Abstract
Hairy cell leukemia (HCL) is characterized by leukemic cells with abundant “hairy” cytoplasm, strong cytoplasmic positivity for tartrate-resistant acid phosphatase (TRAP), characteristic immunophenotype and sensitivity to treatment with purine nucleoside analogs. HCL-variant (HCL-v) encompasses chronic B-cell leukemias resembling classical HCL but exhibiting variant cytomorphology, variant immunophenotype and resistance to conventional HCL therapy. We present the case of a 67-year-old Taiwanese male with HCL-v who had leukocytosis and splenomegaly. His hairy leukemic cells were weakly positive for TRAP and expressed CDllc and CD103 but not CD25. He received oral chemotherapy with chlorambucil and in complete hematological remission in 9 months but relapsed 2 months later. Literature review revealed 9 cases of HCL and 3 cases of HCL-v including current case from Taiwan. All patients were adults with splenomegaly. The HCL patients had a significantly higher frequency of leukopenia (p = 0.024) and monocytopenia (p = 0.008) and a lower frequency of leukocytosis (p = 0.018) than HCL-v patients. All 8 HCL patients responded favorably to 2-chlorodeoxyadenosine with or without splenectomy. The 3 HCL-v patients had leukocytosis and received chemotherapy with variable outcome. HCL and HCL-v are rare in Taiwan and their pathological and immunophenotypical features were not fully characterized. A multimodality approach incorporating hematological findings, cytomorphology, histopathology, cytochemistry, complete immunophenotypingand clinical features is needed to identify and characterize such cases in Taiwan.
Keywords: CDllc, CD25, CD103, hairy cell leukemia, hairy cell leukemia variant, Taiwan
Introduction
Hairy cell leukemia (HCL) is a rare and indolent form of small mature B-cell leukemia characterized by oval or indented (bean-shaped) nuclei and abundant “hairy” cytoplasm involving peripheral blood (PB), bone marrow (BM) and spleen [1]. The neoplastic cells exhibit strong cytoplasmic positivity for tartrate-resistant acid phosphatase (TRAP) and express CDllc, CD25, CD103, DBA.44 and annexin Al in addition to B-cell markers. HCL is sensitive to treatment with either α-interferon or purine nucleoside analogs such as cladribine. HCL-variant (HCL-v), a district clinicopathological entity, encompasses cases of chronic B-cell lymphoproliferations that resemble classical HCL but exhibit variant cyto-morphological features, variant immunophenotype and resistance to conventional HCL therapy with purine nucleoside analogs [2,3]. Compared to classical HCL, HCL-v is a more aggressive disease. It is no longer considered to be biologically related to HCL and has been included in the 2008 World Health Organization (WHO) classification as a provisional entity [2]. HCL and HCL-v are very rare in Taiwan with only a few cases been reported [4-10]. Here we present a new case of HCL-v and the results of our literature review of HCL and HCL-v in Taiwan.
Case Report
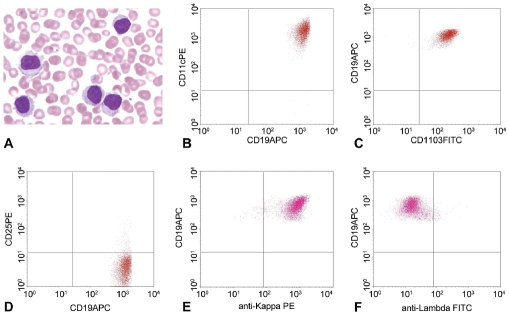
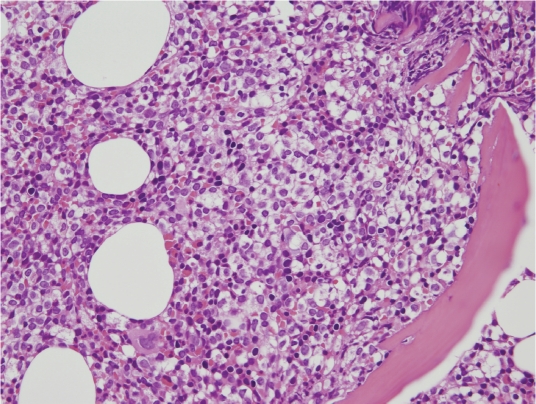
