Abstract
Osteosarcoma is the most frequent primary malignant bone tumor. Distinct histological features are distinguishable based on the morphology of the tumor. Differences in nuclei size and shape are often observed in osteosarcoma reflecting its broad histopathological heterogeneity. This study explores the relevance of two nuclear parameters in osteosarcoma: large area and round shape. Computerized nuclear morphometry was performed in 56 conventional osteosarcoma preoperative biopsies. The mean patient follow-up time was 35.1 months. Based on the nuclear area, no significant difference (P = 0.09) in overall survival between patients with large (> 42.5 μm2) and small (< 42.5 μm2) tumor nuclei was found. However, when cases with large and round nuclei were analyzed jointly (> 42.5 μm2 and coefficient of nuclear roundness > 0.7), these two parameters together were likely to be a predictive factor (P = 0.05). Osteosarcoma patients with large and round tumor nuclei had a better outcome than patients with small and polymorphic (ovoid or spindle-shaped) nuclei. In this study, nuclear morphometry proved to be a useful tool to shed light on the biology of osteosarcoma showing that some morphometric parameters can be easily applied to help identifying patients with a good prognosis.
Keywords: Large nuclei, round nuclei, nuclear morphometry, osteosarcoma
Introduction
Osteosarcoma is the most frequent primary malignant bone tumor that primarily affects children and adolescents with an incidence of 4-5 per million [1]. Several histological subtypes are distinguishable based on the morphology of the tumor. The most common subtype is the conventional osteosarcoma, which accounts for 75% of the cases [2]. During daily routine, a broad histopathological feature is observed. Some of them are well known prognostic factor [3] (e.g., chemotherapy response, tumor size) and others are still unraveled. The presence of large tumor nuclei is described in osteosarcoma, especially in the anaplastic subtype [4]. However its biological relevance is still unclear. There has been report that cyto- or histomor-phometric analysis may give reliable histopathological information regarding tumor behaviour [5,6]. In 1982 Diamond et al. first used a measurement of the cell nucleus for estimation of prognosis in prostate cancer [7]. Later on, several other studies were carried out to establish the role of nuclear morphometry as a prognostic factor [6,8,9]. It has been shown that nuclear morphometry is valid and accurate in predicting relapse in early-stage renal cell carcinoma [10] and prognosis in rhabdomyosarcoma [6]. Taking advantage of the computerized nuclear morphometry previously described [6,11], the aim of this study was to clarify the relevance of two nuclear parameters: large area and round shape. In osteosarcoma, large and round tumor nuclei are likely to predict patient longer survival.
Materials and methods
56 preoperative biopsies were obtained from 56 patients with high grade osteosarcoma of long bones enrolled in the Brazilian osteosarcoma treatment group study 2000 [12], from january 2000 to may 2004, and treated in the Instituto de Oncologia Pediatrica, GRAACC/ UNIFESP (Grupo de Apoio ao Adolescente e à Criança com Câncer/Universidade Federal de São Paulo). Patient data were obtained by reviewing pathological reports or clinical charts. All the cases were obtained from the Department of Pathology of the Universidade Federal de São Paulo and handled according to the ethical guidelines of that institution.
All the biopsies were fixed in formalin, decalcified and embedded in paraffin according to the standard procedures. 4 urn-thick sections stained with Hematoxylin and Eosin (H&E) were used on the nuclear morphometric analysis.
Assessment of patients- and tumor-related variables
The following variables were evaluated: presence or absence of metastases at diagnosis, tumor size (< 12 cm or > 12 cm), histological subtype, local recurrence, histological response to preoperative chemotherapy (good or poor responders) and overall survival. The subtype was classified following the criteria of the World Health Organization (WHO) classification for conventional osteosarcoma [1]. Good responder was defined as 90% tumor necrosis or more in the surgical specimen after preoperative chemotherapy, and poor responder as less than 90% necrosis [13]. Overall survival was defined as the time interval between the date of enrollment onto the study and death from any cause, including secondary malignancies, or the most recent follow-up contact.
