ABSTRACT
The development of a pleural effusion or ascites in patients with underlying malignancy typically heralds end-stage disease and often results in a significant reduction in the patient&'s quality of life. The goal of treatment is the safe and effective palliation of symptoms with minimal inconvenience to the patient. Malignant fluid collections in the chest and abdomen are amenable to percutaneous management with either intermittent thoracentesis or paracentesis or by placement of temporary or permanent drainage catheters.
Keywords: Malignant pleural effusion, malignant ascites, drainage catheter
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion (MPE) develops in about half of all cancer patients who have disseminated disease and is diagnosed in more than 150,000 patients each year in the United States.1,2 The most common etiologies are lung and breast carcinoma followed by lymphoma, ovarian cancer, gastrointestinal cancer, mesothelioma, and malignancy of unknown primary.2 The 30-day mortality rate is as high as 50% and the median survival time is 6 to 12 months for patients with MPE,1,2 so the goal of treatment is cost-effective palliation of symptoms with minimal discomfort and inconvenience for the patient.
More than 75% of patients with MPE are symptomatic and most present with dyspnea, although patients may also complain of cough, pleuritic pain, or a feeling of pressure or heaviness in the chest.3 These symptoms diminish the patient's quality of life and will not improve without treatment. The etiology of the MPE and the patient's prognosis, severity of symptoms, and performance status should be considered when planning therapy. Although MPE secondary to lymphoma or small cell lung carcinoma may respond to systemic chemotherapy, most malignant effusions require local palliative therapy. The local treatment options include frequent thoracentesis, placement of nontunneled or tunneled drainage catheters, pleurodesis, pleuroperitoneal shunting, pleurectomy, and decortication.
Thoracentesis
Thoracentesis is typically the first step in the management of a newly diagnosed pleural effusion. In a symptomatic patient, the presence of an effusion can be confirmed with a chest radiograph, and a lateral decubitus film can demonstrate whether the pleural fluid is free flowing or loculated. Depending on the size and nature of the effusion, a thoracentesis may be performed with or without sonographic guidance.
Ultrasound-guided thoracentesis can be performed from a posterior intercostal approach with the patient seated or from a midaxillary approach with the patient in a supine, slightly oblique position. After sterile preparation, the skin and soft tissues at the access site are anesthetized and a small dermatotomy is created. A sheathed needle, such as a 5F Yueh (Cook, Bloomington, IN) or One-Step Centesis (Merit Medical Systems, South Jordan, UT) needle is advanced into the pleural space with continuous sonographic visualization. Care should be taken to enter along the superior margin of the rib to avoid injury to the intercostal artery. Once in the pleural space, the needle is removed and the sheath is attached to a one-way valve system for aspiration of fluid. As a rule of thumb, not more than 1500 mL of fluid should be removed at one time to reduce the risk of reexpansion pulmonary edema.
The initial thoracentesis can be diagnostic as well as therapeutic. Pleural fluid should be sent for cell count, total protein, lactate dehydrogenase (LDH), glucose, pH, and cytology. Although malignant cells are detected in only about half of effusions that ultimately prove to be malignant, an exudative effusion in a patient with a known neoplasm should be considered malignant until proven otherwise.4 The criteria used to diagnose an exudate are as follows: pleural fluid to serum protein ratio > 0.5, pleural fluid LDH > 200, pleural fluid to serum LDH ratio > 0.6, protein > 3 g/dL and pH > 7.3.4 If two thoracenteses fail to diagnose the etiology of the effusion, a thoracoscopy should be performed.3
Thoracentesis is a simple way to achieve acute relief of symptoms as well as to assess the degree of symptomatic improvement experienced by the patient. Unfortunately, MPE recurs in 98 to 100% of patients within 30 days of thoracentesis and thoracentesis will only control MPE for 4 days on average,2,3 so frequent thoracentesis is likely to be required for palliation of symptoms. The risks of the procedure, including bleeding, infection, pneumothorax, fluid loculation, and reexpansion pulmonary edema, increase with repeat thoracenteses. For these reasons, treatment of MPE with thoracentesis alone is usually reserved for patients who are likely to respond quickly to systemic therapy or for those with a very short life expectancy.
