Abstract
D2-40 is a recently available mouse monoclonal antibody specific for human podoplanin and has been used in identifying lymphovascular invasion (LVI) of tumors. Although its expression has been evaluated in other tissues, its use as a marker for myoepithelial cells (MEC) of breast has not been studied. To explore its expression in the MEC of breast, paraffin-embedded tissue blocks from 48 patients with breast diseases were selected to include usual ductal hyperplasia (UDH, 41 cases), atypical ductal hyperplasia (ADH, 4 cases) and ductal carcinoma in situ (DCIS, 17 cases). Normal breast parenchyma and invasive carcinoma coexisting in the tissue sections were also included in the study. Immunohistochemistry for D2-40, calponin and p63 was performed and the staining patterns were reviewed and compared. D2-40 immunohistochemical staining is positive in the cytoplasm of MEC in UDH, ADH, and the majority of DCIS. The staining pattern of D2-40 is comparable with that of calponin, however D2-40 staining of MEC is weaker than that of calponin and with less background. In addition, myoepithelial cells and myofibroblasts at the edge of retraction spaces of DCIS are also stained by D2-40 that could be misinterpreted as tumor LVI. In conclusion, D2-40 immunohistochemistry reliably identifies the MEC of breast in a variety of lesions in a pattern similar to that of calponin and p63, and can be used as an additional MEC marker. Caution should be exercised when interpreting the staining of cells surrounding DCIS and carcinoma with retraction artifact.
Keywords: D2-40, myoepithelial cells, breast, lymphovascular invasion
Introduction
Proliferative lesions of breast, such as florid ductal hyperplasia and sclerosingadenosis, and carcinoma in situ involving lobules, can closely mimic the growth pattern of invasive carcinoma. Identification of myoepithelial cells (MEC) has great value in the accurate diagnosis, as almost invariably the MEC are absent from invasive tumors and present in benign lesions and at the periphery of in situ carcinomas. MEC can be difficult to detect in routine sections, and immunohistochemical staining of MEC is helpful [1-4]. A number of MEC markers have been commonly used, including smooth muscle actin, calponin, smooth muscle myosin heavy chain, CD10 and p63, with varying sensitivity and specificity.
D2-40 is a recently available monoclonal anti-body directed against human podoplanin, a transmembrane mucoprotein that is expressed in lymphatic endothelial cells [5]. The diagnostic value of D2-40 immunohistochemistry has been proven in a variety of lymphovascular neoplasms, such as lymphangioma, Kaposi sarcoma, and hemangioendothelioma. Meanwhile its expression at tumor cells is also identified in nonvascular neoplasms such as epithelioid mesothelioma, seminoma, adrenal cortical carcinoma, skin adnexal carcinomas, follicular dendritic cell tumor, schwannoma and epithelioid MPNST[6-8].
Recently several studies have shown that D2-40 immunohistochemistry labels glandular MEC [9-11]. While using it as the marker for lymphatic endothelial cells, we have also found that it ser-endipitously stains the MEC of the terminal duct lobular units of breast, which triggered this study to further explore the possibility of D2-40 as an additional MEC marker in breast pathology.
Lymphovascular invasion (LVI) in breast carcinoma is an independent predictor of axillary lymph node metastases, which in turn is one of the most important prognostic factors of patients. D2-40 immunohistochemistry has been shown to improve accuracy in detecting LVI of breast carcinoma [12]. However, pitfalls in interpretation were raised in a recent study [11]. Besides focusing on D2-40 expression in normal breast parenchyma, ductal hyperplasia, ADH, ductal carcinoma in situ and invasive carcinoma, we also assessed the potential pitfalls in interpreting tumor LVI in this current study.
Materials and methods
Case selection and clinical information
Paraffin-embedded blocks of breast tissue of 48 patients that were processed between February 2005 and August 2007 were retrieved from the archives of the Department of Pathology and Laboratory Medicine of Temple University Hospital. All patients were female, ranging in age from 25 to 81 years (mean 54 years). The experimental samples included 15 with normal breast parenchyma, 41 with usual ductal hyperplasia (UDH), 4 with atypical ductal hyperplasia (ADH), 17 with ductal carcinoma in situ (DCIS) and 9 with invasive ductal carcinoma (IDC). Immunohistochemistry (IHC) for D2-40, calponin and p63 was performed in all of the cases. This study has been approved by the institutional review board.
