Superior vena cava syndrome (SVCS) is a constellation of symptoms that develops during stenosis or occlusion of the superior vena cava (SVC) or its main tributaries. Obstruction above the azygos vein causes a moderate increase in venous pressure because blood can be diverted through chest-wall veins into the thoracic and iliac veins and then enters the heart by way of the inferior vena cava and azygos system. Blockage below the level of the azygos vein is not well tolerated because a higher venous pressure is required to pump blood back to the heart through the only patent channel, the inferior vena cava.1
High central venous pressure produces a syndrome characterized by dyspnea, facial edema, venous distention of the chest wall and neck veins, and, less often, facial plethora, cough, arm edema, and central nervous system complaints.2,3,4,5 Although SVCS is a medical emergency that requires immediate diagnostic evaluation and therapy, it is rarely a life-threatening emergency. Symptoms are the most severe if the SVC is blocked quickly and venous collaterals do not have time to expand. Thus development of symptoms can be as rapid as a few weeks for a malignant tumor or gradual over several years for fibrosing mediastinitis.1
CASE REPORT
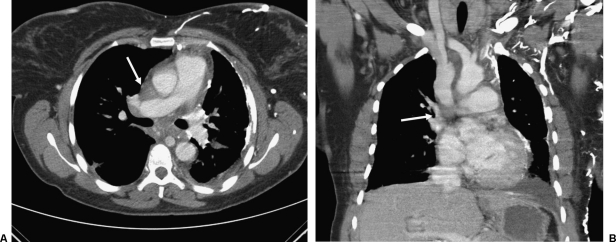
A 40-year-old woman with a history of sickle cell disease and pulmonary fibrosis presented to our emergency department with neck and facial swelling. A contrast-enhanced chest computed tomography revealed obliteration of the left brachiocephalic vein and inferior portion of the SVC with a dilated azygous venous system and significant collateral vessels in the anterior chest wall and mediastinum (Fig. 1). The patient was deemed stable and referred to the Vascular and Interventional Radiology Section for SVC and inferior vena cava venogram and venoplasty.
Figure 1.
Enhanced computed tomography (CT) of superior vena cava (SVC) syndrome. (A) Axial CT image shows numerous collateral veins in the presence of SVC occlusion (white arrow). (B) Coronal reformatted image shows collaterals and low SVC occlusion (white arrow).
After the risks of the procedure were explained to the patient, informed consent was obtained. The patient was placed supine on the procedure table and the right neck was prepped in normal sterile fashion. Due to the patient's condition, general anesthesia was provided for airway protection. The patient's vital signs, electrocardiogram (EKG), and oxygenation were monitored throughout the procedure. Under ultrasound guidance, the right internal jugular vein (IJV) was accessed and an 8F vascular sheath was inserted. A 5F Kumpe catheter (Cook, Bloomington, IN)was placed into the right IJV and digital subtraction angiography (DSA) of the SVC was performed demonstrating occlusion of the SVC with a prominent azygous vein (Fig. 2).
Figure 2.
Superior vena cavagram showing superior vena cava (SVC) occlusion. Digital subtraction angiogram shows SVC occlusion and retrograde flow into the azygous vein (black arrow).
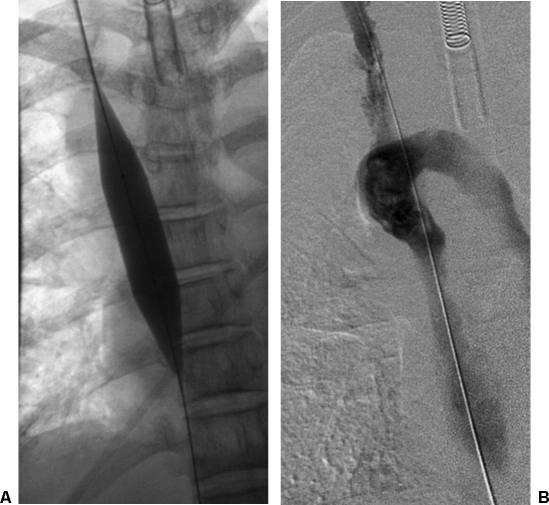
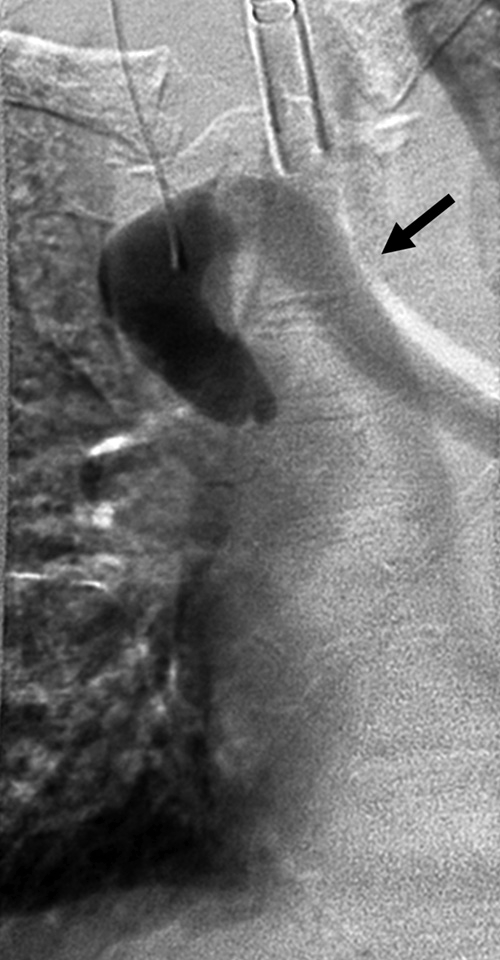
Using the catheter and a 0.035-inch straight hydrophilic wire, the occlusion in the SVC was successfully crossed and access was gained into the inferior vena cava. The catheter was then exchanged for a 5 × 20 mm balloon catheter, and venoplasty of the SVC was performed. Postvenoplasty hand injection of contrast demonstrated persistent high-grade stenosis. The catheter was then exchanged for a 12 × 40 mm angioplasty balloon catheter. Venoplasty of the SVC was performed. Postvenoplasty hand injection of contrast demonstrated persistent high-grade stenosis (Fig. 3). The catheter was then exchanged for an 18 × 40 mm balloon catheter. Venoplasty of the SVC was again performed. Postvenoplasty DSA demonstrated improvement of the stenosis but reflux into the azygous vein, indicating very poor cardiac output (Fig. 4) Shortly after the last dilation, the patient began to have increased carbon dioxide levels. The patient had no palpable pulse and yet had an active sinus rhythm by EKG, indicating pulseless electrical activity (PEA) cardiac arrest. Urgent resuscitation was performed, but the patient was not able to be revived. An autopsy was refused.