If thoracentesis does not result in symptomatic improvement, alternative explanations for the patient's symptoms should be sought. In some patients the compressed lung is relatively stiff and does not reexpand immediately following evacuation of the pleural fluid. Up to 30% of patients have some degree of pneumothorax following thoracentesis that is felt to be due to an ex-vacuo phenomenon related to failure of the lung to reexpand rather than to an air leak.4 In some of these patients, the lung never reexpands because it is “trapped” or encased by a dense peel of malignant tissue surrounding the lung parenchyma. If the lung does reexpand but the patient remains symptomatic, underlying etiologies such as lymphangitic spread of tumor, pulmonary embolism, or malignant airway obstruction may be responsible, and further evaluation with computed tomography of the chest should be pursued.
Pleurodesis
Pleurodesis results in elimination of the pleural space, preventing the reaccumulation of fluid, and can be accomplished with either chemical or mechanical irritation of the pleura. Pleurodesis may be performed via an indwelling chest tube at the bedside or with video-assisted thoracoscopic surgery (VATS). A variety of agents have been used for pleurodesis, some that are intended to cause an inflammatory response and others that are supposed to act as chemotherapeutic agents as well. For pleurodesis to be successful, the lung must reexpand completely following removal of the pleural fluid.
Pleurodesis using a chest tube has historically been an inpatient procedure, often requiring prolonged hospitalization, but recent reports have described successful outpatient sclerosis.4,5 The patient should have a good performance status, and either he or his caregiver should be reliable to care for the chest tube and monitor its output before ambulatory therapy is considered. The technique for chest tube placement is basically the same for both inpatient and outpatient treatment, and, although numerous protocols for administration of the sclerosant have been described, they all involve drainage of the pleural space until it is dry followed by instillation of the agent of choice.
With the patient in a supine, slightly oblique position, ultrasound is used to identify an entry site into the pleural space, ideally along the midaxillary line in the lowest intercostal space that is safely above the diaphragm. If the pleural fluid is loculated, the largest loculation should be targeted for entry. After the skin and soft tissues at the entry site are anesthetized and a small dermatotomy is created, an 18-gauge needle is directed posteriorly into the pleural space using sonographic guidance. Using fluoroscopic guidance, a 0.035 stiff Amplatz wire (Cook, Bloomington, IN) is advanced through the needle. The wire can be used to disrupt loculations if necessary. The needle is exchanged over the wire for sequential tissue tract dilators followed by a locking pigtail drain. The wire and stiffener are removed and the pigtail is formed and locked. The pigtail is optimally positioned near the costophrenic angle and is connected to a Pleur-Evac (Teleflex Medical, Limerick, PA) for inpatient management or a Tru-Close (UseSil, Skokie, IL) gravity drain for outpatient management. Any size chest tube can be used, but 10, 12 or 14F tubes are most commonly placed.
If the patient is admitted, the Pleur-Evac is placed on continuous wall suction at 20 to 30 cm H2O, and drain output is recorded daily. When the drainage decreases to < 100 to 200 mL in 24 hours, a chest radiograph is obtained to document resolution of the effusion and reexpansion of the lung. If there is persistent fluid, the drain should be checked to make sure it is patent and not kinked or otherwise malpositioned. If the drain is clogged or the fluid has become loculated, instillation of a fibrinolytic agent such as tissue plasminogen activator may restore patency of the drain or facilitate drainage by breaking down adhesions within the fluid. Complete drainage of the pleural effusion takes about 5 days on average.4 When drainage is complete, the sclerosant, typically 100 mL of saline and 5 g of talc slurry with or without 20 to 50 mL of 0.5% lidocaine, is instilled and the chest tube is clamped for a period of time, usually 2 hours. The chest tube is returned to drainage, and, if the output is < 100 to 200 mL for the 24 hours after administration of the sclerosant, the tube is removed. If the output is > 200 mL, the process is repeated.
Ambulatory patients are taught how to care for the chest tube and record its output and are given emergency contact information. These patients are instructed to return when drainage is < 100 to 200 mL in a 24-hour period, and, if a chest radiograph demonstrates resolution of the effusion, the sclerosant is administered, the tube is clamped for 2 hours, and the patient is observed. The patient returns for removal of the chest tube on the following day.