A 67 years old male presented with abdominal pain for days in January 2010. Abdominal sonography revealed splenomegaly (11.8 × 9.4 cm) with cystic change. Hemogram showed WBC count at 46.7 ×103/μl, hemoglobin level at 14.1 gm/dl, and platelet count at 143×103/μl. Differential counts showed 13% neutrophils, 10% small lymphocytes, 2% monocytes, 2% eosinophils and 73% abnormal lymphocytes. These abnormal lymphocytes exhibited villous/hairy cytoplasmic projection and small round nuclei with condensed chromatin and a distinct nucleolus (Figure 1). Flow cytometric immunophenotyping revealed that these leukemic cells expressed CDllc, CD19, CD20 and CD103 but not CD5, CD10, CD23, CD25, CD43 or FMC7. They were monotypic for surface immunoglobulin kappa light chain expression (Figure 1). Cytochemical stain for TRAP was weakly positive in the leukemic cells. The marrow trephine biopsy was hypercellular with 70% cellularity and a marked interstitial infiltration by atypical small lymphocytes accounting for half of the marrow cells. In focal areas, the tumor cells exhibit abundant clear cytoplasm with a fried-egg appearance (Figure 2). There was no reticulin fibrosis. Immunohistochemically these atypical lymphocytes expressed CD20, CD43, bcl-2, IgD and IgM but not CD3, CD5, CD23, CD123, CD138, bcl-6 or cyclin D1. Cytogenetic study using marrow aspirate showed normal karyo-type, 46, XY[cp9]. This patient received oral chemotherapy with chlorambucil (Leukeran) without splenectomy. Nine months later, his hemogram became normal without lymphocytosis except for mild thrombocytopenia. Unfortunately, 2 months later, the disease relapsed (normal hemoglobin, WBC count at 21.3 ×103/μl and platelet count at 99 ×103/μl), and abdominal CT scan revealed marked splenomegaly with partial infarction.
Figure 1.
The current case of HCL-v with the peripheral blood smear showing leukemic cells with a prominent nucleolus and hairy cytoplasm, which are weakly positive for TRAP stain (not shown). Flow cytometric immunophenotyping reveals expression of CDllc, CD19 and CD103 but not CD25. The leukemic cells are monotypic for surface immunoglobulin kappa light chain expression.
Figure 2.
Photomicrography of the morrow biopsy shows infiltration of the atypical lymphocytes with abundant cytoplasm forming a “fried-egg” appearance (H&E stain, original magnification ×400).
Literature review of HCL and HCL-v in Taiwan
Table 1 summarizes the pertinent laboratory and clinicopathological features of the HCL and HCL-v cases of Taiwanese patients. Cases 1 to 9 were HCL patients including 6 males and 3 females with a median age of 48 (range, 27-81). All patients had splenomegaly. One of 2 and 2 of 6 patients had hepatomegaly and lymphadenopathy, respectively. Hemogram showed leukopenia (88%), monocytopenia (100%), anemia (67%) and thrombocytopenia (100%). Interestingly, leukemic cells in PB were identified in only 5 (56%) patients. The staining intensity for TRAP was strong in 1 case (Case 9) while that of the other 4 cases was not specified. Eight patients had BM aspiration/biopsy and 6 underwent splenectomy. The pathological and immunophenotypical findings were described only very briefly. Marrow fibrosis was identified in 4 of 6 cases. The tumor cells of all cases were positive for CD20 and/or CD19. The only case (Case 8) with tumor cells stained for DBA44 in the splenic specimen was positive. Cases 4 and 9 were negative for CD23. Case 4 was negative for cyclin Dl. There was no data on the expression of CDllc, CD25, CD103 or FMC-7. Case 1 underwent splenectomy with supportive treatment [4]. Unfortunately he developed acute febrile neutrophilic dermatoses (Sweet's syndrome) 5 months later. The patient died from uncontrolled infection and pneumonia, 10 months after the diagnosis of HCL. Five of the remaining 8 patients underwent splenectomy. All 8 patients responded favorably to 2-chlorodeoxyadenosine (Cladribine) and were in complete remission with a median follow-up of 21 months (range, 4-96).
Table 1.