Immunohistochemistry
IHC was performed using standard procedures with a Venta Benchmark XT Automated Stainer. Briefly, 5 μm tissue sections were deparaf-finized. Sections for p63 were heated in a pressure cooker for 15 minutes in EDTA (pH8.0) for antigen retrieval. Antigen retrieval for D2-40 and calponin was performed in a steamer using citrate buffer (pH 6.0) for 30 minutes. After blocking with hydrogen peroxide and normal goat serum, the sections were incubated with primary monoclonal antibodies (Dako, USA) against D2-40 (1:50), calponin (1:200) and p63 (1:800) for 30 minutes at 37°C. The sections were incubated sequentially with biotinylated goat anti-mouse immunoglobulin and peroxi-dase-conjugated streptavidin. Primary antibody was replaced by phosphate-buffered saline in negative control sections. Color was developed by incubation with 3, 3'-diaminobenzidine tetra-hydrochloride and slides were conterstained by Hematoxylin.
D2-40 and calponin immunohistochemistry stains the cytoplasm of MEC and p63 immunohistochemistry has a nuclear staining of MEC. The staining was scored semiquantitatively as follows: negative (no staining at all); 1+ (weakly positive); 2+ (moderately positive); and 3+ (strongly positive). The staining intensity and pattern were assessed separately in samples with more than one type of pathological entity, and the D2-40 stain was compared to calponin and p63 stains on the serial sections in the same case.
Results
Benign breast parenchyma and UDH
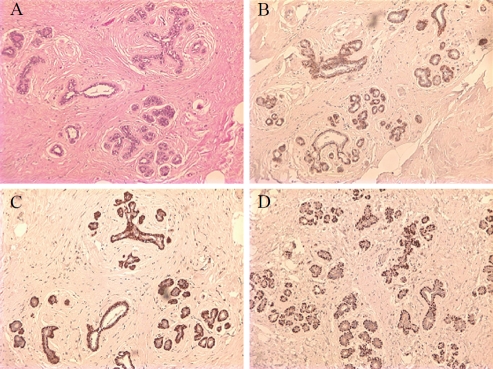
MEC have a peripheral location alongthe breast lobules and terminal ducts, and were labeled by D2-40 immunohistochemistry in all the cases with strong intensity. The luminal epithelial cells were negative for D2-40 (Figure 1).
Figure 1.
Benign breast tissues. Hematoxylin and Eosin stain (A). Myoepithelial cells react with D2-40 (B), calponin (C), and p63 (D). Note weaker cytoplasm staining of D2-40 than that of calponin. × 100
The UDH manifested as marked to florid ductal hyperplasia and D2-40 consistently stained the peripherally located MEC and demonstrated a mosaic pattern of MEC within the luminal lesions intermingling with the proliferative epithelial cells. MEC were moderately to strongly positive for D2-40 in majority of the cases (36/42).
The identification of MEC by D2-40 was the same as calponin and p63. In comparison, the staining intensity of MEC with D2-40 was slightly less than that of calponin (Table 1 and Figure 1), and same was true for the background nonspecific stain. The nuclear stain of p63 had the cleanest background and was the easiest to interpret.
Table 1.