The most common side effects of talc pleurodesis are pain and fever, but respiratory complications, including talc pneumonitis and adult respiratory distress syndrome (ARDS), can occur. Respiratory failure has been reported in 4% of patients after slurry and 8% after poudrage.6 The incidence of respiratory complications appears to be dose related, with most cases involving doses > 5 g.3
A recent review of all available data on pleurodesis in MPE sought to determine the best agent and the optimal protocol for administration of that agent. Meta-analysis of 31 randomized controlled trials (RCTs) comparing sclerosing agents showed a slight trend toward talc as a more effective sclerosant than bleomycin or tetracycline, the other most commonly used agents. Talc is the least expensive and probably the safest of all the agents as well. Three RCTs that addressed bedside instillation of talc slurry versus VATS insufflation of talc poudrage were analyzed and seemed to favor VATS but not enough for the authors of the review to strongly recommend VATS over bedside pleurodesis. VATS is obviously the procedure of choice if a pleural biopsy is also needed for diagnosis. Other interesting conclusions of this review are that rolling the patient following pleurodesis does not make it more effective, drainage for shorter periods of time is as effective as protracted drainage prior to pleurodesis, and small chest tubes are just as effective as large ones.7
In one series of patients treated with ambulatory pleurodesis, six of eight patients reported symptomatic improvement at 30 days, one patient failed sclerosis, and one patient died 26 days after sclerosis despite radiographic resolution of the effusion.5 In a slightly larger series, 10 of 19 patients had a complete radiographic response while 5 of 19 had a partial response. All patients experienced symptomatic improvement at 30 days, and no major complications were reported.8
Nontunneled Drainage Catheters
Although nontunneled drainage catheters are almost always used in conjunction with a sclerosing agent for pleurodesis, a nontunneled chest tube may be placed for palliation of symptoms in a patient who has an extremely short life expectancy and is not a candidate for other treatment options. Although chest tube placement with complete drainage of the fluid has been reported to control MPE in up to 77% of patients,1 nontunneled chest tubes are more likely to become dislodged or infected than tunneled chest tubes and are not optimal for the long-term management of MPE.
Tunneled Drainage Catheters
Tunneled drainage catheters have become an appealing alternative for management of MPE. Tunneled catheters are cost effective and well tolerated by patients, have high success and low complication rates, and give patients some control over their disease by allowing them to drain recurrent fluid at home.1,2,8,9,10,11 Patients who have a good symptomatic response following thoracentesis for MPE, a reasonable life expectancy, and a reliable caregiver should be considered for placement of a tunneled catheter.
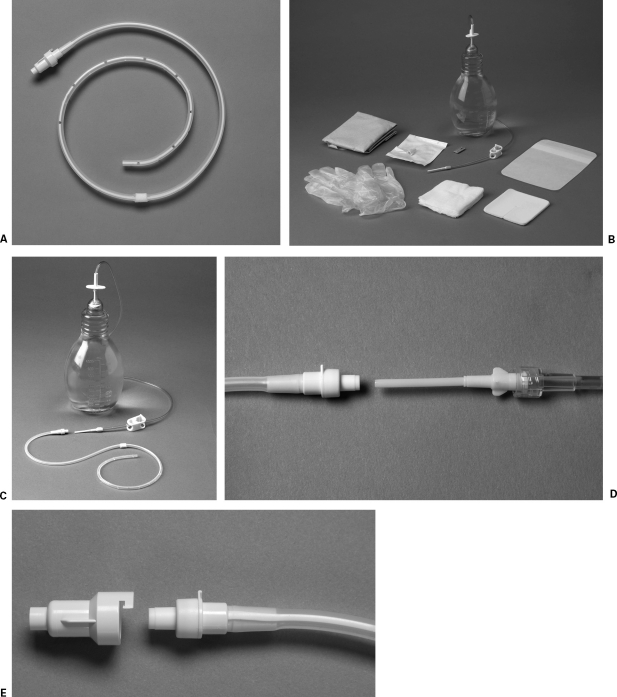
The Pleurx (Denver Biomedical, Golden, CO) is a tunneled catheter designed specifically for use in patients with MPE and approved by the U.S. Food and Drug Administration (FDA) for that indication in 1997. The Pleurx is a 15.5F 66 cm silicone catheter with side holes along the distal 24 cm, a single polyester cuff, and a valved hub (Fig. 1) The catheter is flexible and soft, making it comfortable for the patient. The cuff is intended to decrease migration of the catheter, and the valve prevents leakage from the catheter as well as entry of air into the pleural space.
