ABSTRACT
Interventional radiology procedures often play an integral role in the diagnosis and treatment of patients with cancer. In the latter stages of cancer treatment, palliative care therapies may be sought for improvement in the quality of remaining life for oncology patients. Increased awareness among interventionalists and referring oncologists regarding minimally invasive treatments for palliation is desirable to provide additional options for patients. In particular, endovascular therapies can provide control of symptoms and complications related to incurable malignancies.
Keywords: Palliative care, endovascular therapy, interventional radiology
Over the years, interventional radiologists have played an increasingly important role in the diagnosis and management of patients with cancer. In many medical centers, interventional radiology (IR) techniques are now viewed as indispensable in the care of patients with malignancies. From image-guided biopsies to catheter-directed therapies, the emerging subspecialty coined “interventional oncology” has opened the door to novel approaches in the way many treatment regimens are formulated. Much of the recent focus in the IR literature has been primarily directed toward the active management and potential for cure or increased survival utilizing locoregional tumor therapy. Relatively less attention has been devoted to the particular needs of patients in the latter stages of cancer treatment. This article highlights certain endovascular therapies that can be used to provide palliative and end-of-life care for the interventional oncology patient.
Historically, medicine has achieved remarkable overall successes across a wide spectrum of previously untreatable or poorly treated malignancies. Modern chemotherapeutic regimens combined with surgery and radiation therapy have markedly improved survival and decreased rates of recurrence. As life expectancy with a malignancy increases, patients are often faced with chronic or unusual manifestations of their disease process. Innovative minimally invasive procedures may be sought to provide patients with symptomatic relief. Often, the challenge lies not necessarily in finding a cure, which may remain elusive, but rather in providing interventions to improve the quality of remaining life for patients.1
The general medical community has often used the term palliative care to describe the treatment for this subset of patients regardless of the severity of their illness or their anticipated life expectancy. However, it has been difficult, even within the oncological community, to clarify the definition of palliative care such that specific treatment aims can be applied. In 1999, the European Society of Medical Oncology established the Palliative Care Working Group to improve the quality and set standards for supportive care and palliative care delivery. To that end, a series of definitions were established to help clarify commonly used terms including supportive care, palliative care, and end-of-life care.
Supportive care is defined as care that aims to optimize comfort, function, and social support of the patient and their family at all stages of the illness. This emphasizes the oncologist's role in optimizing quality of life for all patients, including those with potentially curable illness.
Palliative care is defined as care that aims to optimize the comfort, function, and social support of the patients and their families when cure is not possible. This emphasizes the special needs of the patient whose illness is either incurable or is unlikely to be cured.
Finally, end-of-life care is defined as palliative care when death is imminent.2
Image-guided endovascular therapy offers a wide range of procedures that can be offered to the oncology patient. It is important to distinguish those that are palliative versus those that may be supportive, such that patients and their referring physicians can make informed decisions regarding potential outcomes. Some of these treatments are aimed at local control of disease (with concomitant reduction of tumor-related symptoms such as pain or compression of adjacent structures); others are directed at distant organs adversely affected by the malignant process. Endovascular therapy offers a subset of options for cancer patients that will be further highlighted. Examples of these procedures broadly include the following:
Endovascular embolotherapy for control of symptoms associated with primary or metastatic disease.
Treatment of venous and arterial thromboses related to malignancy, with particular emphasis on large venous occlusions.
Placement of intravascular devices for convenient intravenous (IV) access.
TUMOR CONTROL WITH EMBOLOTHERAPY FOR PALLIATION
Embolotherapy has long been a mainstay in the interventionalist's toolbox. Applications for embolization in the palliative care setting are varied and include its use for control of hormonally active metastatic disease, temporary relief of refractory tumor pain and compression symptoms, and cessation of bleeding from tumors.