Nuclear morphometry
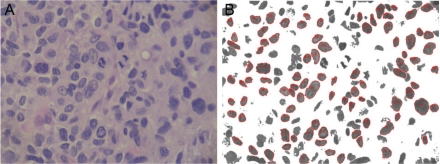
Nuclear morphometry was performed semiautomatically on H&E-stained sections using a computer connected to an Olympus BX 40 microscope with a x40 magnification lens as previously described [6,11]. The nuclei were manually selected and automatically measured by ImageJ software (NIH Image, Bethesda, MD). The color threshold module was used to remove parts of the image that not fall within the nuclear color range (Figure 1). For the nuclear morphometric evaluation the following parameters were measured: 1). Area of cell nucleus (μm2); 2). Coefficient of nuclear roundness (CNR) - it defines the degree of similarity of nuclear shape to the circle: if the shape of the nucleus is more round, the coefficient is closer to 1.
Figure 1.
Computerized nuclear morphometry. A. Osteosarcoma with large tumor nuclei before image processing. H&E, high power. B. Example of the output after image processing. The color threshold module was used to remove the background which allowed a precise selection of the nuclei. Nuclear measurements were automatically performed regarding area and roundness.
To reduce bias, the selection of the tumor nuclei was carried out without knowledge of the clinical or pathological report. For every sample at least 100 nuclei were evaluated and the mean of all measured nuclei was used (Table 1). As the histological response to chemotherapy is a good prognostic factor in osteosarcoma patients [1], the mean of the nuclear area of the good responder cases was used as a cut-off to define the following types of cell nuclei: small nuclei and large nuclei. Nucleus was considered round when the roundness coefficient was above 0.70.
Table 1.
Distribution of clinicopathological parameters and cross-tabulations with nuclear area and coefficient of nuclear roundness (CNR)
| Nuclear Area | Nuclear Area and CNR | ||||||
|---|---|---|---|---|---|---|---|
| Variable | No. of cases | Small nuclei | Large nuclei | P-value | Small and ovoid/spindleshaped nuclei | Large and round nuclei | P-value |
| Roundness coefficient | |||||||
| round nuclei | 23 | 13 | 10 | .00 | - | - | - |
| non- round nuclei | 33 | 29 | 4 | - | - | ||
| Necrosis grade | |||||||
| Poor responders | 40 | 34 | 6 | .00 | 36 | 4 | .01 |
| Good responders | 16 | 8 | 8 | 10 | 6 | ||
| Tumor size | |||||||
| < 12 cm | 25 | 17 | 8 | .27 | 19 | 6 | .28 |
| >12cm | 31 | 25 | 6 | 27 | 4 | ||
| Local recurrence | |||||||
| Yes | 33 | 22 | 11 | .08 | 26 | 7 | .43 |
| No | 23 | 20 | 3 | 20 | 3 | ||
| Metastasis at diagnosis | |||||||
| Yes | 26 | 22 | 4 | .12 | 24 | 2 | .06 |
| No | 30 | 20 | 10 | 22 | 8 | ||
| Death | |||||||
| Yes | 26 | 17 | 9 | .12 | 27 | 3 | .09 |
| No | 30 | 25 | 5 | 19 | 7 | ||
Statistical analysis
For all statistical tests, SPSS 16.0 software was used. Survival curves were plotted using the Kaplan-Meier procedure and multivariable analysis was performed by using the Multivari-ate Cox Regression method. Correlations were studied by contingency tables and Pearson's chi -square (χ2) test or one-way ANOVA. P-values <0.05 were considered statistically significant.
Results
Clinical features
The ages of the patients ranged from 8 to 26 years (mean age, 20 years) and 50% of them (28 of 56) were in the second decade of life. 38 patients were male and 18 were female. In 52 cases the lesion was located in long bones of the lower extremity (34 femurs, 17 tibias and 1 fibula). Additionally, twenty-four patients (42%) had lung metastasis at the time of diagnosis. The mean follow-up time was 35.1 months (range, 9 to 74 months).