Figure 1.
(A) Pleurx catheter. (B) Sterile supply kit provided for drainage. (C,D) The connector at the end of the drainage bottle fits inside the catheter, opening the one-way valve in the hub of the Pleurx. (E) The Pleurx should be capped when not in use.
The RCT that led to approval of the Pleurx by the FDA involved 144 patients, two thirds of whom were treated with the Pleurx while one third was treated with a chest tube and doxycycline pleurodesis. Equivalent efficacy and safety were shown for both groups, with no difference in median survival. The median hospital stay of one day for the Pleurx patients, the same length as that mandated by the study protocol, was significantly shorter than the median stay of 6.5 days in the sclerotherapy group. Spontaneous pleurodesis occurred in 46% of Pleurx patients; pleurodesis was achieved in 54% of sclerotherapy patients. The early complication rate of 10 to 14% was similar in both groups. The late complications reported for the Pleurx patients included local cellulitis in six, tumor seeding of the catheter track in three, catheter obstruction in two, and hospitalization for pleural infection in one.11
In a series involving treatment of 31 hemithoraces with the Pleurx catheter in 28 patients (9 of whom were included in the FDA trial), the technical success rate for placement with image guidance was 100%, immediate improvement in symptoms occurred in 94%, and 91% remained improved at 30 days. Spontaneous pleurodesis occurred in 42% at a median time of 19 days. Five patients required ancillary procedures to maintain catheter function. Four of the catheters became separated from the effusion by fibrosis and were removed and replaced, and one catheter migrated outside the chest and was replaced. Six patients had pain associated with drainage that was severe enough to require premedication with narcotics, and this pain persisted < 1 week in four of the six. One patient developed tumor seeding along the track, and one patient was treated with antibiotics for fever and growth of mixed flora on a pleural fluid culture.1
A recent retrospective review of 250 sequential tunneled catheter insertions for MPE in 233 patients describes symptom control as complete in 38.8%, partial in 50%, and none in 3.6% at a 2-week follow-up visit. Spontaneous pleurodesis occurred in 42.9% at a median time of 59 days. Additional procedures were required in 9.9% and included repeat tunneled catheter placement in 9 patients, thoracentesis in 6, standard chest tube in 5, and fibrinolysis in 4. The most common complication was symptomatic loculation of fluid, which developed in 8.4%. The most severe complication was empyema, which was seen in 3.2% and treated with intravenous antibiotics, fibrinolytics, and additional chest tubes as needed. The other complications listed included pneumothorax in 2.4%, cellulitis in 1.6%, catheter displacement in 1.2%, tumor seeding in 0.4%, and pain requiring removal in 0.4%. The conclusion of the authors of this study was that tunneled pleural catheters should be considered as a first-line treatment option for MPE.9
The technique for placement of a tunneled catheter is similar to that for a nontunneled catheter with a few additional steps. The pleural space should be entered from a low intercostal space in a direction that will allow the catheter to lie across the posterior costophrenic sulcus. The entry site is dilated over a 0.035 stiff Amplatz with the tapered 16F peel-away sheath provided with the Pleurx kit. The wire and peel-away are left in place while the subcutaneous tunnel is created. The tunnel should be directed anteriorly so the patient can easily access the catheter, taking care to avoid placement of the exit site where the patient's waistband would typically lie. The length of the tunnel should be measured so the cuff is 1 to 2 cm from the exit site when the most proximal side hole of the catheter is safely within the pleural space. After the subcutaneous tissues are anesthetized with 1% lidocaine, the catheter is brought through the tunnel in an antegrade fashion.