The liver is a common site for metastatic disease, particularly in cases of primary gastrointestinal cancers. The presence of metastatic deposits in the liver by itself does not necessary cause clinically evident symptoms and may not require treatment with locoregional therapy. Problems may become clinically evident when normal liver function is impaired by a large tumor burden. Symptoms can be dramatic in cases of gastrointestinal carcinoid, an uncommon tumor with an incidence of 0.5 to 2.0 per 100,000 people.3 Carcinoid tumors arise from embryonic endodermal tissue and are characterized by their production of serotonin and neuropeptides. These hormones are metabolized by the liver during first pass and can cause symptoms when they are produced by metastatic deposits in the liver. The hormones produced by these hepatic metastases can still be metabolized by the liver, but when the metastatic tumor burden overtakes the metabolic capacity of the liver, these hormones are released into the systemic circulation and patients can develop malignant serotonin syndrome. This syndrome is characterized by flushing, diarrhea, cardiac valvular damage, and bronchoconstriction with asthma-like symptoms. Symptoms can be medically controlled with the use of octreotide, a somatostatin analogue; however, this measure only affects symptoms and does not alter hormone production or reduce hepatic metastasis.4 Carcinoid tumors frequently do not respond well to systemic chemotherapy, with some studies quoting regression rates seldom exceeding 20%.5 Surgical resection or percutaneous ablation may not be a viable option because most patients have extensive tumor burden at this stage.
Transcatheter hepatic artery embolization of carcinoid metastasis is effective in the management of carcinoid syndrome, as well as the metastatic deposits themselves. Both chemoembolization and bland embolization have been performed with comparable results.6,7 A 2002 study by Schell et al4 reviewed 24 patients in whom bland embolization with lipiodol and Gelfoam was performed. A total of 101 selective hepatic artery embolizations were performed. Following treatment, hepatic tumor burden was reduced in 79% and remained stable in 17%. A good number of patients (64%) were rendered asymptomatic following treatment and average daily octreotide requirements were reduced; 46% were able to discontinue octreotide completely. Five-year survival was calculated to be 72% for all patients, and 54% when only looking at patients with malignant serotonin syndrome.
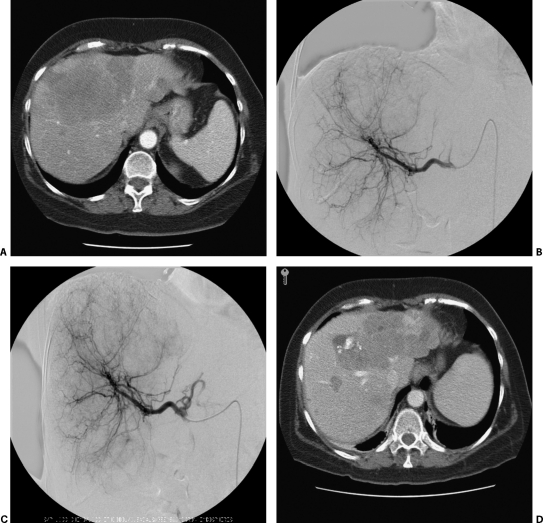
This study advocates the use of hepatic artery particle embolization as a primary therapy for control of symptoms associated with unresectable metastatic carcinoid disease manifesting as the serotonin syndrome. Due to the extensive nature of the metastatic disease at the time of presentation, the cytoreductive property of embolization does not add a significant curative advantage. Hepatic artery embolization can be reasonably recommended as a palliative therapy in this setting (Fig. 1).
Figure 1.
(A) Computed tomography (CT) scan of a patient with symptoms of carcinoid syndrome despite treatment with a high-dose long-acting octreotide. Demonstrated are multiple bilateral liver metastases including a dominant lesion bridging the right and left lobes. (B) Selective hepatic angiogram delineating the arterial supply to the hypervascular liver metastases. (C) Immediate follow-up hepatic angiogram postchemoembolization (Mitomycin/Adriamycin/Cisplatin mixed with Ethiodol). (D) Three-month follow-up CT shows mixed areas of Ethiodol uptake, necrosis, and hypervascularity. Of clinical importance, the patient's octreotide dose had been gradually tapered off and symptoms were well controlled at follow-up.
Pancreatic islet cell tumors arising from the endocrine pancreas can be functional or nonfunctional, benign or metastatic. Symptoms can be profound after a functional tumor has metastasized to the liver. The symptoms vary depending on the tumor cell type, which includes insulinoma, gastrinoma, glucagonoma, somatostatinoma, and VIPoma. As the primary tumor tends to be indolent, the metastatic tumor burden can be quite large at the presentation of symptoms. Islet cell tumors often have a better response to systemic chemotherapy than carcinoid tumors, ranging from 30 to 70%; however, less than a third have a lasting response. Functional liver metastasis from islet cell tumors is responsive to hepatic artery embolization. A retrospective study by Gupta et al8 showed that the average duration of symptomatic relief from hepatic artery embolization was ~16 months.