Clinicopathological features of hairy cell leukemia (HCL) and HCL-variant (HCL-v) in Taiwan
| Case/sex/age | l/M/63 | 2/M/48 | 3/F/44 | 4/F/53 | 5/M/47 | 6/M/27 | 7/M/77 | 8/F/43 | 9/M/81 | 10/M/59 | ll/F/59 | 12/M/67 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dx | HCL | HCL | HCL | HCL | HCL | HCL | HCL | HCL | HCL | HCL-v | HCL-v | HCL-v |
| WBC(ul) | 1,000 | 2,010 | 6,000 | 2,860 | 2,100 | 2,920 | 590 | 13,200 | 7,950 | 33,900 | 10,100 | 46,700 |
| Neutropenia | + | + | NA | + | + | + | + | - | + | - | - | - |
| Monocytopenia | + | NA | NA | + | + | + | + | + | + | - | - | - |
| Leukemic cells in PB | + | - | + | + | - | + | + | + | + | + | ||
| Hairy cytoplasm | + | NA | + | + | NA | NA | NA | NA | + | + | + | + |
| Prominent nucleolus | NA | - | NA | NA | NA | NA | NA | NA | + | - | + | |
| TRAP | + | NA | + | + | NA | NA | NA | + | + (strong) | + (weak) | - | + (weak) |
| Anemia (Hb; g/dL) | + (5.3) | -(14.2) | + (5.9) | -(12.2) | -(14.2) | + (8.7) | + (6.4) | + (8.5) | + (8.9) | + (8.9) | -(13.0) | -(14.1) |
| Thrombocytopenia (Pit; 103/μl) | + (38) | + (97) | + (50) | + (95) | + (97) | + (58) | + (43) | + (31) | + (34) | + (129) | + (74) | - (143) |
| Splenomegaly (cm) | + | + (12.5) | + | + | + (15) | + (50) | + (18) | + (45) | + (22) | + | + (30) | + (19) |
| Hepatomegaly | - | NA | NA | NA | NA | NA | NA | NA | + | - | - | - |
| Lymphadenopathy | - | NA | NA | NA | + | - | - | - | + | - | - | - |
| LDH | NA | NA | NA | NA | WNL | WNL | WNL | WNL | NA | NA | WNL | Elevated |
| Marrow fibrosis | + | + | NA | NA | + | - | + | - | NA | - | - | - |
| CDllc | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | + | + |
| CD19 | + | NA | + | NA | NA | NA | NA | NA | + | + | + | + |
| CD20 | + | + | + | + | + | + | + | + | + | NA | + | + |
| CD23 | NA | NA | NA | - | NA | NA | NA | NA | - | NA | - | - |
| CD25 | + | NA | NA | NA | NA | NA | NA | NA | + | - | - | - |
| CD43 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | - |
| CD103 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | - | + |
| DBA44 | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | + | NA |
| HLA-DR | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | + | + |
| Cytogenetics | NA | NA | NA | NA | NA | NA | NA | NA | NA | Complex | Normal | Normal |
| Treatment | Splenectomy Supportive | Splenectomy 2-CdA | 2-CdA | 2-CdA | Splenectomy 2-CdA | Splenectomy 2-CdA | Splenectomy 2-CdA | Splenectomy 2-CdA | 2-CdA | Chlorambucil, COP, R/T | Splenectomy | Chlorambucil |
| Outcome (month) | DOI (10) | CR(4) | CR(4) | CR (12) | CR (96) | CR (49) | CR(29) | CR(21) | CR (NA) | Progressive; DOD (33) | PR; AWD (60) | AWD (12) |
| Author (yr) | Chang (1999) | Yang and Ho (2001) | Chen et al. case 1 (2005) | Chen et al. case 2 (2005) | Yu et al case 1 (2007) | Yu et al case 2 (2007) | Yu et al case 3 (2007) | Yu et al case 5 (2007) | Huang and Kao (2010) | Dunn et al (1995) | Chuang et al (2007) | Current case (2010) |
Abbreviation: 2-CdA: 2-chlorodeoxyadenosine; AWD, alive with disease; COP, cyclophosphamide, vincristine and prednisolone; CR, complete remission; DOD, died of disease; DOI, died of infection complicating Sweet's syndrome; Hb, hemoglobin level; LDH, lactate dehydrogenase; NA, not available; Pit, platelet count; PR, partial remission; R/T, radiotherapy; TRAP, tartrate-resistant acid phosphatase; WNL, within normal limits.