Pathologic features
Pathological data for each of the 56 patients are summarized in Table 2. 62.5% of the cases were subclassified as osteoblastic, 23.2% as chondroblastic, 5.4% as fibroblastic and 8.9% as other rare variants. The proportion of good responders to preoperative chemotherapy was 46% and of poor responders was 54%.
Table 2.
Mean values of nuclear morphometry in the histological subtypes of osteosarcoma
| Area (μm2) | Coefficient of nuclear roundness | |
|---|---|---|
| Osteoblastic (n = 35) | 35.17±10.59 | 0.67±0.03 |
| Chondroblastic (n = 13) | 31.47±7.86 | 0.67±0.03 |
| Fibroblastic (n = 3) | 34.91±12.44 | 0.65±0.02 |
| Others (n = 5) | 44.77±6.85 | 0.72±0.05 |
Nuclear morphometry
The mean values of the nuclear morphometry are summarized in Table 1. No significant difference in the mean value of nuclear area between the osteosarcoma subtypes was observed (P = 0.10) (Table 2). Based on the mean of the nuclear area of the good responder cases, a cutoff was used to define cases with large (> 42.5 μm2) and small (< 42.5 μm2) nuclei. 42 osteosarcomas (75%) were classified as cases with small nuclei and 14 (25%) as cases with large nuclei (Figure 2). The results are shown in Table 2.
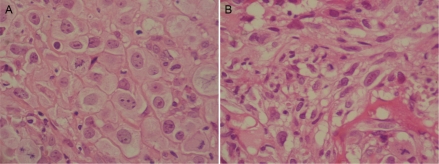
Figure 2.
Osteosarcoma can display a variety of histopathological features. A. Osteosarcoma with large and round tumor nuclei. H&E, high power. B. Osteosarcoma with small tumor cell nuclei. H&E, high power.
As nuclear polymorphism is described in osteosarcoma, in all the studied samples, round, ovoid and spindle-shaped nuclei were observed. The coefficient of nuclear roundness was used to define tumors with round (> 0.70) or non-round shape (< 0.70). 10 tumors (18%) were classified as cases with large and round nuclei (mean area value > 42.5 μm2 and CRN > 0.7) and 46 (82%) as cases with small and polymorphic (ovoid or spindle-shaped) nuclei (median area value < 42.5 μm2 and CRN < 0.7). The results are shown in Table 2.
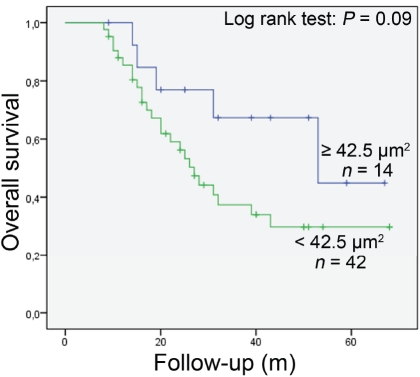
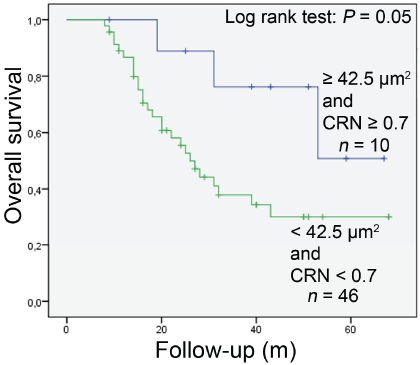
Among the 56 patients analyzed, 30 were alive at the time of the analysis (53.6%) and 26 had died (46.4%). No significant difference in overall survival between patients with large and small tumor nuclei was seen (P = 0.09) (Figure 3). However, when cases with large and round nuclei were analyzed jointly, these two parameters together were likely to be a predictive factor (P = 0.05) (Figure 4). Osteosarcoma patients with large and round tumor nuclei had a better out-come than patients with small and polymorphic (ovoid or spindle-shaped) nuclei (Figure 4). Multivariate Cox regression analysis showed that large and round tumor nuclei was not an independent predictive factor for the overall survival of these patients.