Gupta et al also examined the efficacy of chemoembolization compared with bland particle embolization in the treatment of metastatic neuroendocrine tumors. Originally, it was thought that arterial ischemia could be potentiated by intra-arterial injection of mixtures of iodized oil and cytotoxic drugs (such as doxorubicin), followed by embolization with an absorbable gelatin sponge.9 Several studies have implicated that chemoembolization is more effective in the treatment of both carcinoid and islet cell carcinomas; however, these studies appear to lack statistical power as well as cohort heterogeneity. Gupta and coauthors found that chemoembolization was more effective than bland embolization in the treatment of islet cell carcinoma metastases, whereas chemoembolization was less effective than bland embolization in the treatment of carcinoid metastases. Currently, there is not universal agreement on which procedure should be considered as first-line treatment of neuroendocrine metastases, and further investigation is warranted.
Arterial embolization of tumors has been described for palliative control of local symptoms. For example, osseous metastases from various malignancies may cause symptoms such as refractory pain, bleeding, and neurological compromise. For patients with bone metastasis refractory to conventional symptomatic therapy, the most common treatment modalities involve either systemic chemotherapy or radioactive isotope therapy, with or without localized external beam radiation. In cases of localized symptoms refractory to conventional therapy, locoregional treatments should be considered.
Transcatheter embolization of hypervascular osseous metastases is reported and can be effective in select patients. Renal cell carcinoma and thyroid carcinoma are both tumors that typically exhibit hypervascular metastatic bone lesions and can be potentially responsive to endovascular treatment. In a study including 51 patients by Barton et al, patients receiving transcatheter embolization experienced decreased pain and bleeding complications from their disease without the need for surgical management. Selective embolization of painful hypervascular bony metastatic disease may be an appropriate palliative therapy to provide symptom relief. Responses can be rapid, although transient, with median symptom relief duration from 6.5 to 9 months.10,11
Similarly, painful osseous metastases from thyroid carcinoma can be difficult to treat effectively. Usually, these lesions do not show significant uptake of iodine-131, leaving surgical resection or external beam therapy as the only remaining traditional palliative measures. Transcatheter embolization of these lesions gives rapid relief of both pain and neurological symptoms.12,13 The effects, however, are not curative because the symptoms tended to recur within 6 months without additional therapies. Many of the patients required multiple treatments over time to continue their palliation. Given the alternatives, this is a relatively noninvasive therapy that may offer significant symptomatic relief of localized tumor pain.
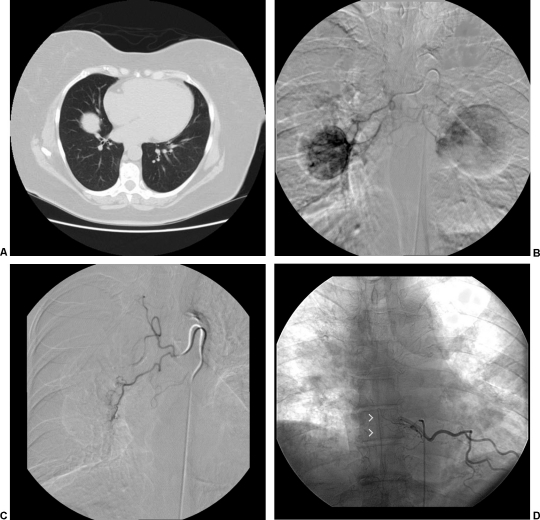
The use of transcatheter embolization and endovascular stenting is well described for the control of bleeding from malignancies. For example, transcatheter embolization can be used for palliative control of massive hemoptysis arising from unresectable lung tumors. Historically, bland embolic materials including Gelfoam, polyvinyl alcohol particles, microspheres, and endovascular coils were used to occlude bleeding tumor vessels (Fig. 2). A 2000 study by Witt et al14 demonstrated that bronchial artery embolization was useful for patients with recurrent hemoptysis when compared with patients managed with conservative measures alone. In this study, all patients with recurrent bleeding that were managed conservatively died, whereas those that were embolized again survived a mean of 159 days longer. Embolization for palliative control of gastrointestinal bleeding and spontaneous hemorrhage from solid organ malignancies has been well described. In the liver, hepatocellular carcinoma, as well as hypervascular metastatic disease, can be controlled with endovascular therapy.15,16,17
Figure 2.
