Abstract
Transactive response DNA-binding protein of 43 kDa (TDP-43), an RNA and DNA binding protein involved in transcriptional repression, RNA splicing and RNA metabolism during the stress response, is the major component of neuronal inclusions in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin inclusions, now referred to as FTLD-TDP. While initially thought to be relatively specific to ALS and FTLD-TDP, TDP-43 pathology has now been detected in a number of other neurodegenerative diseases, many associated with tau pathology, including Guam Parkinson dementia complex and Alzheimer's disease (AD). TDP-43 pathology is detected in 25% to 50% of AD cases, especially those with more severe clinical phenotype and greater Alzheimer type pathology, as well as AD cases with hippocampal sclerosis (HS). HS is characterized by selective neuronal loss affecting CA1 sector of the hippocampus, and most cases of HS, with or without AD, have TDP-43 pathology. Whether TDP-43 pathology is merely an incidental finding in AD or actually contributing to the more severe clinical phenotype remains unresolved. Presence of TDP-43 in normal elderly, who are at increased risk for AD, would strengthen the argument that it is not merely a secondary or incidental finding in end stage AD. Limited studies suggest that TDP-43 pathology is infrequent in neurologically normal elderly (3% or less). We provide an overview of what is known about TDP-43 in AD, normal aging and in other disorders and suggest that TDP-43 proteinopathies be considered in two classes - primary and secondary.
Keywords: Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD), neurofibrillary tangles (NFT), progranulin, tau, transactive response DNA-binding protein 43 (TDP-43)
Introduction
Transactive response DNA-binding protein 43 (TDP-43) was initially described based upon its binding to the regulatory element, TAR, in the human immunodeficiency virus type 1 (HIV-1) long terminal repeat, ultimately affecting HIV-1 gene expression [1]. A decade later, its functions include, but are not limited to, acting as a transcriptional repressor, binding both RNA and DNA, and modulating gene splicing [2-6]. It is also involved in RNA metabolism during the stress response [7-9]. The marked interest in TDP-43 is related to its role in neurodegenerative diseases [10-16].
Structure and function
TDP-43 is a predominantly nuclear protein, 414 amino acids in length, with an estimated molecular mass of approximately 43 kDa [1]. The TARDBP gene on chromosome 1, which encodes TDP-43, is 6 exons in length and has up to 11 different alternative splice forms [17], the predominant being the 43 kDa form [4, 17]. Both mRNA and protein expression seem to be ubiquitous, as TDP-43 is detected in the pancreas, placenta, spleen, testis, ovary, lung, kidney, spinal cord and brain [3]. This distribution holds true for both rodents and humans although the actual levels of expression may vary amongst these tissues and also between species [3]. Evolutionarily speaking, the TARDBP gene is highly conserved and has been found in all higher species, as well as in Drosophila melanogaster and Caenorhabditis elegans [18], signifying the importance of its function. In addition, TARDBP knockouts are embryonic lethal due to peri-implantation defects [19].
The primary structure of TDP-43 resembles that of a heterogeneous nuclear ribonucleoprotein family member [1]. This type of structure includes two RNA recognition motifs and a glycine -rich C-terminal tail [17]. One of the RNA recognition motifs has been shown to bind to the gene for the cystic fibrosis transmembrane conductance regulator (CFTR), allowing for skipping of exon 9 through alternative RNA splicing, contributing to cystic fibrosis [17]. The glycine rich C-terminal tail contains most of the known mutations, suggesting that neurotoxic effects of TDP-43 are driven by this domain [20-23]. Im-munohistochemical staining of C-terminal fragments are enriched in TDP-43 inclusions [24]. In vitro work has also shown these fragments to be toxic [21, 25].
Numerous functions have been proposed for TDP-43 through studies in cell culture experiments, animal models and biochemical assays [26-29]. Most functions suggest a role of TDP-43 in transcriptional repression, RNA metabolism and gene splicing. These roles involve -TDP -43 binding to both RNA and DNA. These interactions converge around a conserved poly- UG sequence contained in RNA [30]; however, DNA binding domains have not been elucidated, suggesting a more indirect effect. Recent studies have suggested that it is also a component of stress granules induced by cell stress, such as oxidative or osmotic stress [7-9].