One of the cases in the series of Yu et a I was a Vietnamese with HCL-v and was excluded from this analysis [8]. There were only 3 HCL-v cases, 2 males and 1 female, including our current case. Two patients were 59 years old and the other, 67. All 3 patients presented with splenomegaly without hepatomegaly or lymphadenopathy. Hemogram of all 3 patients showed leukocytosis with PB involvement by leukemic cells. The hemogram of one patient was anemic, 2 were thrombocytopenic, while none had monocytopenia. The hairy leukemic cells were weakly positive for TRAP cytochemical stain in 2 of the 3 cases tested. There was no myelofibrosis in all patients. Immunophenotyping showed that the leukemic cells expressed CDllc, CD19 and HLA-DR in all 3 cases. The other expressed antigens were CD20 (2/2), CD43 (1/2), CD103 (1/2), and DBA.44 (1/1). The leukemic cells did not express CD5 (3 cases), CD23 (1), CD25 (3), FMC-7 (1), cyclin D1 (2) or annexin Al (1). Patient no. 10 received chlorambucil and COP (cyclophosphamide, vincristine and prednisolone) chemotherapy with local radiation of the spleen and died of progressive disease at 33 months. Patient no. 11 received only splenectomy and was alive with low levels of leukemic cells (WBC at 9,600/ul with 45.3% lymphocytes) at the last follow-up in September 2010, 60 months after diagnosis. The last patient received chlorambucil and was in complete hematologic remission in 9 months but relapsed 2 months later.
Table 2 summarizes the comparison of the major clinicopathological features between these 2 groups of patients. The HCL patients had a significantly higher frequency of leukopenia (p = 0.024) and monocytopenia (p = 0.008) and a lower frequency of leukocytosis (p = 0.018) than HCL-v patients (Fisher's exact test). There was no statistical difference in age (p = 0.373; Mann-Whitney U test), sex, presence of leukemic cells in the peripheral blood, anemia, thrombocytopenia, TRAP positivity, splenomegaly, lymphadenopathy or marrow fibrosis (Fisher's exact test) between these two groups.
Table 2.
Comparison of major clinicopathological features between HCL and HCL-v
| Type (no.) | HCL (n= 9) | HCL-v (n= 3) | P value |
|---|---|---|---|
| M: F | 6:3 | 2: 1 | 1.000 |
| Leukocytosis | 1/9 (11%) | 3/3 (100%) | 0.018 |
| Leukopenia | 7/8 (88%) | 0/3 (0%) | 0.024 |
| Monocytopenia | 7/7 (100%) | 0/3 (0%) | 0.008 |
| Leukemia in PB | 5/9 (56%) | 3/3 (100%) | 0.490 |
| Anemia | 6/9 (67%) | 1/3 (33%) | 0.523 |
| Thrombocytopenia | 9/9 (100%) | 2/3 (67%) | 0.250 |
| TRAP positivity | 5/5 (100%) | 2/3 (67%) | 0.375 |
| Splenomegaly | 9/9 (100%) | 3/3 (100%) | 1.000 |
| Lymphadenopathy | 2/6 (33%) | 0/3 (0%) | 0.500 |
| Marrow fibrosis | 4/6 (67%) | 0/3 (0%) | 0.167 |
Fisher's exact test.
