Abstract
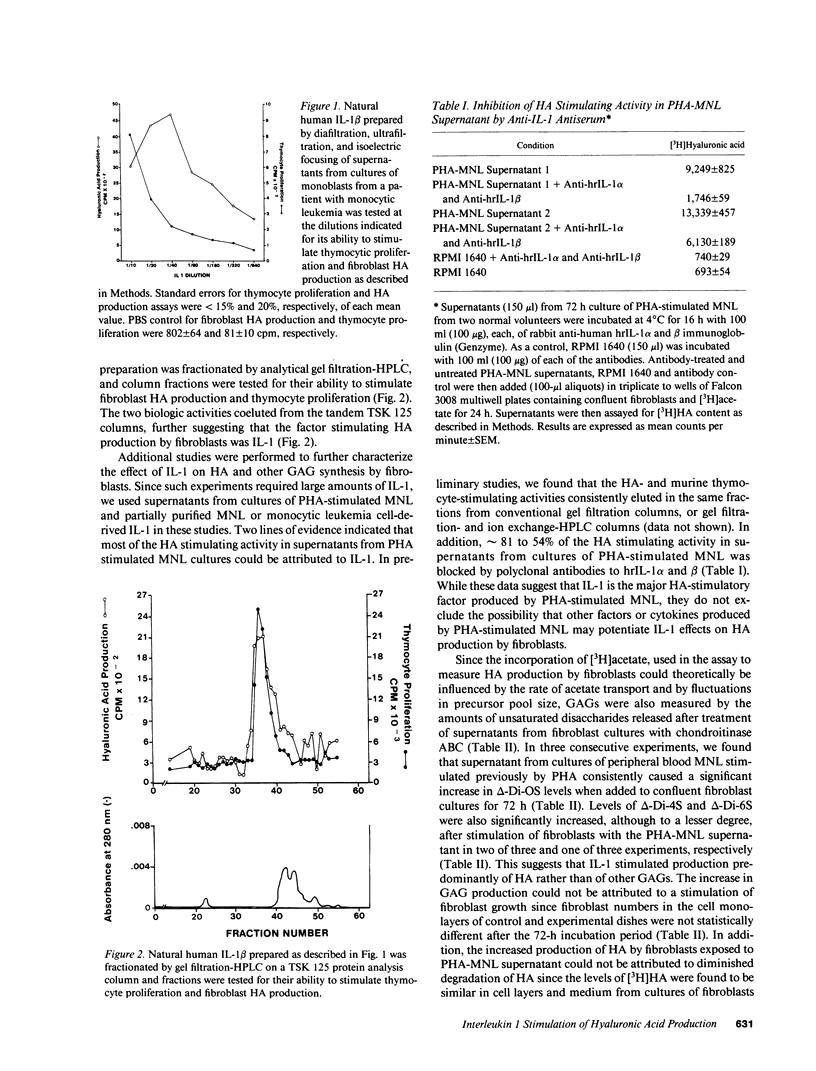
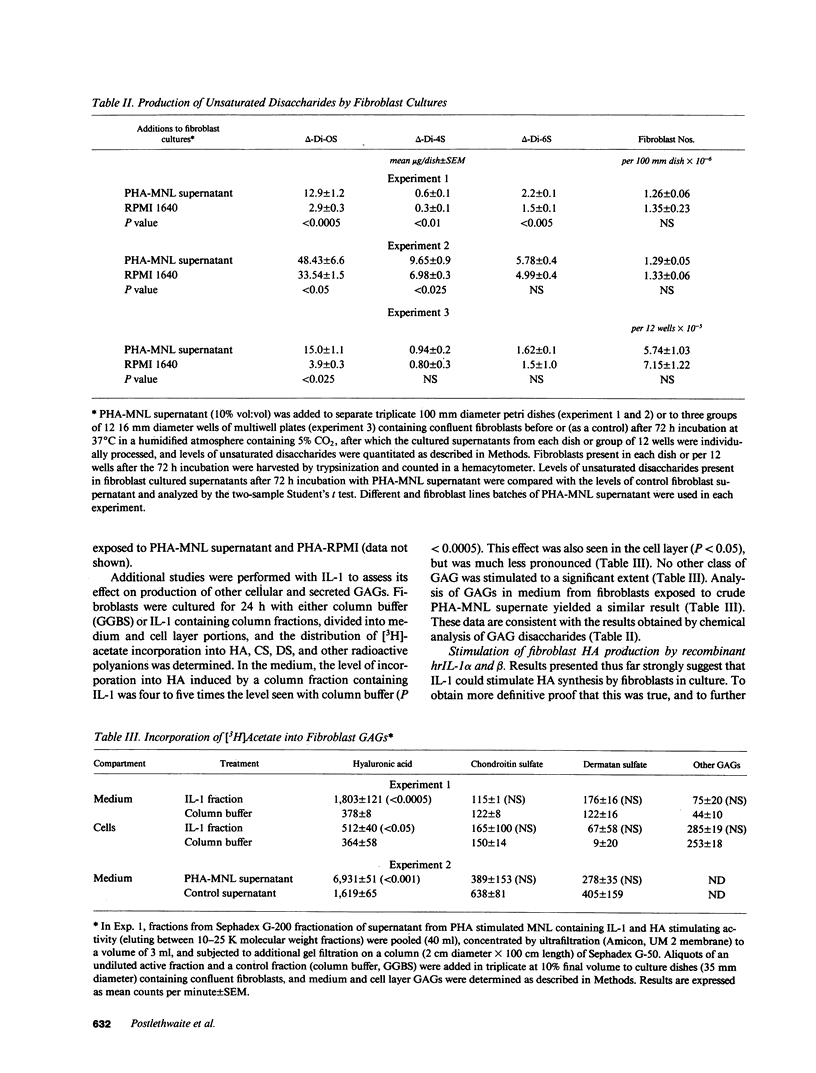
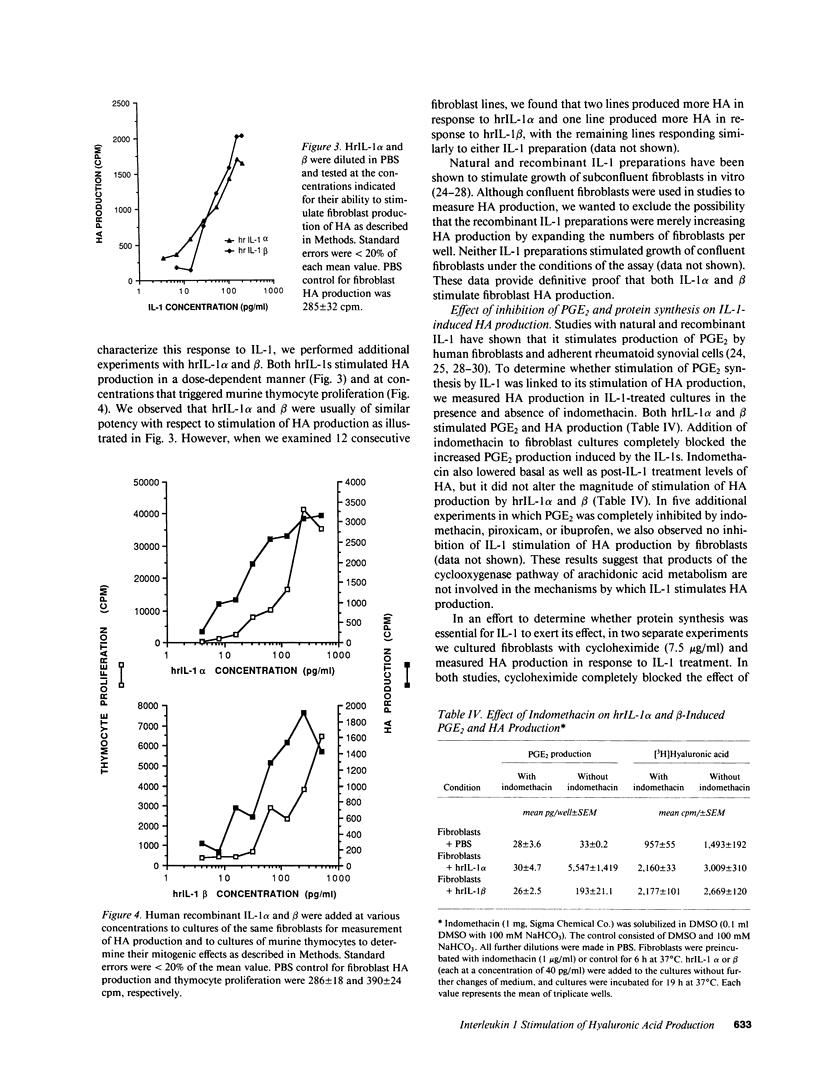
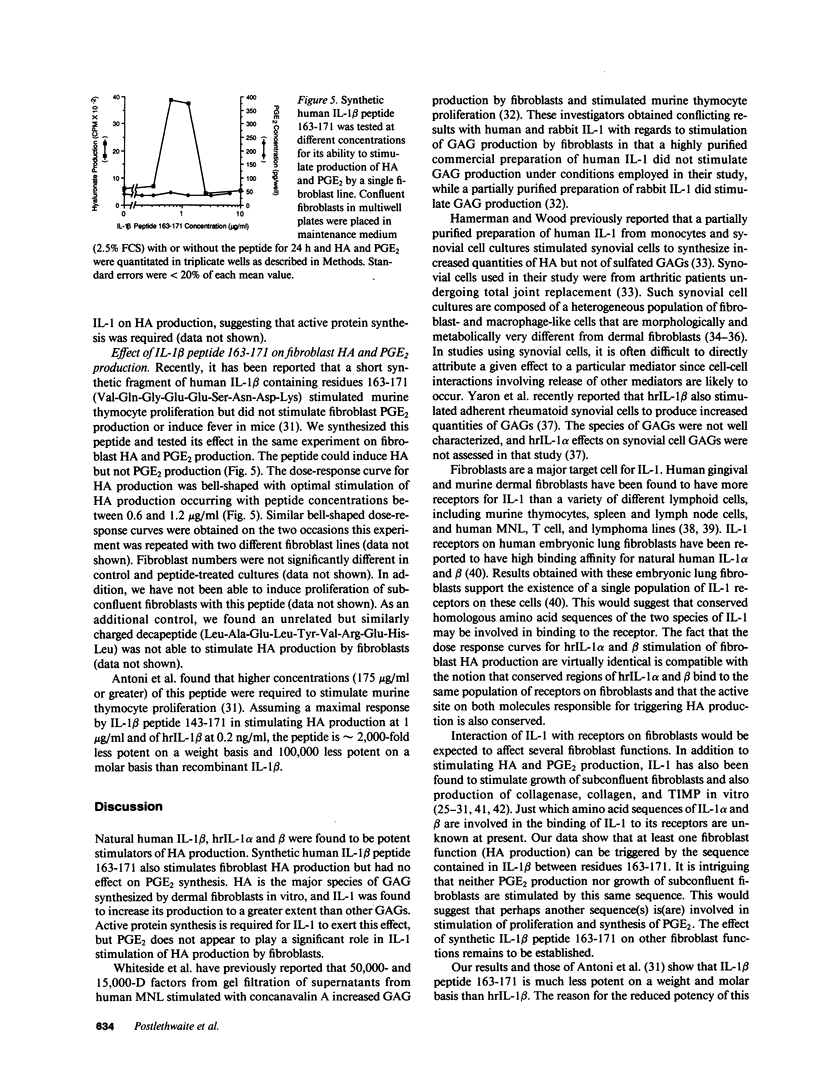
Hyaluronic acid (HA) is believed to play a critical role in wound healing and in morphogenesis. Factors controlling the production of HA by fibroblasts in normal and pathological states are not completely understood. In this report we have observed that natural human interleukin (IL-1)1 beta and human recombinant (hrIL)-1 alpha and beta are potent stimulators of HA production by fibroblasts in vitro. Hyaluronic acid is the major species of glycosaminoglycan (GAG) stimulated by IL-1 in fibroblasts. PGE2 does not appear to be involved directly in this IL-1 effect on fibroblasts, but stimulation of HA production by IL-1 is dependent on protein synthesis. The synthetic human IL-1 beta peptide 163-171 (Val-Gln-Gly-Glu-Glu-Ser-Asn-Asp-Lys), which has been previously shown to stimulate thymocyte proliferation but not fibroblast PGE2 production, is also able to stimulate fibroblast HA production. The synthesis and secretion of IL-1 by mononuclear phagocytes at sites of inflammation and immune reactions in vivo could potentially serve as a signal for fibroblasts to synthesize HA, which in turn could serve to facilitate and modulate reparative and immune processes by virtue of its ability to alter cell-cell, cell matrix, and cell-membrane receptor interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. A., Donoff R. B. The glycosaminoglycans of open wounds. J Surg Res. 1980 Nov;29(5):422–429. doi: 10.1016/0022-4804(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Antoni G., Presentini R., Perin F., Tagliabue A., Ghiara P., Censini S., Volpini G., Villa L., Boraschi D. A short synthetic peptide fragment of human interleukin 1 with immunostimulatory but not inflammatory activity. J Immunol. 1986 Nov 15;137(10):3201–3204. [PubMed] [Google Scholar]
