.3 (4.0) | 11.1 (2.8) | 7.5 (3.8) | F = 0.02 | 1, 92 | NS, 0.904 |
| IOWA/C teacher inattention/overactivity | 10.3 (4.1) | 7.2 (3.6) | 11.2 (3.5) | 9.8 (3.9) | F = 4.17 | 1, 86 | p = 0.044 |
| IOWA/C teacher oppositional defiant | 8.5 (5.1) | 9.9 (4.0) | 5.6 (4.6) | 7.8 (5.5) | F = 0.74 | 1, 86 | NS, 0.393 |
Analyses were based on repeated measure analysis of covariances using ADHD medication use at intake as a covariate.
CBCL = Child Behavior Checklist; TRF = Teacher Report Form; IOWA/C = IOWA/Conners' Rating Scale; SD = standard deviation; NS = not statistically significant; df = degrees of freedom; ADHD = attention-deficit/hyperactivity disorder.
Discussion
This study examines correlates of medication use at intake and study medication refusal among children with ADHD referred for treatment of their disruptive disorders. Parental variables were among the most salient correlates, as decreased medication acceptability was related to ADHD medication status at intake and during study intervention. These findings remained significant when excluding those already on medications at intake. Additional parental factors reflected that increased parental self-efficacy and the parents' emotional support were associated with medication refusal during the treatment study phase.
Medication-refusing (vs. accepting) parents viewed their children as having fewer ADHD symptoms and being equally disruptive, but teachers perceived medication refusers as more disruptive. The variation by adult informant here may reflect a host of moderating influences, such as the setting of observation or the degree of parent–teacher communication, among other possibilities. These findings may relate to discrepancies between professional and lay explanatory models for disruptive behavior and whether the child's behaviors were interpreted as “normal adolescence” or “a problem” behavior that may be based on explanatory models of the child as well as the family as “bad” versus “ill.” Problem identification is the critical first step in the help-seeking stage when considering professional intervention (Bussing and Faye 2001). Our finding that increased parental self-efficacy and parents' emotional support were associated with medication refusal was unexpected as they were not included in our initial hypotheses. This finding may reflect a form of parental resiliency that led to a lowered sense of impairment related to the child's symptom severity and, as a result, less of a perceived need for a medication intervention. In assessing social networks for elementary school parents of students at high risk for ADHD, Bussing et al. (2003) also found that higher levels of instrumental support lowered the likelihood, whereas parental strain increased the likelihood, of ADHD treatment.
Contrary to our hypotheses, few demographic, family, or insurance factors were associated with medication refusal. The fact that minority status and lower SES were significant correlates at intake and not during the study intervention may reflect issues of treatment accessibility, feasibility, treatment alliance, and timing of recommendations. As no differences were seen for medication acceptance in clinic versus community settings, further investigations of provider alliance, type of psychoeducation, and cultural sensitivity may be helpful. Moreover, even though some parental and clinical variables were found to be associated with medication refusal, only the COTS medication acceptability variable was consistently found across several analyses and remained significant after Bonferroni correction for the number of dependent variables. These results also support Nock and Kazdin's (2001) finding that parental expectancies predict participation and premature termination over and above several family, parent, and child characteristics.
Somewhat surprisingly, medication did not produce any effect for disruptive behaviors during the course of treatment, though it showed some benefit for symptoms of inattention and overactivity. A possible explanatory variable here is that medication adherence was not rigorously monitored herein, given that our medication management occurred in the context of a modular treatment study. Rates of adherence to stimulant medication regimens are similar to rates found among children or adults with other chronic diseases with average rates of about 50% and with decrements in adherence occurring with time (Osterberg and Blaschke 2005). Hence, ADHD medication management is probably best addressed within a chronic disease model and viewed along a continuum of care.
Limitations
We conducted secondary data analyses, which precluded the use of more standardized measures for certain variables. The COTS variable of medication acceptability could have been psychometrically tested. Additionally, we could have used other instruments such as the ADHD Knowledge and Opinion Scale (Rostain et al. 1993), the Attitudes, Satisfaction, Knowledge, and Medication Experiences survey (DosReis et al. 2003), or the Treatment Acceptability Questionnaire (Krain et al. 2005). We did not determine whether those without prior treatment were even offered medication treatment previously. Examination of feasibility, perceptions of treatment relevance, intensity of therapy demands, and quality of the therapist relationship could have been explored. Additionally, child measures of medication attitudes and the interactional effects with their parents' attitudes could also extend our understanding of medication acceptability and adherence.