Discussion
HCL is a rare disease comprising about 2% of lymphoid leukemia in the West [1]. Its incidence is lower in Asia and even lower in Taiwan. HCL-v is defined as cases of small B-cell lymphoproliferative disorders resembling classical HCL but exhibiting variant features in hemogram (leukocytosis without monocytopenia), cytomorphology (neoplastic cells with prominent nucleoli, with blastic or convoluted nuclei and/or absence of circumferential shaggy contours), cytochemistry (weak or negative reaction for TRAP), immunophenotype (absence of CD25, annexin Al or TRAP), and resistance to conventional HCL therapy (lack of dramatic response to cladribine) [2,11]. In an analysis of 52 HCL-v cases, Matutes et al reported that the median age of the patients was 71 and 90% patients had raised WBC counts and all had circulating hairy leukemic cells with a prominent nucleolus [12]. Immunophenotypically, in addition to the nearly universal expression of FMC-7, CD19, HLA-DR and CD22, the leukemic cells were positive for CDllc (87% of cases) and CD103 (60%) and were consistently negative for CD5, CD23 and CD25 [12]. FMC-7 is frequently expressed in HCL and HCL-v cases and is of no particular use for differential diagnosis. As compared to classical HCL, HCL-v patients were older without male predominance and there was a marked lymphocytosis with no monocytopenia and neu-tropenia. The median survival was 9 years. They suggested that splenectomy was the best option because it corrected hypersplenism and removed the bulk of disease. From our literature review and analysis, we confirm that HCL-v in Taiwan is distinct from HCL by leukocytosis without leukopenia or monocytopenia, similar to the findings in the West. A Japanese variant of HCL (HCL-jv) had been proposed [3,13,14]. The leukemic hairy cells exhibited an inconspicuous nucleolus and they showed weak expression of surface immunoglobulin G (IgG) with kappa-chain predominance. Furthermore, spleen sections revealed diffuse leukemic infiltration in the red pulp. HCL-v is listed as a provisional entity in the 2008 WHO classification of lymphoid neoplasms, probably including cases of HCL-jv. HCL-v is no longer considered biologically related to HCL [2].
In our current patient, the “variant” features from HCL included leukocytosis without monocytopenia, CD25-negativity, and weak TRAP positivity. Furthermore, the patient responded well to oral chemotherapy with chlorambucil, further supporting our diagnosis of HCL-v. This is the second case of HCL-v at our institution in a 21-year period with 1,032 cases of lymphomas. We have not encountered any case of classical HCL in our institution. The leukemic cells of the first HCL-v case in our institution, Case no. 9 in Table 1, did not exhibit a prominent nucleolus and they diffusely infiltrated the splenic red pulp [8]. The tumor cells expressed CDllc and DBA.44 but not CD25 or CD103. Interestingly, these features were more similar to the HCL-jv than to the HCL-v as defined by the WHO classification [2]. It is probably reasonable to put HCL-jv under the WHO provisional entity of HCL-v to include cases with or without a distinct nucleolus, disregarding the ethnic origin of patients.
Of the 9 cases of HCL, cytochemical stain for TRAP was positive in 5 cases including 1 with intense reactivity characteristic of HCL and 4 with unspecified intensity. The remaining 4 cases (Cases no. 2 and 5-7) were negative for leukemic cells and TRAP stain. The number of circulating hairy cells in leukopenic HCL cases can be very low, and these cells are often present at the feathery edge of regular smears with distorted morphology and can be easily missed.
To properly identify the circulating hairy cells, we should prepare blood smears from the buffy coat with the aid of TRAP stain, which shows strong cytoplasmic reaction in hairy cells.
HCL is also rare in Honk Kong Chinese. Au et al reported 18 cases of HCL in a 12-year period from Hong Kong with an estimated incidence of 0.035/100,000 populations per year, at least one log lower than that reported in the Western series [15]. In that study, all 9 cases tested for CD103 were positive. Nine of 10 and 3 of 6 cases expressed CD25 and FMC-7, respectively. Interestingly the single case negative for CD25 had marked leukocytosis (195 ×103/ul) and had persistent disease following interferon treatment. Although there were no morphological features for confirmation, this case is most likely HCL-v.
The major weakness of this review is that the case numbers in both groups are too small, especially the HCL-v. From our literature review we have noted very limited and incomplete immunophenotyping data of HCL and HCL-v cases from Taiwan as compared to those reported by the Japanese or Western series. One of the reasons might be that most of these cases were reported by hemato-oncologists with emphasis mainly on the clinical course and treatment outcome. The other reason might be that these diseases are very rare in Taiwan so the diagnostic laboratories do not stock up the rarely used markers such as DBA.44, annexin Al, CD103 and TRAP, particularly with a very low payment for flow cytometric immunophenotyping by the National Health Insurance, Taipei, Taiwan. Although Taiwan is nearly a developed country, the National Health Insurance payment for flow cytometric immunophenotyping of a leukemia case is incredibly low as compared to that in the United States (US$ 65 vs. 700). In order to identify such rare cases and to differentiate them from other low-grade B-cell lymphoproliferations, a multimodality approach incorporating hematological findings, cytomorphology, histopathology, cytochemistry, complete immunophenotyping including immunohistochemistry and clinical features is needed. An effort to raise the reimbursement fee from the National Health Insurance for complete immunophenotyping of leukemia cases seems necessary. One or a few referral laboratories for flow cytometric immunophenotyping might be a solution for such rare cases.