Figure 3.
Kaplan-Meier overall survival curves in all analysed osteosarcoma patients according to nuclear area of tumour cells (large nuclei: ≥ 42.5 μm2; small nuclei: < 42.5 μm2). No differences in outcome between the patients was observed.
Figure 4.
Kaplan-Meier overall survival curves in all analysed osteosarcoma patients according to nuclear area and coefficient of nuclear roundness (CRN) of tumour cells (large and round nuclei: ≥ 42.5 μm2 + CRN ≥0.7; small and ovoid/spindle-shaped nuclei: < 42.5 μm2 + CRN < 0.7). Patients with large and round nuclei had better prognosis.
Discussion
Computerized nuclear morphometry has been shown as a good and cheap tool to predict patient outcome in several tumors [6,8]. During daily practice, differences in nuclei size and shape are often observed in osteosarcoma reflecting its broad histopathological heterogeneity. Large cells, up to 100 μm in diameter, are described in the anaplastic subtype [14]. However the relevance of larger nuclei in osteosarcoma is still unclear. On the other hand, small cell osteosarcoma has a slightly worse prognosis [15]. It has shown that cell size and shape can predict the effect of chemotherapy in osteosarcoma [16].
In this study, large nuclei were not associated to the chondroblastic subtype indicating that this feature was present in others variants. Large nuclei, defined only by the nuclear area, can have a round-, ovoid- or spindle-shape. The coefficient of nuclear roundness was used to identify cases with large and round nuclei. The presence of large and round tumor nuclei in osteosarcoma is likely to be associated with longer survival. Interestingly, only large nuclei could not predict good outcome. In this series, patients with large and round nuclei responded better to the preoperative chemotherapy and had a longer survival which might indicate that these tumors were more drug-sensitive. The association between two nuclear parameters, large area and round shape, might reflect a subset of poorly differentiated osteosarcoma with more anaplastic features: abundant eosinophilic cytoplasm, minimal osteoid and cartilage production, multiple nucleolated nuclei with prominent nucleoli. In preoperative biopsy, a list of differential diagnosis might be considered in the cases with large and round cell/nuclei with minimal osteiod formation. This list has to include malignant lymphoma, malignant melanoma (metastatic), metastatic carcinoma and malignant fibrous histiocytoma. The immunore-activity for osteocalcin might be useful in diagnosing osteosarcomas [14].
In rhabdomyosarcoma, a tumor also derived from mesenquimal tissue, nuclear morphometry has shown that the clinical outcome is better if the nuclei seen in biopsies are more spindle-shaped and, in the embryonal subtype, patients with small nuclei had a better outcome then patients with large nuclei [6]. It suggests that the relationship between nuclear size and prognosis seems to be tumor specific.
Taken all the findings together, nuclear morphometry proved to be a useful tool to shed light on the biology of osteosarcoma showing that some morphometric parameters can be easily applied to help identifying patients with a good prognosis.
Acknowledgments
The authors thank the pathology department of the Federal University of São Paulo for the support and useful discussions. This study was partially supported by CAPES (Brazilian Higher Education Staff Training Agency).