This study took place when the first extended release stimulant medications were brought to market. It is possible that the availability of these agents for the study could have resulted in increased acceptability, decreased “refusal” of medication, and/or increased adherence. As the refusers had access to a potentially efficacious treatment for DBDs, this could have influenced their decision. Not all children with DBDs may have access to such psychosocial treatment and may feel more compelled to try medications. Finally, it is possible that participants and their families volunteering to participate in such an intervention study do not represent children with disruptive behavior disorders who are treated in the community.
Clinical Implications
The majority of medication refusers in our study never met the psychiatrist, which highlights the role of the clinician in treatment alliance, assessment, and execution of a treatment plan, including acceptance of referral to at least discuss medication management. One may consider use of the psychiatrist earlier in the intervention to review the treatment plan and foster a collaborative team approach with the family before any focus on medication management per se. Additionally, addressing parental concerns about inadequate assessment and fear of side effects may be warranted as suggested by Monastra (2005). Once a trial has been initiated, longer-acting stimulants and frequent appointments may help to promote adherence (Charach and Gajaria 2008).
A majority of youth with ADHD do not receive medication because of either declining a medication trial or poor adherence. A substantial number of families (30% in our study) decline medication management even though they are treatment seeking and have access to care. Parental factors appear to be largely associated with medication acceptability and the initiation of a medication trial. Bussing et al. (2005) state that “beliefs about the causation of misbehavior will likely influence parental decisions about what type of interventions to pursue,” and work such as this on parental help-seeking is critical in order to more fully understand the continuum from problem identification to acceptance of a medication trial. Future studies should aim to further understand medication refusers, which in turn may impact how we initiate medication trials and promote adherence, and should also include better studies to understand why families seek care and how this impacts their willingness to accept and adhere to treatment.
Footnotes
This study was funded by a grant from the National Institute of Mental Health (No. MH57727).
Disclosures
Since the time of the original data collection (but no conflicts in the past year), Oscar G. Bukstein has been a member of the Speakers Bureau of Shire Pharmaceuticals, McNeil Pediatrics, Novartis, Quintiles; consultant for Shire Pharmaceuticals, McNeil Pediatrics, Cephalon, Quintiles; and research support for Shire Pharmaceuticals, OrthoMcNeil Jannsen. Drs. Demidovich and Kolko and Mr. Hart have no conflicts of interest.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 & 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991a. [Google Scholar]
- Achenbach TM. Manual for the Teacher's Report Form & 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991b. [Google Scholar]
- American Academy of Child and Adolescent Psychiatry (AACAP) Practice parameter for the use of stimulant medications in the treatment of children, adolescents, adults. J Am Acad Child Adolesc Psychiatry. 2002;41(Suppl):26–49. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry (AACAP) Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- Beck AT. Ward CH. Mendelson M. Mock J. Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bennett DS. Power TJ. Rostain AL. Carr DE. Parent acceptability and feasibility of ADHD interventions: Assessment, correlates, and predicative validity. J Pediatr Psychol. 1996;21:643–647. doi: 10.1093/jpepsy/21.5.643. [DOI] [PubMed] [Google Scholar]
- Brown RT. Borden KA. Clingerman SR. Adherence to methylphenidate therapy in a pediatric population: A preliminary investigation. Psychopharmacol Bull. 1985;21:28–36. [PubMed] [Google Scholar]
- Brown RT. Borden KA. Wynne ME. Spunt AL. Clingerman SR. Compliance with pharmacological and cognitive treatments for attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1987;26:521–526. doi: 10.1097/00004583-198707000-00010. [DOI] [PubMed] [Google Scholar]
- Brown RT. Borden KA. Wynne ME. Spunt AL. Clingerman SR. Patterns of compliance in a treatment program for children with attention deficit disorder. J Compliance Health Care. 1988;3:23–29. [Google Scholar]
- Bukstein OG. Satisfaction with treatment for attention-deficit/hyperactivity disorder. Am J Manag Care. 2004;10(Suppl 4):107–116. [PubMed] [Google Scholar]