- Aycock R. S., Raghow R., Stricklin G. P., Seyer J. M., Kang A. H. Post-transcriptional inhibition of collagen and fibronectin synthesis by a synthetic homolog of a portion of the carboxyl-terminal propeptide of human type I collagen. J Biol Chem. 1986 Oct 25;261(30):14355–14360. [PubMed] [Google Scholar]
- Britz J. S., Hart G. W. Biosynthesis of glycosaminoglycans by epithelial and lymphocytic components of murine thymus. J Immunol. 1983 Apr;130(4):1848–1855. [PubMed] [Google Scholar]
- Chin J., Cameron P. M., Rupp E., Schmidt J. A. Identification of a high-affinity receptor for native human interleukin 1 beta and interleukin 1 alpha on normal human lung fibroblasts. J Exp Med. 1987 Jan 1;165(1):70–86. doi: 10.1084/jem.165.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A. Molecular composition and origin of substrate-attached material from normal and virus-transformed cells. J Supramol Struct. 1976;5(2):239–255. doi: 10.1002/jss.400050210. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby M. A. Analysis of glycosaminoglycans within the extracellular environments encountered by migrating neural crest cells. Dev Biol. 1978 Oct;66(2):321–336. doi: 10.1016/0012-1606(78)90241-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Diz D. I., Baer P. G., Nasjletti A. Angiotensin II-induced hypertension in the rat. Effects on the plasma concentration, renal excretion, and tissue release of prostaglandins. J Clin Invest. 