References
- 1.World Health Organization Classification of Tumours, editor. Lyon: IARC Press; 2002. Pathology and Genetics of Tumours of Soft Tissue and Bone. [Google Scholar]
- 2.Unni KK. Osteosarcoma of bone. J Orthop Sci. 1998;3:287–94. doi: 10.1007/s007760050055. [DOI] [PubMed] [Google Scholar]
- 3.Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009;35:1030–6. doi: 10.1016/j.ejso.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin DC, Unni KK. 4 ed. Springfield, Illinois: C.C. Thomas Publ; 1986. Bone tumors. General aspects and data on 8,542 cases. [Google Scholar]
- 5.Ishida T, Kikuchi F, Machinami R. Histological grading and morphometric analysis of cartilaginous tumours. Virchows Arch A Pathol Anat Histopathol. 1991;418:149–55. doi: 10.1007/BF01600290. [DOI] [PubMed] [Google Scholar]
- 6.Kazanowska B, Jelen M, Reich A, Tarnawski W, Chybicka A. The role of nuclear morphometry in prediction of prognosis for rhabdomyosarcoma in children. Histopathology. 2004;45:352–9. doi: 10.1111/j.1365-2559.2004.01948.x. [DOI] [PubMed] [Google Scholar]
- 7.Diamond DA, Berry SJ, Jewett HJ, Eggleston JC, Coffey DS. A new method to assess metastatic potential of human prostate cancer: relative nuclear roundness. J Urol. 1982;128:729–34. doi: 10.1016/s0022-5347(17)53158-4. [DOI] [PubMed] [Google Scholar]
- 8.Veltri RW, Miller MC, Isharwal S, Marlow C, Makarov DV, Partin AW. Prediction of prostate-specific antigen recurrence in men with long-term follow-up postprostatectomy using quantitative nuclear morphometry. Cancer Epidemiol Biomarkers Prev. 2008;17:102–10. doi: 10.1158/1055-9965.EPI-07-0175. [DOI] [PubMed] [Google Scholar]
- 9.Bol MG, Baak JP, Rep S, et al. Prognostic value of proliferative activity and nuclear morphometry for progression in TaT1 urothelial cell carcinomas of the urinary bladder. Urology. 2002;60:1124–30. doi: 10.1016/s0090-4295(02)01906-4. [DOI] [PubMed] [Google Scholar]
- 10.Carducci MA, Piantadosi S, Pound CR, Marx WL, Kruse AJ, Bos SD, Kisman O, Voorhorst FJ. Nuclear morphometry adds significant prognostic information to stage and grade for renal cell carcinoma. Urology. 1999;53:44–9. doi: 10.1016/s0090-4295(98)00440-3. [DOI] [PubMed] [Google Scholar]
- 11.De Andrea CE, Bleggi-Torres LF, Alves MTS. Nuclear morphometric analysis: description of the methodology and the role of image-editing softwares. J Bras Patol Med Lab. 2008;44:51–7. [Google Scholar]
- 12.Benites EC, Paiva MG, Cappellano AM. Clinical, serum cardiac troponin T and echocardio-graphic evaluation for prediction of late doxorubicin cardiotoxicity. J Clin Oncol [abstract] 2006;24(18S):9536. [Google Scholar]
- 13.Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38:1218–25. doi: 10.1016/s0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Marui T, Mizuno K, Kizaki T, Minami R, Hanioka K, Hayashi Y. Anaplastic osteosarcoma with abundant eosinophilic cytoplasm and minimal osteoid production. Pathol Int. 2000;50:553–7. doi: 10.1046/j.1440-1827.2000.01077.x. [DOI] [PubMed] [Google Scholar]
- 15.Ayala AG, Ro JY, Raymond AK, Jaffe N, Chawla S, Carrasco H, Link M, Jimenez J, Edeiken J, Wallace S. Small cell osteosarcoma. A clinico-pathologic study of 27 cases. Cancer. 1989;64:2162–73. doi: 10.1002/1097-0142(19891115)64:10<2162::aid-cncr2820641031>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Apel R, Delling G, Krumme H, Winkler K, Salzer-Kuntschik M. Nuclear polymorphism in osteosarcomas as a prognostic factor for the effect of chemotherapy. A quantitative study. Virchows Arch A Pathol Anat Histopathol. 1985;405:215–23. doi: 10.1007/BF00704373. [DOI] [PubMed] [Google Scholar]