- Bussing R. Faye G. Practice guidelines and parental ADHD treatment evaluations: Friends or foes? Harv Rev Psychiatry. 2001;9:223–233. doi: 10.1080/10673220127905. [DOI] [PubMed] [Google Scholar]
- Bussing R. Koro-Ljungberg ME. Gary F. Mason DM. Garvan CW. Exploring help-seeking for ADHD symptoms: A mixed-methods approach. Harv Rev Psychiatry. 2005;13:85–101. doi: 10.1080/10673220590956465. [DOI] [PubMed] [Google Scholar]
- Bussing R. Zima BT. Gary FA. Mason DM. Leon CE. Sinha K. Garvan CW. Social networks, caregiver strain, and utilization of mental health services among elementary school students at high risk for ADHD. J Am Acad Child Adolesc Psychiatry. 2003;7:842–850. doi: 10.1097/01.CHI.0000046876.27264.BF. [DOI] [PubMed] [Google Scholar]
- Charach A. Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8:1563–1571. doi: 10.1586/14737175.8.10.1563. [DOI] [PubMed] [Google Scholar]
- Charach A. Ickowicz A. Schachar R. Stimulant treatment over five years: Adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43:559–567. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- Corkum P. Rimer P. Schachar R. Parental knowledge of attention-deficit hyperactivity disorder and opinions of treatment options: Impact on enrollment and adherence to a 12 month treatment trial. Can J Psychiatry. 1999;44:1043–1048. doi: 10.1177/070674379904401011. [DOI] [PubMed] [Google Scholar]
- Derogatis L. Rickels K. Rock AF. The SCL-90 and the MMPI: A step in validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- dosReis S. Zito JM. Safer DJ. Soeken KL. Mitchell JW. Ellwood LC. Parental perceptions and satisfaction with stimulant medication for attention-deficit/hyperactivity disorder. J Dev Behav Pediatr. 2003;24:155–162. doi: 10.1097/00004703-200306000-00004. [DOI] [PubMed] [Google Scholar]
- Evans ME. Boothroyd RA. Armstrong MI. Development and implementation of an experimental study of the effectiveness of intensive in-home crisis services for children and their families. J Emot Behav Disord. 1997;5:93–105. [Google Scholar]
- Firestone P. Factors associated with children's adherence to stimulant medication. Am J Orthopsychiatry. 1982;52:447–457. doi: 10.1111/j.1939-0025.1982.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Gage JD. Wilson LJ. Acceptability of attention-deficit/hyperactivity disorder interventions: A comparison of parents. J Atten Disord. 2000;4:174–182. [Google Scholar]
- Gau SS. Shen H. Chou M. Tang C. Chiu Y. Gau C. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16:286–297. doi: 10.1089/cap.2006.16.286. [DOI] [PubMed] [Google Scholar]
- Grcevich S. Hodgkins P. Broken M. Capone N. Prescription fill rates, adherence to ADHD medications. Presented at the American Academy of Child and Adolescent Psychiatry Annual Meeting; San Diego, CA. 2006. (poster). [Google Scholar]
- Hazzard A. Christensen A. Margolin G. Children's perceptions of parental behaviors. J Abnorm Child Psychol. 1983;11:49–59. doi: 10.1007/BF00912177. [DOI] [PubMed] [Google Scholar]
- Hoagwood K. Horwitz S. Stiffman A. Weisz JR. Bean D. Rae D. Compton W. Cottler L. Bickman L. Leaf P. Concordance between parent reports of children's mental health services and records: The services assessment for children and adolescents (SACA) J Child Fam Stud. 2000;9:315–331. [Google Scholar]
- Hollingshead A. Redlich F. Social Class and Mental Illness: A Community Study. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim ES. Rates of adherence to pharmacological treatment among children and adolescents with attention deficit hyperactivity disorder. Hum Psychopharmacol. 2002;17:225–231. doi: 10.1002/hup.406. [DOI] [PubMed] [Google Scholar]
- Jensen PS. Bhatara VS. Vitiello B. Hoagwood K. Feil M. Burke LB. Psychoactive medication prescribing practices for U.S. children: Gaps between research and clinical practice. J Am Acad Child Adolesc Psychiatry. 1999;38:557–565. doi: 10.1097/00004583-199905000-00017. [DOI] [PubMed] [Google Scholar]
- Johnson C. Fine S. Methods of evaluating methylphenidate in children with attention deficit hyperactivity disorder: Acceptability, satisfaction, and compliance. J Pediat Psychol. 1993;18:717–730. doi: 10.1093/jpepsy/18.6.717. [DOI] [PubMed] [Google Scholar]
- Johnston C. Hommersen P. Seipp C. Acceptability of behavioral and pharmacological treatments for attention-deficit/hyperactivity disorder: Relations to child and parent characteristics. Behav Ther. 2008;39:22–32. doi: 10.1016/j.beth.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. Kaufman NL. Kaufman Brief Intelligence Test (K-BIT) Manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Ryan N. Kiddie-SADS-Present, Lifetime Version (K-SADS-PL): Diagnostic Interview. Pittsburgh, PA: Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center; 1996. [Google Scholar]
- Kauffman RE. Smith-Wright D. Reese CA. Simpson R. Jones F. Medication compliance in hyperactive children. Pediatr Pharmacol. 1981;1:231–237. [PubMed] [Google Scholar]