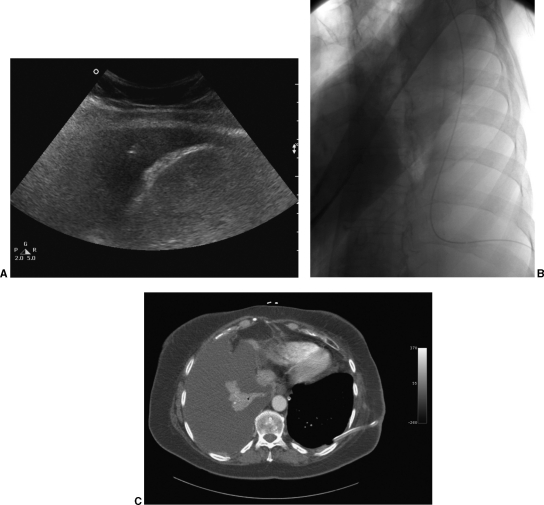
Temporary occlusion of the side holes of the catheter helps minimize the amount of air that enters the pleural space as well as avoid loss of a large amount of fluid and can be accomplished by placing a 6.3 F van Andel catheter (Cook, Bloomington, IN) through the most proximal side hole and out the distal end of the Pleurx. If placement of the Pleurx over the wire is desired, the van Andel can be shortened. When the dilator is removed from the peel-away, the peel-away should be pinched to prevent entry of a large amount of air into the chest. The Pleurx can be advanced through the peel-away either with or without the wire, although use of the wire helps direct the course of the catheter within the pleural space. The wire, van Andel, and peel-away are removed. The entry site is closed and the catheter is secured at the exit site with 2-0 nonabsorbable suture. After placement of the catheter, up to 1500 mL of pleural fluid is aspirated to confirm the catheter functions well and the patient tolerates drainage in this manner. A fluoroscopic image demonstrating the course of the catheter should be obtained (Fig. 2).
Figure 2.
(A) Ultrasound-guided placement of an 18-gauge needle into the pleural space just above the diaphragm. (B) Fluoroscopic image demonstrates good position of the Pleurx catheter, which courses posteromedially along the costophrenic sulcus and then toward the apex of the chest. (C) Computed tomography confirms the position of the left Pleurx catheter and demonstrates no pleural fluid on the left. The patient subsequently underwent placement of a Pleurx on the right.
Following placement of the Pleurx, the patient is usually observed for 4 hours. If a chest radiograph is obtained prior to discharge, a small pneumothorax often is present. If the patient is hemodynamically stable and symptomatically improved, the patient is discharged home. Explicit instructions regarding the care and use of the catheter are given to the patient and the patient's caregiver prior to discharge. Most importantly, contact numbers are provided should any questions or problems arise.
Spontaneous pleurodesis with the Pleurx is considered to have occurred when the output is < 100 mL on three consecutive attempts at drainage and a chest radiograph demonstrates no significant effusion. Patients should be instructed to call if output decreases to this extent. If the chest radiograph does not demonstrate some other explanation for the decreased drainage, the catheter can be removed at the bedside using local anesthesia.
The use of a chemical sclerosant in conjunction with the Pleurx catheter has been reported in a few patients but has not been formally evaluated.1,7 This approach has been successful in patients who have not achieved spontaneous pleurodesis but who no longer wish to have the catheter in place or who can no longer drain the catheter as required; however, the complication of empyema following administration of talc slurry via a Pleurx has been reported.9
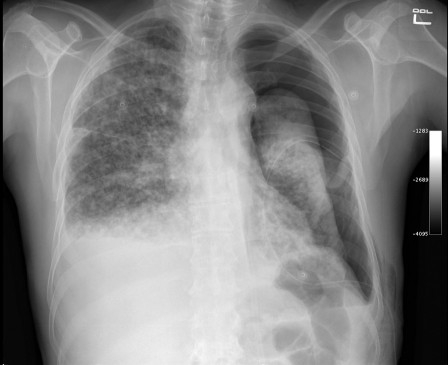
Patients with trapped lung in addition to MPE are unlikely to respond to either tube thoracostomy and sclerosis or VATS pleurodesis because the lung parenchyma is unable to expand enough to allow apposition of the parietal and visceral pleura. Many of these patients will not experience symptomatic relief following thoracentesis, but use of a Pleurx catheter has been proposed as an effective alternative for management in those patients who do improve following drainage. In a small series of 11 patients, all reported symptomatic benefit following Pleurx placement, and 10 of these remained in place until the patient's death.12 In a slightly larger series involving 34 patients with trapped lung, all patients felt the Pleurx catheter improved their quality of life, was easy to use, and was worth recommending to others10 (Fig. 3).