Pathology of TDP-43 in FTLD-TDP and ALS
In affected neurons and glia in neurodegenerative disorders, TDP-43 is absent from its normal nuclear location and found in the cytoplasm in the form of inclusion bodies, which are associated with insoluble forms of the protein in biochemical extracts of affected tissue [12]. Pathological aggregates in FTLD-TDP with or without motor neuron disease and in amyotrophic lateral sclerosis (ALS) contain protein with posttranslational modifications, including phos-phorylation, ubiquitination and proteolytic cleavage [12, 24, 31-33]. These forms of TDP-43 have been shown to accumulate in cytosolic and nuclear fractions [34]. Abnormal forms of TDP-43 have been shown with immunoelectron microscopic to accumulate as intracellular filamentous inclusions in neurons and glia [35, 36].
The morphology and anatomical pattern of TDP-43 inclusions shows disease specificity that correlate with clinical and genetic phenotypes [14, 37]. Table 1 summarizes features of FTLD-TDP subtypes as originally defined by Mackenzie and colleagues based on clinical features and distribution of abnormal TDP-43 [37] . More recently, this scheme has been validated and extended to subcortical regions [14]. Table 1 also includes limited studies of TDP-43 pathology in normals. The commonality in all FTLD-TDP subtypes is pathology in frontal and temporal neocortical regions, but not in occipital cortex or cerebellum [38]. The relative amount of inclusions in different cellular structures differs among the FTLD-TDP types. For example, Type 1 is associated with widespread cortical and subcortical neuronal cytoplasmic inclusions (NCI), dystrophic neurites (DN) and neuronal intraneuronal inclusions (NII), while Type 2 has predominantly DN in the cortex and Pick-body like NCI in the dentate fascia, amygdala and basal ganglia, and Type 3 has mainly NCI in medial temporal lobe structures and in cases with motor neuron disease, NCI in motor neurons [14].
Table 1.
| Pathologic classification | Clinical presentation | Genetic links | TDP-43 cellular locations | Frequency in disease |
|---|---|---|---|---|
| FTLD-TDP type 1 | FTDbv or PNFA | Progranulin (RGN) mutations | DN, NCIs and NII in layer II of frontal and temporal cortex. | 32% of FTLD-TDP43 |
| FTLD-TDP type 2 | Semantic dementia | None | DN predominant in lower layers of cerebral cortex | 27% of FTLD-TDP43 |
| FTLD-TDP type 3 | Motor neuron disease | Familial cases linked to chromosome 9 | NCI in cerebral cortex and hippocampus | 42% of FTLD-TDP43 |
| AD | Unknown | Unknown | DN and NCI in amygdala and hippocampus | 23% |
| Normal | N/A | Unknown | DN, NCI and NII in amygdala and hippocampus | 3% |
Classification according to Mackenzie, et al. [72] Abbreviations: dystrophic neurites (DN), neuronal cytoplasmic inclusions (NCI), intraneuronal inclusions (NII), progressive non-fluent aphasia (PNFA), FTDbv = behavioral variant of frontotemporal dementia; N/A = not appropriate.
TDP-43 and Alzheimer's disease
In it increasingly clear that abnormal TDP-43 immunoreactivity is common in AD [15, 16, 38-41]. Presence of TDP-43 neuronal and glial inclusions is estimated to be approximately 25-30% of sporadic AD cases [15, 16, 39], but perhaps lower (14%) in familial AD and Down's syndrome [41]. The wide range in frequency of TDP -43 pathology in various disorders is in part related to methodology, since higher frequency of TDP-43 pathology is detected in AD when immunohistochemistry is performed with antibodies that are specific to pathologic forms of TDP-43, such as abnormal phospho-epitopes [42] or carboxyl-terminal epitopes [21, 24].
The potential clinical implications of TDP-43 immunoreactivity in AD have been explored by investigating associations between presence of abnormal TDP-43 and both imaging and behavioral features. These studies suggest that presence of TDP-43 is associated with greater brain atrophy (in particular hippocampal atrophy) and more severe clinical deficits [40]. Hippocampal atrophy is also associated with hippocampal sclerosis in the elderly [43, 44], a relatively common coexistent finding in elderly with dementia [45]. Given that TDP-43 is frequent in hippocampal sclerosis [16, 46-48] its role in the worse hippocampal atrophy needs to be considered. After controlling for concomitant pathology, hippocampal atrophy continued to be greater in AD with TDP-43 pathology compared to AD without TDP-43 pathology [40].