(A) Computed tomography scan showing lung metastasis in a patient with known renal cell carcinoma and massive hemoptysis. Bronchoscopy localized bleeding to the right side. (B) Bronchial angiogram was performed with selective catheterization of an intercostal bronchial trunk, which gives rise to right and left bronchial arteries as well as right intercostals. Demonstrated is enhancement of a large right lung lesion and partial supply to a left lung lesion. (C) Postembolization angiogram was performed demonstrating stasis of antegrade flow to the right bronchial artery. Embolization was performed via a coaxial system (Renegade Hi-Flo microcatheter [Boston Scientific, Natick, MA] through a Mikaelsson catheter [Cook Medical, Bloomington, IN]) utilizing polyvinyl alcohol particles of 355 to 500 microns. (D) During bronchial angiography, recognition of spinal cord supply via the anterior spinal artery, or artery of Adamkiewicz (arrowheads), is critical to avoid nontarget embolization leading to cord ischemia.
Preliminary use of transcatheter chemoembolization for the palliative management of unresectable lung metastases has been reported. In a study by Vogl and colleagues,18 26 patients were embolized with a mixture of mitomycin C and iodized oil followed by microsphere particles. No major adverse outcomes resulting from the procedure were reported. Follow-up was conducted at 3-month intervals via contrast enhanced computed tomography (CT). In eight patients regression was noted, stable disease was found in six patients, and progression of treated metastases was noted in nine patients. Further study is warranted to confirm the efficacy of this technique. Palliative embolization for other solid organ malignancies such as unresectable renal cell carcinoma for refractory pain or hematuria has also been reported with good overall success rates.19,20,21
ENDOVASCULAR MANAGEMENT OF MALIGNANCY-RELATED VASCULAR THROMBOSES
Patients with a malignancy are in a known hypercoagulable state.22 Complications can range from superficial thrombophlebitis to deep venous thrombosis, or from thrombotic microangiopathy to arterial thrombosis. Complications from vascular thrombosis is the second leading cause of death in patients with overt malignancies.23 Endovascular treatments should be considered in the palliative care of symptomatic patients who fail or are not candidates for conservative medical therapy. At the severe end of the spectrum, large vessels including the superior and inferior vena cava can be involved. Both superior and inferior vena cava syndrome represent significant symptomatic complications often associated with malignancies.
Superior vena cava (SVC) syndrome was first described in 1757 as a complication of syphilitic aortitis. Since then, the term has been used to describe the constellation of symptoms associated with obstruction of the SVC. The primary symptoms associated with the syndrome are facial, periorbital, neck and bilateral arm swelling, and dilated superficial veins over the chest wall. SVC syndrome can be severely debilitating, with progressive symptoms of dysphagia, dyspnea, and cognitive dysfunction due to cerebral venous hypertension.24 More than 85% of the cases are due to an underlying malignancy such as bronchogenic carcinoma, lymphoma, or metastatic disease. Although benign causes only account for 15% of SVC syndrome, the number of cases caused by SVC obstruction secondary to chronic indwelling catheters appears to be on the rise.
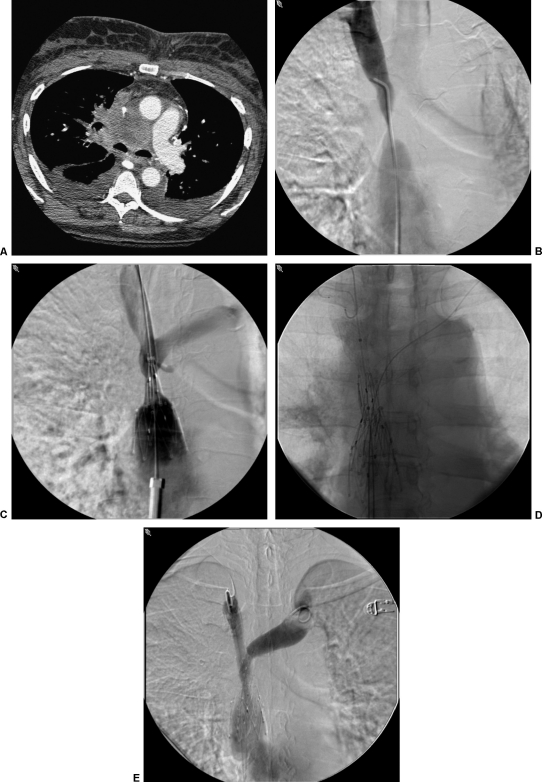
Prior to aggressive endovascular options, the traditional treatment for malignant SVC syndrome was radiation therapy and chemotherapy. External beam radiation has been reported to have a high success rate of > 70%, but recurrence is common and clinical benefits of the therapy may not be seen for up to 4 weeks.24,25 In the era of endovascular management, most patients can now be primarily treated with venous stenting (Fig. 3). A 2000 study by Lanciego et al26 demonstrated that vascular endoprostheses are effective in relieving the symptoms of the SVC syndrome. A total of 52 patients underwent the endovascular therapy, with all patients achieving relief within 72 hours. Thirteen patients required repeated stenting due to recurrent obstruction, migration of the prosthesis, suboptimal placement, or stent shortening. A second procedure was technically successful in all of these patients.