1983 Aug;72(2):466–477. doi: 10.1172/JCI110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukovich M., Severin J. M., White S. J., Yamazaki S., Mizel S. B. Stimulation of fibroblast proliferation and prostaglandin production by purified recombinant murine interleukin 1. Clin Immunol Immunopathol. 1986 Mar;38(3):381–389. doi: 10.1016/0090-1229(86)90248-5. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Edén S., Kostyo J. L., Schwartz J. Ability of growth hormone fragments to complete with 125I-iodinated human growth hormone for specific binding to isolated adipocytes of hypophysectomized rats. Biochim Biophys Acta. 1982 Dec 30;721(4):489–491. doi: 10.1016/0167-4889(82)90106-9. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- HIROHATA K., KOBAYASHI I. FINE STRUCTURES OF THE SYNOVIAL TISSUES IN RHEUMATOID ARTHRITIS. Kobe J Med Sci. 1964 Dec;10:195–225. [PubMed] [Google Scholar]
- Hamerman D., Wood D. D. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc Soc Exp Biol Med. 1984 Oct;177(1):205–210. doi: 10.3181/00379727-177-1-rc1. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Smith G. N., Jr, Kang A. H. Studies on glycosaminoglycans of regenerating rabbit ear cartilage. Dev Biol. 1981 Aug;86(1):198–205. doi: 10.1016/0012-1606(81)90330-4. [DOI] [PubMed] [Google Scholar]
- Hjerpe A., Antonopoulos C. A., Engfeldt B. Determination of sulphated disaccharides from chondroitin sulphates by high-performance liquid chromatography. J Chromatogr. 1979 Apr 1;171:339–344. doi: 10.1016/s0021-9673(01)95313-0. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Krey P. R., Cohen A. S., Smith C. B., Finland M. The human fetal synovium. Histology, fine structure and changes in organ culture. Arthritis Rheum. 