Figure 3.
Chest radiograph demonstrates a large left pneumothorax following placement of a Pleurx in a patient with trapped lung secondary to metastatic pancreatic cancer.
Pleuroperitoneal Shunting
Pleuroperitoneal shunts have fallen out of favor for the treatment of recurrent MPE. Although effective palliation has been reported in 73 to 100% of patients, the incidence of shunt occlusion is as high as 25% at a median time of 2.5 months.7 In addition, these shunts must be pumped frequently during the day, making them burdensome for patients.
Pleurectomy/Decortication
Although surgical pleurectomy is very effective in achieving pleurodesis, it also has a high complication rate, often resulting in a prolonged hospital stay. A perioperative mortality rate of up to 12.5% has been reported, and the incidence of prolonged air leak is as high as 20%.7 Given the comorbidities and relatively short life expectancies of patients with MPE, this surgical approach is no longer recommended in this patient population.3
MALIGNANT ASCITES
Approximately 10% of all cases of refractory ascites are related to malignancy.13 Breast, ovarian, endometrial, gastrointestinal, and pancreatic carcinomas are the primary malignancies in 80% of patients with malignant ascites; the remainder have an unknown primary.13 Several mechanisms for the development of ascites in these patients are proposed, including neoplastic production of exudative fluid, peritoneal carcinomatosis, obstruction of lymphatic drainage, hepatic congestion due to tumor infiltration, and changes in vascular permeability.14
The development of large-volume ascites can cause significant morbidity, markedly decreasing the patient's quality of life. These patients frequently suffer from painful abdominal distension, early satiety, nausea, dyspnea, and lower extremity edema due to the mass effect caused by the ascites. As with malignant effusions, the goal of management is the effective relief of symptoms in the safest, most convenient manner for the patient. Although dietary modification and diuretics may play a minor role in controlling fluid accumulation, serial paracentesis or placement of a drainage catheter is often necessary. Creation of peritoneovenous, peritoneogastric, and peritoneourinary routes of drainage and placement of a transjugular intrahepatic portosystemic shunt (TIPS) have also been described for management of malignant ascites.
Serial Paracentesis
Repeated large-volume paracentesis (LVP) is the most frequently employed therapy for refractory ascites because it can be performed safely as an outpatient procedure and results in the rapid, albeit temporary, relief of symptoms.15,16 The mean duration of symptomatic relief in patients with malignant ascites was 10.4 days in one series,17 meaning that frequent trips to the hospital or clinic are required. Patients often extend the time interval between procedures until their symptoms are absolutely unbearable to avoid the inconvenience of the procedure as well as the pain associated with it for as long as possible.
The complications associated with LVP include bleeding, infection, hypotension, development of loculations, bowel perforation, and prolonged drainage after the procedure. In one series of 67 patients who underwent 392 LVPs, the overall complication rate was 1.3% per procedure and 7.5% per patient.15 Although the complication rate is extremely low, death related to irreversible hypotensive shock has been reported.16
Although sonographic guidance typically is not required for LVP, it facilitates drainage in patients with loculated fluid collections or significant peritoneal implants, and it is used routinely when paracentesis is performed in the interventional radiology suite. Ultrasound is used to identify the most appropriate site for access, and the skin is prepped and draped in sterile fashion. Care should be taken to avoid injury to the inferior epigastric artery, which runs along the lateral border of the rectus sheath (Fig. 4). The pain associated with paracentesis can be significantly decreased by sonographically guided instillation of a local anesthetic along the peritoneal lining. A 5F sheathed needle such as a Yueh or One-Step needle is advanced into the fluid and the needle is removed. Rapid drainage is accomplished by connecting the sheath to a large volume Vacutainer bottle via a male-to-male connector and a 16-gauge needle.