- Kazdin AE. Crowley MJ. Moderators of treatment outcome in cognitively based treatment of antisocial children. Cogn Ther Res. 1997;21:185–207. [Google Scholar]
- Kelley ML. Heffer RW. Gresham FM. Elliot SN. Development of a modified treatment evaluation inventory. J Psychopathol Behav Assess. 1989;11:235–247. [Google Scholar]
- Kolko DJ. Conduct disorder. In: Hersen M, editor; Ammerman RT, editor; Sisson L, editor. Handbook of Aggressive and Destructive Behavior in Psychiatric Patients. New York: Plenum Press; 1994. pp. 21–50. [Google Scholar]
- Kolko DJ. Treatment development, assurance of treatment fidelity, specificity. Presented at the American Academy of Child and Adolescent Psychiatry Annual Meeting; New Orleans, LA. 1995. (symposium). [Google Scholar]
- Kolko DJ. Dorn LD. Bukstein OG. Pardini J. Holden EA. Hart J. Community vs. clinic-based modular treatment of children with early-onset ODD or CD: A clinical trial with 3-year follow-up. J Abnorm Child Psychol. 2009;37:591–609. doi: 10.1007/s10802-009-9303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolko DJ. Swenson CC. Assessing and Treating Physically Abused Children and Their Families: A Cognitive-Behavioral Approach. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Krain AL. Kendall PC. Power TJ. The role of treatment acceptability in the initiation of treatment for ADHD. J Attn Disord. 2005;9:425–434. doi: 10.1177/1087054705279996. [DOI] [PubMed] [Google Scholar]
- Loney J. Milich R. Hyperactivity, inattention, aggression in clinical practice. In: Wolraich M, editor; Routh DK, editor. Advances in Developmental and Behavioral Pediatrics. Greenwich: JAB Press; 1982. pp. 113–147. [Google Scholar]
- McConnaughy EA. Prochaska JO. Velicer WF. Stages of change in psychotherapy: Measurement and sample profiles. Psychother: Theory Res Pract. 1983;20:368–375. [Google Scholar]
- Monastra VJ. Overcoming the barriers to effective treatment for attention-deficit/hyperactivity disorder: A neuro-educational approach. Int J Psychophysiol. 2005;58:71–80. doi: 10.1016/j.ijpsycho.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Moos RH. Insel PM. Humphrey B. Family Work and Group Environment Scales. Palo Alto, CA: Consulting Psychologists Press; 1974. [Google Scholar]
- Multimodal Treatment Study of ADHD (MTA) Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Nock MK. Kazdin AE. Parent expectancies for child therapy: Assessment and relation to participation in treatment. J Child Fam Stud. 2001;10:155–180. [Google Scholar]
- Olson DH. Porter J. Bell RQ. FACES II: Family Adaptability and Cohesion Evaluation Scales. St. Paul, MN: Family Social Sciences, University of Minnesota; 1982. [Google Scholar]
- Osterberg L. Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Pharmacotherapy for children with attention-deficit hyperactivity disorder. Sch Psychol Rev. 1993;22:199–227. [Google Scholar]
- Pelham WE. Milich R. Murphy DA. Murphy HA. Normative data on the IOWA Connors Teachers Rating Scale. J Clin Child Psychol. 1989;18:259–262. [Google Scholar]
- Rostain AL. Power TJ. Atkins MS. Assessing parents' willingness to pursue treatment for children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1993;32:175–181. doi: 10.1097/00004583-199301000-00025. [DOI] [PubMed] [Google Scholar]
- Shelton KK. Frick PJ. Wooten J. Assessment of parenting practices in families of elementary school-age children. J Clin Child Psychol. 1996;25:317–329. [Google Scholar]
- Sleator EK. Ullmann RK. Von Neumann A. How do hyperactive children feel about taking stimulants and will they tell the doctor? Behav Pediatr. 1982;21:474–479. doi: 10.1177/000992288202100805. [DOI] [PubMed] [Google Scholar]
- Spitzer RL. Williams JW. Gibbons M. First MB. Structured Clinical Interview for DSM-III-R—Patient Edition (SCID-P, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Straus MA. Hamby SL. Finkelhor D. Moore DW. Runyan D. Identification of child maltreatment with the parent-child conflict tactics scales: Development and psychometric data for a national sample of American parents. Child Abuse Negl. 1998;22:249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Summers J. Caplan P. Laypeople's attitudes toward drug treatment for behavioral control depend on which disorder and which drug. Clin Pediatr. 1987;26:258–263. doi: 10.1177/000992288702600509. [DOI] [PubMed] [Google Scholar]
- Swanson J. Compliance with stimulants for attention-deficit/hyperactivity disorder: Issues and approaches for improvement. CNS Drugs. 2003;17:117–131. doi: 10.2165/00023210-200317020-00004. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam D. Charach A. Schachar R. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:922–928. doi: 10.1097/00004583-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Wilson LJ. Jennings JN. Parent's acceptability of alternative treatments for attention-deficit hyperactivity disorder. J Atten Disord. 1996;1:114–121. [Google Scholar]