1971 May-Jun;14(3):319–341. doi: 10.1002/art.1780140303. [DOI] [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Lachman L. B., Shih L. C., Brown D. C. Interleukin 1 from human leukemic monocytes. Methods Enzymol. 1985;116:467–479. doi: 10.1016/s0076-6879(85)16038-6. [DOI] [PubMed] [Google Scholar]
- Liao Z., Grimshaw R. S., Rosenstreich D. L. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med. 1984 Jan 1;159(1):126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Akahoshi T., Yamada M., Furutani Y., Oppenheim J. J. Properties of a specific interleukin 1 (IL 1) receptor on human Epstein Barr virus-transformed B lymphocytes: identity of the receptor for IL 1-alpha and IL 1-beta. J Immunol. 1986 Jun 15;136(12):4496–4502. [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin. An immunosuppressive 85-kilodalton glycoprotein isolated from human pregnancy urine is a high affinity ligand for recombinant interleukin 1 alpha. J Biol Chem. 1986 Oct 15;261(29):13404–13407. [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Nozoe M., Dennis M. V., Herman J. H. Modulation of human lymphocyte response by cartilage proteoglycans and glycosaminoglycans--a comparison between normal subjects and patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1979 Apr;12(4):369–381. doi: 10.1016/0090-1229(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Kang A. H. Induction of fibroblast proliferation by interleukin-1 derived from human monocytic leukemia cells. Arthritis Rheum. 1984 Sep;27(9):995–1001. doi: 10.1002/art.1780270905. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Ghadially F. N., Crane W. A. Synovial membrane in traumatic effusion. Ultrastructure and autoradiography with tritiated leucine. Ann Rheum Dis. 1966 May;25(3):259–271. doi: 10.1136/ard.25.