Figure 3.
(A) Computed tomography scan of a patient with metastatic bronchogenic carcinoma. The superior vena cava (SVC) is encased in this patient with dyspnea and facial swelling. (B) SVC gram demonstrates severe stenosis of the SVC from external compression. (C) SVC gram during deployment of a 25-mm diameter Gianturco Z stent (off-label use; Cook Medical, Bloomington, IN). (D) Self-expanding kissing brachiocephalic vein stents were utilized to preserve the venous confluence. (E) Completion venogram demonstrating technical improvement in SVC patency. This patient's symptoms were significantly improved within 48 hours.
Confirmatory studies support high success rates for endovascular treatment of SVC syndrome: 90 to 100% immediate technical success27,28,29 and primary patency of 70 to 80% at 6 months have been reported. Secondary patency was ~80 to 90% at 6 months, which is most often sufficient for palliative end-of-life care. Complications of SVC recanalization/stenting have a reported range of 4 to 10% and include bleeding secondary to thrombolysis, recurrent laryngeal nerve paralysis, congestive heart failure, pulmonary emboli, pacemaker dysfunction, and cardiac tamponade. Nevertheless, an endovascular approach should be considered as a first-line option in the management of SVC syndrome due to its benefit of a rapid clinical response.
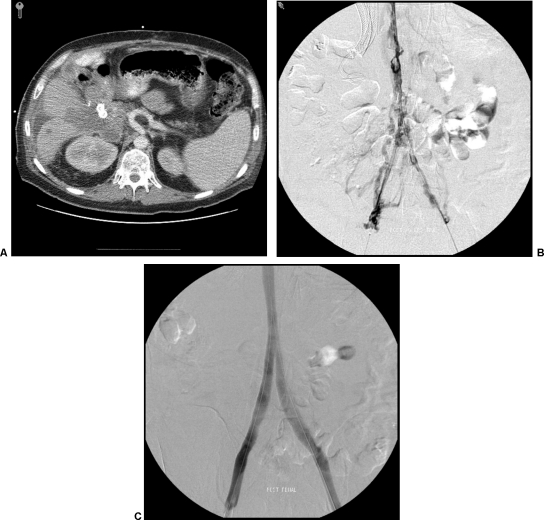
Whereas endovenous therapy in SVC syndrome is relatively well described and may already be a known option among referring oncologists, treatment of inferior vena cava (IVC) occlusion has received less attention. Symptomatic occlusion of the IVC can produce a variety of symptoms, including massive lower extremity edema, ascites, back pain, and venous stasis complications. If thrombus extends to involve renal or hepatic veins, this can lead to organ dysfunction from venous congestion as well. Aggressive endovascular recanalization of properly selected patients with IVC occlusions may provide symptomatic relief and prevent late-stage complications such as phlegmasia cerulea dolens or secondary organ failure. Stenting is performed either primarily or in combination with thrombolytic therapy, and technical success was 88% in some series with primary patency rates of up to 80% at 19 months. A mean follow-up of 18 months and secondary patency of 87% was described in one series.30 As with the SVC, IVC intervention should be considered in patients who are symptomatic and require a relatively durable and minimally invasive therapy (Fig. 4).
Figure 4.