Acknowledgments
This work was partly supported by a research grant CMFHR9958 from Chi-Mei Medical Center, Tainan, Taiwan.
References
- 1.Foucar K, Falini B, Catovsky D, Stein H. Hairy cell lymphoma. In: Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haemtopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 188–190. [Google Scholar]
- 2.Piris M, Foucar K, Mollejo M, Campo E, Falini B. Splenic B-cell lymphoma/leukemia, unclassifiable. In: Swerdlow SH CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haemtopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 191–193. [Google Scholar]
- 3.Robak T. Hairy-cell leukemia variant: Recent view on diagnosis, biology and treatment. Cancer Treat Rev. 2010 doi: 10.1016/j.ctrv.2010.05.003. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Chau WK, Liu MT, Ho CH. Acute febrile neutrophilic dermatosis (Sweet's syndrome) in hairy cell leukemia: a case report. Chin Med J (Taipei) 1999;62:467–471. [PubMed] [Google Scholar]
- 5.Dunn P, Shin LY, Ho YS, Tien HF. Hairy cell leukemia variant. Acta Haematol. 1995;94:105–108. doi: 10.1159/000203984. [DOI] [PubMed] [Google Scholar]
- 6.Yang MH, Ho CH. 2-chlorodeoxyadenosine (cladribine) in the treatment of hairy cell leukemia. J Chin Med Assoc. 2001;64:54–58. [PubMed] [Google Scholar]
- 7.Chen L, Chung Y, TY C. Hairy cell leukemia: experience of two cases in Taiwan. J Med Sci. 2005;25:157–160. [Google Scholar]
- 8.Chuang SS, Chang ST, Huang WT, Li CY, Campo E. Variant hairy cell leukemia without distinct nucleoli. Leuk Lymphoma. 2007;48:1050–1052. doi: 10.1080/10428190701245146. [DOI] [PubMed] [Google Scholar]
- 9.Yu YB, Li CY, Chen CC, et al. Combined treatment with splenectomy and cladribine in hairy cell leukemia in Taiwan: a clinicopathologic study of 5 cases. J Chin Med Assoc. 2007;70:551–555. doi: 10.1016/S1726-4901(08)70059-5. [DOI] [PubMed] [Google Scholar]
- 10.Huang TC, Kao WY. An aged man with massive hepatosplenomegaly. Gastroenterology. 2010;139:1467–1799. doi: 10.1053/j.gastro.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 11.Matutes E, Wotherspoon A, Catovsky D. The variant form of hairy-cell leukaemia. Best Pract Res Clin Haematol. 2003;16:41–56. doi: 10.1016/s1521-6926(02)00086-5. [DOI] [PubMed] [Google Scholar]
- 12.Matutes E, Wotherspoon A, Brito-Babapulle V, Catovsky D. The natural history and clinicopathological features of the variant form of hairy cell leukemia. Leukemia. 2001;15:184–186. doi: 10.1038/sj.leu.2401999. [DOI] [PubMed] [Google Scholar]
- 13.Katayama I, Mochino T, Honma T, Fukuda M. Hairy cell leukemia: a comparative study of Japanese and non-Japanese patients. Semin Oncol. 1984;11:486–492. [PubMed] [Google Scholar]
- 14.Machii T, Tokumine Y, Inoue R, Kitani T. Predominance of a distinct subtype of hairy cell leukemia in Japan. Leukemia. 1993;7:181–186. [PubMed] [Google Scholar]
- 15.Au WY, Kwong YL, Ma SK, et al. Hairy cell leukemia in Hong Kong Chinese: a 12-year retrospective survey. Hematol Oncol. 2000;18:155–159. doi: 10.1002/1099-1069(200012)18:4<155::aid-hon668>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]