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Smith G. N., Jr, Newsome D. A. The nature and origin of the glycosaminoglycans of the embryonic chick vitreous body. Dev Biol. 1978 Jan;62(1):65–77. doi: 10.1016/0012-1606(78)90093-3. [DOI] [PubMed] [Google Scholar]
- Smith G. N., Jr, Toole B. P., Gross J. Hyaluronidase activity and glycosaminoglycan synthesis in the amputated newt limb: comparison of denervated, nonregenerating limbs with regenerates. Dev Biol. 1975 Apr;43(2):221–232. doi: 10.1016/0012-1606(75)90022-6. [DOI] [PubMed] [Google Scholar]
- Toole B. P. Developmental role of hyaluronate. Connect Tissue Res. 1982;10(1):93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Gross J. The extracellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971 May;25(1):57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Jackson G., Gross J. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1384–1386. doi: 10.1073/pnas.69.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L., Worrall J. G., Prince R. K., Buckingham R. B., Rodnan G. P. Soluble mediators from mononuclear cells increase the synthesis of glycosaminoglycan by dermal fibroblast cultures derived from normal subjects and progressive systemic sclerosis patients. Arthritis Rheum. 1985 Feb;28(2):188–197. doi: 10.1002/art.1780280214. [DOI] [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Yaron M. Human recombinant interleukin-1 beta stimulates glycosaminoglycan production in human synovial fibroblast cultures. Arthritis Rheum. 1987 Apr;30(4):424–430. doi: 10.1002/art.1780300410. [DOI] [PubMed] [Google Scholar]