(A) Computed tomography scan in a patient with unresectable pancreatic cancer and massive bilateral lower extremity swelling. Biliary stents are in place. (B) Venogram via bilateral popliteal vein access after overnight transcatheter thrombolysis with tissue plasminogen activator. Significant stenoses secondary to chronic iliocaval thrombus remains. (C) Self-expanding inferior vena cava and kissing iliac vein stents were deployed with good technical result on venography.
CENTRAL VENOUS ACCESS
When discussing endovascular interventions, attention to procedures such as placement of central venous catheters and implantable ports for convenient IV access can be paramount to patient comfort. Many, particularly those near the end of life, are unable to maintain effective oral intake. For those patients, the maintenance of durable, long-term vascular access is critical for the administration of fluids, medications, and/or total parenteral nutrition. Although not typically thought of as a palliative measure, these are procedures that can significantly alter the quality of life for many patients. The majority of IV access procedures are rather straightforward to the interventionalist using ultrasound and fluoroscopic guidance. Technical challenges remain, however, in those patients with multiple venous stenoses/thromboses. Alternative venous access sites or recanalization techniques should be considered in these cases. For a good overview of these options, refer to Lorenz's article in the Seminars in Interventional Radiology, September 2006.31
CONCLUSIONS
Palliative care has developed into a complex multidisciplinary field that has advanced far beyond rudimentary pain control measures. Interventionalists are able to provide effective control of patient symptoms in a targeted, minimally invasive manner. This brief overview of a few endovascular options that can be offered is not meant in any way to be comprehensive. Countless other therapies, from stenting or coil embolization of arteriovenous fistulas and vascular fistulas with hollow viscous to catheter-directed brachytherapy for palliation, are beyond the scope of this article. Rather, the goal is to familiarize readers with the application of common or novel interventional techniques that may be of particular benefit in the palliative care setting. Through these procedures, patients may be afforded alternatives that can provide significant improvement in the quality of remaining life.
REFERENCES
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- Cherny N I, Catane R, Kosmidis P. ESMO takes a stand on supportive and palliative care. Ann Oncol. 2003;14:1335–1337. doi: 10.1093/annonc/mdg379. [DOI] [PubMed] [Google Scholar]
- Bax N D, Woods H F, Batchelor A, Jennings M. Clinical manifestations of carcinoid disease. World J Surg. 1996;20:142–146. doi: 10.1007/s002689900022. [DOI] [PubMed] [Google Scholar]
- Schell S R, Camp R, Caridi J G, Hawkins I F. Hepatic artery embolization for control of symptoms, octreotide requirements and tumor progression in metastatic carcinoid tumors. J Gastrointest Surg. 2002;6:664–670. doi: 10.1016/s1091-255x(02)00044-6. [DOI] [PubMed] [Google Scholar]
- Brown K T, Koh B Y, Brody L A, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol. 1999;10:397–403. doi: 10.1016/s1051-0443(99)70055-2. [DOI] [PubMed] [Google Scholar]
- Clouse M E, Perry L, Stuart K, Stokes K R. Hepatic arterial chemoembolization for metastatic neuroendocrine tumors. Digestion. 1994;55(suppl 3):92–97. doi: 10.1159/000201208. [DOI] [PubMed] [Google Scholar]
- Lunderquist A, Ericsson M, Nobin A, Sanden G. Gelfoam power embolization of the hepatic artery in liver metastasis of carcinoid tumors. Radiologe. 1982;22:65–70. [PubMed] [Google Scholar]
- Gupta S, Johnson M M, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- Therasse E, Breittmayer F, Roche A, et al. Transcatheter chemoembolization of progressive carcinoid liver metastasis. Radiology. 1993;189:541–547. doi: 10.1148/radiology.189.2.7692465. [DOI] [PubMed] [Google Scholar]
- Barton P P, Waneck R E, Karnel J F, Ritschl P, Kramer J, Lechner G. Embolization of bone metastasis. J Vasc Interv Radiol. 1996;7:81–88. doi: 10.1016/s1051-0443(96)70738-8. [DOI] [PubMed] [Google Scholar]