In the setting of AD the most common pattern of TDP-43 pathology is that in which it is limited to limbic regions of the brain, including the hippocampus, amygdala and adjacent cortices [15, 49, 50]. These distribution overlaps with tau pathology in AD in the form of neuropil threads and neurofibrillary tangles (NFT) [51]. In fact, some of the TDP-43 pathology in AD has been shown to be within neurons with NFT using double labeling immunofluorescent microscopy and double labeling immunoelectron microscopy [16]. Such a relationship has not been found for amyloid plaques. Several studies have addressed the proportion of TDP-43 pathology that is associated with NFT, with results ranging from more than 14% [50] to almost none [15]. Clearly, there is a wide range of TDP-43 pathology in AD, with some indistinguishable from that seen in FTLD-TDP, including presence of neuronal cytoplasmic and intranuclear inclusions as well as dystrophic neurites to cases in which the brunt of the TDP-43 is in neurons vulnerable to NFT. Given that other tauopathies such as Guam Parkinson dementia complex [10], corticobasal degeneration [15] and argyrophilic grain disease [52] have TYDP-43 pathology, more biochemical evidence is needed to determine if there is protein-protein interaction between tau and TDP-43.
Genetics
Common and rare variants in the gene for TDP-43 (TARDBP) have not been studied extensively in AD and other disorders with TDP-43 pathology. With respect to AD, one recent study investigated 8 different TARDBP single-nucleotide polymorphisms (SNPs) in a Japanese population [53] and found no significant association between 181 AD patients and 130 age-matched controls. In addition, no synergistic effects were observed between APOE genotypes and the TARDBP SNPs for the AD cohort [53]. The most common cause of familial FTLD-TDP is mutation in the gene for progranulin (GRN) [54-56]. Of interest is the fact that common variants in the 3'-untranslated region of GRN in a possible micro-RNA binding site have been shown to associated with risk of FTLD-TDP [57] and hippocampal sclerosis in the elderly [46]. It remains to be determined if it is associated with TDP-43 pathology in AD, although one study suggested a trend for this [46]. How changes in progranulin levels lead to TDP-43 pathology remains an unanswered research question, but given that progranulin is a growth factor and that growth factor withdrawal is associated with programmed cell death [58], it is tempting to speculate that activation of programmed cell death pathways may play a role, as suggested by some in vitro experiments [31]. Further studies are needed to determine if this is a viable mechanism in human neurodegenerative disorders.
TDP-43 in normal brain
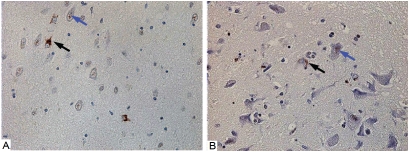
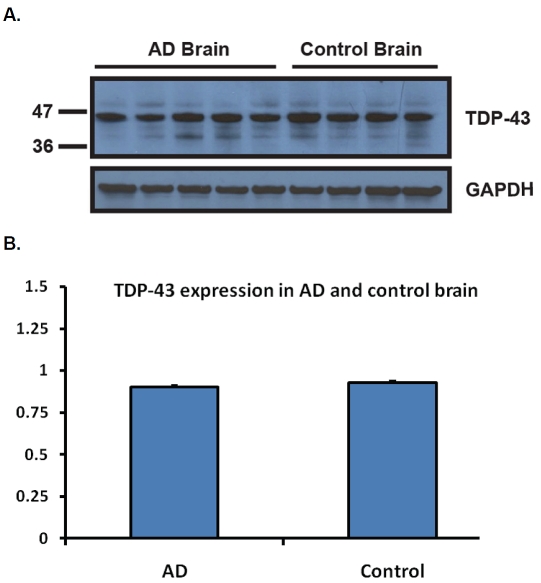
The majority of the studies on TDP-43 have focused on its abnormal distribution in disease, including FTLD-TDP, ALS and a range of other disorders [10, 15, 38, 48, 52, 59]. There have been far fewer studies looking at changes in TDP-43 associated with aging. To better understand the frequency of TDP-43 inclusions in normal individuals, TDP-43 immunohistochemistry was used to study 63 neurologically normal individuals ranging in age from 23 to 94 years at death (median 71). Immunohistochemistry was performed as in other studies [16, 60] and the region used for screening was the amygdala, a region known to be predominately affected in AD [61]. Two of the 63 controls (3%) had TDP-43 inclusions - an 81-year-old man and an 83-year-old woman. Both were cognitively normal without any clinical features of motor neuron disease. Additional immunohistochemistry of medial and superior frontal cortex, superior temporal, cingulate cortex, anterior and posterior hippocampus, striatum, midbrain, pons, medulla and spinal cord of the two positive cases showed that abnormal TDP-43 was restricted to limbic regions (Figure 1). These findings are similar to the only other report on the frequency of abnormal TDP-43 in normal controls, which demonstrated 1 case out of 33 controls (3%) and TDP-43 inclusions limited to hippocampus and entorhinal cortex [62]. To study possible biochemical changes in TDP-43 expression in AD and normals, frontal cortical tissue samples from four neurologically normal controls and five AD cases were homogenized and extracts subjected to western blot analysis. No significant differences were observed in relative expression levels between AD and controls (Figure 2). None of the AD or controls had abnormal lower molecular weight (25 kDa and 35 kDa) cleavage fragments comparable to those found in FTLD-TDP and ALS [12].