Figure 3.
Initial superior vena cava (SVC) dilation. (A) Fluoroscopic image demonstrates 12-mm balloon dilation of SVC. (B) Superior vena cavagram after dilation shows antegrade flow in the SVC with residual occlusion.
Figure 4.
Final superior vena cava (SVC) dilation. (A) Fluoroscopic image shows 18-mm balloon dilation of the SVC. (B) Final venogram after dilation shows minimal residual stenosis and retrograde filling of azygous vein, indicating very poor cardiac output.
DISCUSSION
Obstruction of blood flow in SVCS can be caused by neoplastic invasion of the venous wall, thrombosis from indwelling catheters, extrinsic compression from mediastinal structures, or a combination of these mechanisms. Currently nonmalignant causes account for 15 to 22% of SVC obstructions.2,3,4,5,6 Of the nonmalignant causes of SVCS, infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis are most common.2 Malignancy is responsible for 78 to 85% of SVCS. The most common cause of malignancy-related SVCS is bronchogenic cancer, which accounts for nearly 80% of cases; lymphoma accounts for ~10 to 15% of malignant cases and metastatic cancers the rest.2,3,4,5,6
In the case of malignancy, stenting has become widely accepted in the management of superior vena cava occlusion (SVCO) because of its high efficacy and low range of complications and because it does not prelude subsequent radiation and chemotherapy. Rowell's review of both randomized and nonrandomized controlled trials, in which patients with carcinoma of the bronchus and SVCO have been treated with any combination of steroids, chemotherapy, radiotherapy, or insertion of an expandable metal stent, revealed that stent insertion may provide relief in a higher proportion of patients and more rapidly than chemotherapy and/or radiotherapy. Other reports have showed similarly high rates of success.7,8,9
Uberoi's review of stenting in SVCS reported a mean complication rate of 5.8%. Complications are favorable to chemotherapy and radiotherapy and included SVC rupture, hemorrhage, hemoptysis, epistaxis, pericardial tamponade, cardiac failure, recurrent laryngeal palsy, stent migration, pulmonary emboli, and groin hematoma.9 Mortality following stent insertion was greater if thrombolytics were administered.7 Repeated stenting is possible in most cases, and there are no absolute contraindications, including total occlusion, to stenting.9
It is vital for an interventionalist to be familiar with the mechanisms and treatment of sudden cardiac complications during interventional procedures. This patient experienced PEA: presence of coordinated electrical activity, other than ventricular tachycardia and ventricular fibrillation, in the absence of a detectable pulse. The etiology of PEA can be generalized as either primary or secondary. Primary PEA is usually associated with abnormal automaticity and conduction, which results in bradycardia and a wide and/or slow QRS complex. It commonly occurs after electrical resuscitation from cardiac arrest and is associated with hyperkalemia, hypothermia, and drug overdose. Secondary PEA has a narrow QRS complex and results from abrupt mechanical changes that decrease venous return. Common causes include hypovolemia, tension pneumothorax, pericardial tamponade, massive pulmonary embolism, malfunction of prosthetic valves, and papillary muscle or myocardial wall rupture.10