Immunohistochemistry of D2-40, calponin and p63
| D2-40 | calponin | p63 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 2 | 1 | N | 3 | 2 | 1 | N | 3 | 2 | 1 | N | |
| Benign | 24/42 | 12/42 | 6/42 | 0 | 33/42 | 8/42 | 1/42 | 0 | 28/41 | 12/41 | 1/41 | 0 |
| ADH/DCIS | 5/21 | 8/21 | 6/21 | 2/21 | 7/21 | 12/21 | 2/21 | 0 | 11/21 | 7/21 | 3/21 | 0 |
| Invasive carcinoma | 0 | 0 | 0 | 9/9 | 0 | 0 | 0 | 9/9 | 0 | 0 | 0 | 9/9 |
Note: 3. Strongly positive; 2. Moderately positive; 1. Weakly positive; N. No staining
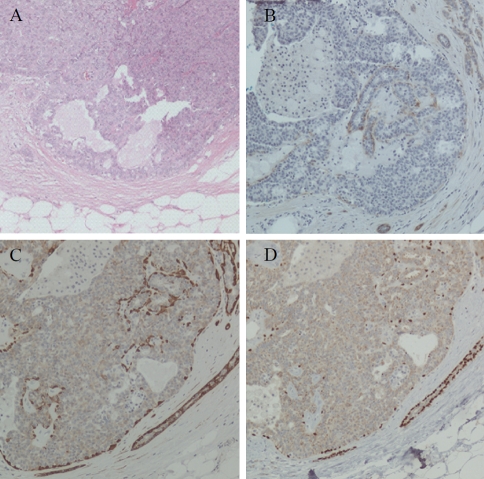
Atypical ductal hyperplasia and ductal carcinoma in situ
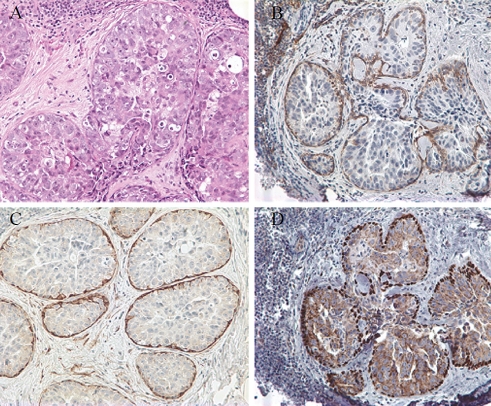
Four cases contained foci of ADH, and in 3 of them D2-40 immunostain demonstrated MEC in the areas without architectural or cytologicalatypia. The distribution of myoepithelial cells was confirmed by calponin and p63 expression; however, MEC were identified in all four cases by the latter two markers (Table 1 and Figure 2). D2-40 expression in MEC of neoplastic ducts was identified in 16 of 17 DCIS with variable positive cell numbers and staining intensity (Table 1 and Figure 3). The positive cells were only present at the periphery of the ducts. MEC were demonstrated with similar number and distribution in 16 cases by calponin and in all of the 17 cases by p63, although with different expression levels (Table 1 and Figure 3).
Figure 2.
Staining of myoepithelial cells in atypical ductal hyperplasia (ADH). Hematoxylin and eosin stain (A). The myoepithelial cells are positive for D2-40 (B), calponin (C) and p63 (D). × 100
Figure 3.
Staining of myoepithelial cells in ductal carcinoma in situ (DCIS). Hematoxylin and eosin stain (A). The myoepithelial cells are positive for D2-40 (B), p63 (C) and calponin (D). × 200
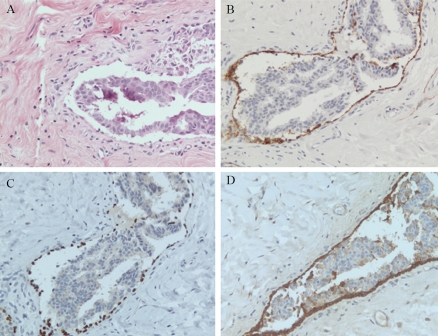
D2-40 was strongly expressed in the cytoplasm of lymphatic endothelial cells, as expected. In addition, stromal myofibroblasts were also labeled by D2-40, although in a weaker intensity. Particularly noted was that in 5 DCIS cases with retraction artifact, the positive MEC and myofibroblasts at the edge of the artifact mimicked lymphatic endothelial cells, especially if the retraction occurred a long the entire circumference of a duct involved by DCIS (Figure 4). The fibro-blasts were not labeled by p63 and calponin.
Figure 4.
Retraction artifact. The retraction of DCIS cells in a duct of breast (A, H&E). The peripherally located myoepithelial cells and myofibroblasts are labeled by D2-40 (B). Calciponin (C) and p63 (D) immunohistochemistry highlights the myoepithelial cells, but not the f ibroblasts. × 200
Invasive carcinoma
D2-40, calponin and p63 were negative in all 9 invasive carcinomas.