Figure 1.
TDP-43 immunoreactivity in normals. TDP-43 immunohistochemistry in the amygdala of the positive cases at a 40X magnification. Black arrows indicate pathological TDP-43 inclusions, while blue arrows indicate normal nuclear TDP-43. A. An 83-year-old woman with medial temporal tangles and grains. B. An 81-year-old man with minimal entorhinal tangles.
Figure 2.
TDP-43 expression in age-matched AD and control subjects. A. Five age-matched AD and four control brain samples were homogenized and run on a 10-20% Tricine Gel. The membrane was probed with the monoclonal TDP-43 antibody (EnCor Biotechnology, Gainesville, FL). One clean band migrating at the expected molecular weight was observed for all samples. The membrane was stripped and re-probed with the goat polyclonal GAPDH antibody (Santa Cruz, CA) as a loading control. B. Intensities of the signal were quantified using the NIH Image J software. There was no difference in TDP-43 expression between AD and control brain samples, after normalization to GAPDH.
Conclusion
Studies examining both genetic and pathological influences of TDP-43 in FTLD-TDP and ALS suggest that it is a primary factor in these disorders (i.e. primary TDP-43 proteinopathies), but also that it is a pathological hallmark of a neurodegenerative process that can occur in association with other distinct pathologic processes (i.e. secondary TDP-43 proteinopathies). Intriguing evidence that genetic variants in GRN may be associated with TDP-43 pathology in FTLD-TDP [57] need to be explored in other conditions in which pathologic TDP-43 is found [46]. Other genetic factors that influence progranulin levels, such as genetic variants in the gene (SORT1)[63] for the neuronal receptor for progranulin, sortilin [64], and for genes associated with risk of FTLD-TDP, including TMEM106B [65, 66], need to be explored in AD and other conditions in which TDP-43 pathology is found as part of the growing family of secondary TDP-43 proteinopathies. Additional studies are needed in clarifying biochemical changes in TDP -43 and how they may be exploited as bio-markers for differential diagnosis and early detection of TDP-43 proteinopathies [67-70] and to study the influence of this disease process in other disorders.
Acknowledgments
This study was supported in part by the grants from National Institutes of Health (AG029972 to DSW; P01AG017216, P50NS072187 to DWD) and a grant from Alzheimer Association (IIRG-08 -90524 to DSW).