- O'Reilly G V, Kleefield J, Klein L A, Blume H W, Dubuisson D, Cosgrove G R. Embolization of solitary spinal metastases from renal cell carcinoma: alternative therapy for spinal cord or nerve root compression. Surg Neurol. 1989;31:268–271. doi: 10.1016/0090-3019(89)90050-5. [DOI] [PubMed] [Google Scholar]
- Eustatia-Rutten C F, Romijn J A, Guijt M J, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3184–3189. doi: 10.1210/jc.2003-030231. [DOI] [PubMed] [Google Scholar]
- Smit J WA, Vielvoye G J, Goslings B M. Embolization for vertebral metastases of follicular thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:989–994. doi: 10.1210/jcem.85.3.6436. [DOI] [PubMed] [Google Scholar]
- Witt Ch, Schmidt B, Geisler A, et al. Value of bronchial artery embolization with platinum coils in tumorous pulmonary bleeding. Eur J Cancer. 2000;36:1949–1954. doi: 10.1016/s0959-8049(00)00188-x. [DOI] [PubMed] [Google Scholar]
- Lai E C, Lau W Y. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Takeda R, Mukaihara S, et al. Treatment of ruptured hepatocellular carcinoma. Int J Clin Oncol. 2001;6:291–295. doi: 10.1007/s10147-001-8030-z. [DOI] [PubMed] [Google Scholar]
- Lok C A, Reekers J A, Westermann A M, der Velden J Van. Embolization for hemorrhage of liver metastases from choriocarcinoma. Gynecol Oncol. 2005;98:506–509. doi: 10.1016/j.ygyno.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Vogl T J, Wetter A, Lindemayr S, Zangos S. Treatment of unresectable lung metastases with transpulmonary chemoembolization: preliminary experience. Radiology. 2005;234:917–922. doi: 10.1148/radiol.2343032091. [DOI] [PubMed] [Google Scholar]
- Guy L, Alfidja A T, Chabrot P, et al. Palliative transarterial embolization of renal tumors in 20 patients. Int Urol Nephrol. 2007;39:47–50. doi: 10.1007/s11255-006-9072-y. [DOI] [PubMed] [Google Scholar]
- Munro N P, Woodhams S, Nawrocki J D, et al. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92:240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- Hallscheidt P, Besharati S, Noeldge G, et al. Preoperative and palliative embolization of renal cell carcinomas: follow-up of 49 patients. Rofo. 2006;178:391–399. doi: 10.1055/s-2006-926538. [DOI] [PubMed] [Google Scholar]
- Goldenberg N, Kahn S R, Solymoss S. Markers of coagulation and angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol. 2003;21:4194–4199. doi: 10.1200/JCO.2003.05.165. [DOI] [PubMed] [Google Scholar]
- Donati M B. Cancer and thrombosis. Thromb Haemost. 1995;74:278–281. [PubMed] [Google Scholar]
- Lochridge S K, Knibbe W P, Doty D B. Obstruction of superior vena cava. Surgery. 1979;85:14–24. [PubMed] [Google Scholar]
- Armstrong B A, Perez C A, Simpson J R, et al. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys. 1987;13:531–539. doi: 10.1016/0360-3016(87)90068-x. [DOI] [PubMed] [Google Scholar]
- Lanciego C, Chacon J L, Julian A, et al. Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2001;177:585–593. doi: 10.2214/ajr.177.3.1770585. [DOI] [PubMed] [Google Scholar]
- Thony F, Moro D, Witmeyer P, et al. Endovascular treatment of superior vena cava obstruction in patients with malignancies. Eur Radiol. 1999;9:965–971. doi: 10.1007/s003300050777. [DOI] [PubMed] [Google Scholar]
- Kee S T, Kinoshita L, Razavi M K, Nyman U RO, Semba C P, Dake M. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998;206:187–193. doi: 10.1148/radiology.206.1.9423671. [DOI] [PubMed] [Google Scholar]
- Wilson E, Lyn E, Lynn A, Kahn S. Radiological stenting provides effective palliation in malignant central venous obstruction. Clin Oncol (R Coll Radiol) 2002;14:228–232. doi: 10.1053/clon.2002.0088. [DOI] [PubMed] [Google Scholar]
- Razavi M K, Hansch E C, Kee S T, Sze D Y, Semba C P, Dake M D. Chronically occluded inferior venae cavae: endovascular treatment. Radiology. 2000;214:133–138. doi: 10.1148/radiology.214.1.r00ja33133. [DOI] [PubMed] [Google Scholar]
- Lorenz J M. Unconventional venous access techniques. Semin Intervent Radiol. 2006;23:279–286. doi: 10.1055/s-2006-948767. [DOI] [PMC free article] [PubMed] [Google Scholar]