Discussion
Pathologists face daily challenges in diagnosing breast proliferative lesions based upon morphologic evidence as benign, borderline or malignant (in situ or invasive), a critical decision in determining the patient's management. Accurate identification of MEC has become one of the most helpful tools in making the diagnosis, as they are present and usually proliferate in benign lesions, become diminished in ADH and DCIS and are lost in invasive carcinomas. MEC are well known for their pleomorphism in size and shape, even in normal tissue, and their recognition can be very difficult in routine tissue sections. The development of immunohisto-chemistry has greatly improved the accuracy of their identification. Several MEC markers have been explored [1-3, 13]. The commonly used markers, including smooth muscle actin (SMA), smooth muscle myosin heavy chain (SM-MHC), calponin and p63, are highly sensitive but have varying specificities. For example, SMA stains scattered epithelial cells and reactive myofibro-blasts in the stroma [13, 14], invasive tumor cells may show focal calponin positivity [15], and p63 may label cells of poorly differentiated carcinoma with squamous differentiation. To increase the specificity and sensitivity, a panel of antibodies is recommended.
D2-40 is a commercially available mouse monoclonal antibody directed against human podo-planin, a mucin-type transmemebrane protein originally reported in lymphatic endothelial cells [16]. Besides staining lymphatic vessels, it has occasionally been reported to stain MEC of salivary gland and breast [9-11]. We have also noted that when D2-40 is used to demonstrate lymphatic vessels, the MEC of normal and benign ducts and lobules of breast are stained. In this extended study, we demonstrated that MEC in benign breast tissues were positive for D2-40, while focally or diffusely diminished MEC were also identified by D2-40 in ADH and DCIS. As expected, no MEC were found in invasive carcinomas by D2-40. The pattern of D2-40 stain was highly correlated with those of calponin and p63. In comparison, the staining intensity of MEC was slightly less than that of calponin, and same was true for the background unspecific stain. The nuclear stain of p63 had the cleanest background and was the easiest to interpret. The study showed that D2-40 immunohistochemistry reliably labels MEC in a variety of breast tissues from benign to pre-malignant lesions, suggesting that it can be considered as an additional marker for MEC of breast.
LVI of breast cancer is an independent adverse prognosticator that is associated with increased regional and distant tumor recurrence and D2-40 immunohistochemisty has proven to enhance the detection of LVI. Braun et al [12] reported that LVI was identified by D2-40 in 70 of 254 (28%) early breast cancer as compared to 40 tumors (16%) by routine HE staining, and D2-40 positivity was the strongest predictor for axillary lymph node metastasis in multivariate analysis. However, LVI detected by D2-40 may disqualify patients with early-stage breast cancer for accelerated partial breast irradiation [17], and potential pitfalls in LVI interpretation by D2-40 immunohistochemistry have been recently discovered. Rabban and Chen [18] found that the spindled, attenuated MEC surrounding solid DCIS resulted in a thin cytoplasmic D2-40 staining pattern that could be misinterpreted as LVI. They proposed that in these difficult cases p63 or SM-MHC should be used to confirm the presence of MEC and thereby exclude the diagnosis of LVI. Retraction artifact results in clear spaces around tumor cell nests and is frequently seen around invasive carcinoma or solid DCIS of breast. This artifact may be difficult to differentiate from LVI. Although a recent study suggested that retraction artifact associated with invasive carcinoma correlates with LVI and poor outcome of early breast carcinoma, the clinical significance of this artifact associated with DCIS is unknown [19].
In our study, 5 cases of DCIS showed retraction artifact of different extent and the artifact space was surrounded by residual MEC and stroma with myofibroblasts. D2-40 stained the MEC attached to the periphery of the DCIS and the myofibroblasts in the stroma. When these cells were located along the artificial cavity, they mimicked lymphatic endothelial cells. However, the myofibroblasts were not labeled by p63 or calponin in our study. This potential mistake in interpretation can be avoided by the application of a panel of markers including another vascular marker, such as CD31, in difficult cases.
In summary, D2-40 immunohistochemistry reliably highlights the MEC of breast in a variety of breast lesions in a pattern similar to that of calponin and p63, and could be considered as an additional MEC marker. Should it be used, a panel with other MEC markers, such as calponin or p63 is recommended. Caution should be exercised when interpreting the staining of cells surrounding DCIS with retraction artifact. In difficult cases, additional markers for MEC and lymphatic vessels should be considered to avoid misinterpretation of LVI.
Acknowledgments
The authors want to acknowledge Dr. Gordon Pringle, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, for his critical review and improvement of the manuscript.