References
- 1.Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 3.Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. Embo J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang IF, Wu LS, Chang HY, Shen CK. TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 6.Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, Shoesmith C. TDP43 is a human low molecular weight neuro-filament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderwyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, Petrucelli L, Wolozin B. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewey CM, Cenik B, Sephton CF, Dries DR, Mayer P, 3rd, Good SK, Johnson BA, Herz J, Yu G. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol. 2010 doi: 10.1128/MCB.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 11.Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- 12.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 13.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–878. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- 18.Ayala YM, Pantano S, D'Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C.elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Wu LS, Cheng WC, Hou SC, Yan YT, Jiang ST, Shen CK. TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis. 2010;48:56–62. doi: 10.1002/dvg.20584. [DOI] [PubMed] [Google Scholar]
- 20.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, Lin WL, Tong J, Castanedes-Casey M, Ash P, Gass J, Rangachari V, Buratti E, Baralle F, Golde TE, Dickson DW, Petrucelli L. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, Clark CM, Elman LB, Miller BL, Grossman M, McCluskey LF, Trojanowski JQ, Lee VM. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM. Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies. J Biol Chem. 2009;284:8516–8524. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, Rochefort D, Bel Hadj S, Durham HD, Vande Velde C, Rouleau GA, Drapeau P. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet. 2010;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- 27.Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, Petrucelli L, Link CD. Neuro-toxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet. 2010;19:3206–3218. doi: 10.1093/hmg/ddq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimoto Y, Ito D, Yagi T, Nihei Y, Tsunoda Y, Suzuki N. Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43. J Biol Chem. 2010;285:608–619. doi: 10.1074/jbc.M109.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Tan CF, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol(Berl) 2007 doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 34.Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- 35.Lin WL, Dickson DW. Ultrastructural localization of TDP-43 in filamentous neuronal inclusions in various neurodegenerative diseases. Acta Neuropathol. 2008;116:205–213. doi: 10.1007/s00401-008-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol. 2009;68:1167–1176. doi: 10.1097/NEN.0b013e3181baacec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King A, Sweeney F, Bodi I, Troakes C, Maekawa S, Al-Sarraj S. Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer's disease. Neuropathology. 2010 doi: 10.1111/j.1440-1789.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 39.Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 40.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Jr, Dickson DW. Abnormal TDP-43 im-munoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lippa CF, Rosso AL, Stutzbach LD, Neumann M, Lee VM, Trojanowski JQ. Transactive response DNA-binding protein 43 burden in familial Alzheimer disease and Down syndrome. Arch Neurol. 2009;66:1483–1488. doi: 10.1001/archneurol.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 43.Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 46.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis. 2010;7:170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 48.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl) 2007;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 52.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009;117:151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 53.Shibata N, Ohnuma T, Baba H, Arai H. Genetic association analysis between TDP-43 polymorphisms and Alzheimer's disease in a Japanese population. Dement Geriatr Cogn Disord. 2009;28:325–329. doi: 10.1159/000251194. [DOI] [PubMed] [Google Scholar]
- 54.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 55.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Van-denberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 56.Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL, 3rd, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 57.Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Caselli RJ, Wszolek ZK, Uitti RJ, Feldman H, Hutton ML, Mackenzie IR, Graff-Radford NR, Dickson DW. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–1165. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- 60.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 61.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 63.Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, DeJesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R. Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet. 2010;87:890–897. doi: 10.1016/j.ajhg.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL, 3rd, Ferrer I, Llado A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, Lopez de Munain A, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, Dekosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, DeCarli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tunon MT, Martinez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, Crook R, Hunter T, Ghidoni R, Benussi L, Crook J, Finger E, Hantanpaa KJ, Karydas AM, Sengdy P, Gonzalez J, Seeley WW, Johnson N, Beach TG, Mesulam M, Forloni G, Kertesz A, Knopman DS, Uitti R, White CL, 3rd, Caselli R, Lippa C, Bigio EH, Wszolek ZK, Binetti G, Mackenzie IR, Miller BL, Boeve BF, Younkin SG, Dickson DW, Petersen RC, Graff-Radford NR, Geschwind DH, Rademakers R. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2010 doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dormann D, Capell A, Carlson AM, Shankaran SS, Rodde R, Neumann M, Kremmer E, Matsuwaki T, Yamanouchi K, Nishihara M, Haass C. Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem. 2009;110:1082–1094. doi: 10.1111/j.1471-4159.2009.06211.x. [DOI] [PubMed] [Google Scholar]
- 68.Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, Johnson N, Weintraub S, Akiyama H, Arai T, Hasegawa M, Bigio EH, Benson FE, Allsop D, Mann DM. Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol. 2009;118:647–658. doi: 10.1007/s00401-009-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.HsiungGY, Fok A, Feldman HH, Rademakers R, Mackenzie IR. rs5848 polymorphism and serum progranulin level. J Neurol Sci. 2011;300:28–32. doi: 10.1016/j.jns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phos-phorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mackenzie IR, Baborie A, Pickering-Brown S, Plessis DD, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006 doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]