In the setting of SVC dilation, either pericardial tamponade or massive pulmonary embolism is the most likely cause of PEA. Pulmonary embolism is uncommon in dilation of chronic obstruction. This patient likely suffered from pericardial tamponade. It is important to remember that the pericardium extends above the heart to the SVC. One good rule of thumb is to use the carina as a marker for the superior extent of the pericardium.11 The pericardium should be evaluated using ultrasound (which is usually immediately available in many interventional radiology suites) for the presence of a pericardial effusion. For pericardiocentesis, a needle is inserted from a left subxiphoid approach using ultrasound guidance directed toward the shoulder at a 15- to 20-degree angle to the abdominal wall. When blood is aspirated, contrast can be injected to confirm position in the pericardial space and a pigtail catheter inserted in standard fashion. In the case of SVC rupture and cardiac tamponade, pericardiocentesis with or without covered stent insertion should be performed and can be life saving. However, Brown and Getrajdman reported a patient that suffered SVC rupture and tamponade who expired despite successful insertion of a pericardial catheter for tamponade.12 This indicates the gravity of the complication and the poor prognosis for recovery.
REFERENCES
- Laskin J, Cmelak A J, Roberts J, Meranze S G, Johnson D H. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editor. Clinical Oncology. 3rd ed. Philadelphia: Elsevier Churchill Livingstone; 2004. Superior vena cava syndrome. pp. 1047–1052.
- Parish J M, Marschke R F, Jr, Dines D E, Lee R E. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc. 1981;56:407–413. [PubMed] [Google Scholar]
- Bell D R, Woods R L, Levi J A. Superior vena caval obstruction: a 10-year experience. Med J Aust. 1986;145:566–568. [PubMed] [Google Scholar]
- Yellin A, Rosen A, Reichert N, Lieberman Y. Superior vena cava syndrome. The myth—the facts. Am Rev Respir Dis. 1990;141:1114–1118. doi: 10.1164/ajrccm/141.5_Pt_1.1114. [DOI] [PubMed] [Google Scholar]
- Lochridge S K, Knibbe W P, Doty D B. Obstruction of the superior vena cava. Surgery. 1979;85:14–24. [PubMed] [Google Scholar]
- Schraufnagel D E, Hill R, Leech J A, Pare J A. Superior vena caval obstruction: is it a medical emergency? Am J Med. 1981;70:1169–1174. doi: 10.1016/0002-9343(81)90823-8. [DOI] [PubMed] [Google Scholar]
- Rowell N P, Gleeson F V. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14:338–351. doi: 10.1053/clon.2002.0095. [DOI] [PubMed] [Google Scholar]
- Chatziioannou A, Alexopoulos T, Mourikis D, et al. Stent therapy for malignant superior vena cava syndrome: should be first line therapy or simple adjunct to radiotherapy. Eur J Radiol. 2003;47:247–250. doi: 10.1016/s0720-048x(02)00207-3. [DOI] [PubMed] [Google Scholar]
- Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006;29:319–322. doi: 10.1007/s00270-005-0284-9. [DOI] [PubMed] [Google Scholar]
- Neumar R W, Ward K R. In: Marx JA, Hockberger RS, Walls RM, editor. Rosen's Emergency Medicine: Concepts and Clinical Practice. 6th ed. Philadelphia: Mosby Elsevier; 2006. Adult resuscitation. pp. 79–90.
- Albrecht K, Nave H, Breitmeier D, Panning B, Tröger H D. Applied anatomy of the superior vena cava: the carina as a landmark to guide central venous catheter placement. Br J Anaesth. 2004;92:75–77. doi: 10.1093/bja/aeh013. [DOI] [PubMed] [Google Scholar]
- Brown K T, Getrajdman G I. Balloon dilation of the superior vena cava resulting in SVC rupture and pericardial tamponade: a case report and brief review. Cardiovasc Intervent Radiol. 2005;28:372–376. doi: 10.1007/s00270-004-0001-0. [DOI] [PubMed] [Google Scholar]