References
- 1.Barbareschi M, Pecciarini L, Cangi MG, Macri E, Rizzo A, Viale G, Doglioni C. p63, a p53 homo-logue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–1060. doi: 10.1097/00000478-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Moritani S, Kushima R, Sugihara H, Bamba M, Kobayashi TK, Hattori T. Availability of CD10 immunohistochemistry as a marker of breast myoepithelial cells on paraffin sections. Mod Pathol. 2002;15:397–405. doi: 10.1038/modpathol.3880536. [DOI] [PubMed] [Google Scholar]
- 3.Kalof AN, Tam D, Beatty B, Cooper K. Immunostaining patterns of myoepithelial cells in breast lesions: a comparison of CD10 and smooth muscle myosin heavy chain. J Clin Pathol. 2004;57:625–629. doi: 10.1136/jcp.2003.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werling RW, Hwang H, Yaziji H, Gown AM. Immunohistochemical distinction of invasive from noninvasive breast lesions: a comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol. 2003;27:82–90. doi: 10.1097/00000478-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 6.Kalof AN, Cooper K. D2-40 immunohistochemistry–so far! Adv Anat Pathol. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]
- 7.Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- 8.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 9.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type trans-membrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Araujo VC, Altemani A, Furuse C, Martins MT, de Araujo NS. Immunoprofile of reactive salivary myoepithelial cells in intraductal areas of carcinoma ex-pleomorphic adenoma. Oral Oncol. 2006;42:1011–1016. doi: 10.1016/j.oraloncology.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 11.KannerWA, Galgano MT, Atkins KA. Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. Appl Immunohistochem Mol Morphol. 2010;18:226–230. doi: 10.1097/PAI.0b013e3181c65141. [DOI] [PubMed] [Google Scholar]
- 12.Braun M, Flucke U, Debald M, Walgenbach-Bruenagel G, Walgenbach KJ, Holler T, Polcher M, Wolfgarten M, Sauerwald A, Keyver-Paik M, Kuhr M, Buttner R, Kuhn W. Detection of lymphovascular invasion in early breast cancer by D2-40 (podoplanin): a clinically useful predictor for axillary lymph node metastases. Breast Cancer Res Treat. 2008;112:503–511. doi: 10.1007/s10549-007-9875-2. [DOI] [PubMed] [Google Scholar]
- 13.Lerwill MF. Current practical applications of diagnostic immunohistochemistry in breast pathology. Am J Surg Pathol. 2004;28:1076–1091. doi: 10.1097/01.pas.0000126780.10029.f0. [DOI] [PubMed] [Google Scholar]
- 14.El-Zammar OA, Haidar A. Immunoreactivity of ductal cells with putative myoepithelial markers: a potential pitfall in breast carcinoma. Ann Diagn Pathol. 2003;7:335–1. doi: 10.1016/j.anndiagpath.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Jones C, Nonni AV, Fulford L, Merrett S, Chaggar R, Eusebi V, Lakhani SR. CGH analysis of ductal carcinoma of the breast with basaloid/ myoepithelial cell differentiation. Br J Cancer. 2001;85:422–427. doi: 10.1054/bjoc.2001.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, diem K, weninger W, tschachler E, Alitalo K, kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood andlymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debald M, Polcher M, Flucke U, Walgenbach-Brunagel G, Walgenbach KJ, Holler T, Wolfgarten M, Rudlowski C, Buttner R, Schild H, Kuhn W, Braun M. Increased detection of lymphatic vessel invasion by D2-40 (podoplanin) in early breast cancer: possible influence on patient selection for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2010;77:1128–1133. doi: 10.1016/j.ijrobp.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 18.Rabban JT, Chen YY. D2-40 expression by breast myoepithelium: potantial pitfalls in distinquishing intralymphatic carcinoma from in situ carcinoma. Hum Pathol. 2008;39:175–183. doi: 10.1016/j.humpath.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Acs G, Dumoff KL, Solin LJ, Pasha T, Xu X, Zhang PJ. Extensive retraction artifact correlates with lymphatic invasion and nodal metastasis and predicts poor outcome in early stage breast carcinoma. Am J Surg Pathol. 2007;31:129–140. doi: 10.1097/01.pas.0000213316.59176.9b. [DOI] [PubMed] [Google Scholar]