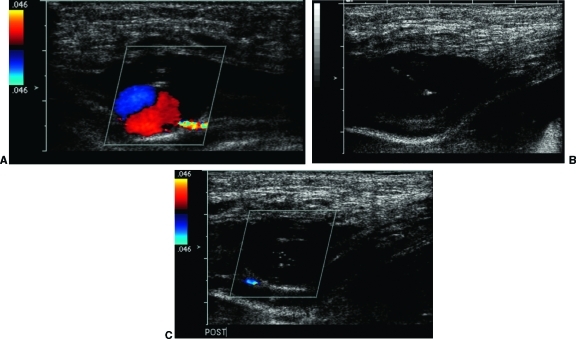
Figure 4.
(A) Color Doppler demonstrates a pseudoaneurysm of the inferior epigastric artery after a non-image-guided paracentesis. (B) Ultrasound-guided placement of a 25-gauge needle in the pseudoaneurysm. (C) Following injection of thrombin, there is no flow in the pseudoaneurysm.
Hypotensive shock is less likely to occur following LVP in oncology patients than it is in cirrhotic patients; however, the administration of plasma volume expanders should be considered any time LVP is performed, and the routine use of albumin in all paracenteses has been described.15 An infusion of albumin (Buminate 25%; Baxter Healthcare, Deerfield, IL) can be started with the initiation of fluid drainage so that ~25 g of albumin are given per 3L removed.
Paracentesis is felt to be safe even in patients with uncorrected coagulopathy, and there are no strict guidelines requiring that the platelet count and prothrombin time should be checked or corrected prior to the procedure. In one series of 1100 non-image-guided paracenteses, a large majority of the procedures were performed in patients with an international normalized ratio > 1.5 and a platelet count < 50,000, and no bleeding complications were reported.15
Nontunneled Drainage Catheters
Nontunneled abdominal drainage catheters are associated with an infection rate as high as 35%, tube blockage in up to 30%, and leakage in 20%.18 Because of the high complication rate, nontunneled drainage catheters often are not considered for the management of malignant ascites; however, two studies suggest that nontunneled catheters may be useful in patients with short life expectancies. In one series of 45 catheters, the mean time for symptomatic infection was 42 days;18 another study of 21 patients reported a mean lifespan of 6.8 weeks following nontunneled catheter placement with no symptomatic bacterial peritonitis observed.19 The catheters were tolerated well by patients in both studies.
Placement of a small-bore locking pigtail catheter in abdominal ascites is easily accomplished. After an access site is chosen with ultrasound, the abdomen is prepped and draped in sterile fashion. A local anesthetic is instilled and an 18-gauge needle is advanced into the fluid with sonographic guidance. The needle is exchanged over a 0.035 stiff Amplatz wire for tissue tract dilators followed by a 10 or 12F pigtail drain, and the pigtail is formed and locked. The catheter is connected to a gravity drainage bag, and the patient is taught to empty the bag as needed.
Tunneled Drainage Catheters
Several different types of tunneled drainage catheters are available for treatment of malignant ascites. Cuffed catheters such as peritoneal dialysis catheters and the Pleurx catheter allow for intermittent external drainage of ascitic fluid, and subcutaneous venous access ports and peritoneovenous shunts are completely internalized. All of these devices have the advantage of effective alleviation of symptoms on an entirely outpatient basis.
Successful placement of cuffed peritoneal dialysis catheters in interventional radiology has been well described.20 Survival rates for peritoneal dialysis catheters placed percutaneously have proven to be at least equal to those placed surgically; complication rates are slightly lower and patient recovery times faster.21 Good results with placement of peritoneal dialysis catheters specifically for management of malignant ascites have also been reported.22,23 A straight Tenckhoff peritoneal dialysis catheter (Bard, Salt Lake City, UT) was used in a series of 30 catheter placements in 29 patients with malignant ascites.22 All procedures in this series were technically successful, there were no major complications, and all patients were satisfied with the convenience and comfort of the tunneled catheter.22 In another series, 20 of 24 patients were treated at home with no further procedures required. Four patients developed symptomatic bacterial peritonitis with positive cultures, three of whom were treated successfully with intravenous antibiotics and one required removal of the catheter because of a coexisting tunnel infection.23
The Pleurx catheter is a cuffed, silastic tunneled catheter specifically designed for use in MPE, but it can also be applied to the management of malignant ascites. The Pleurx catheter was cleared for use in the abdomen by the Food and Drug Administration in November 2005, and kits that allow drainage of up to 1000 mL per bottle are now available for more cost-effective management of large-volume ascites.
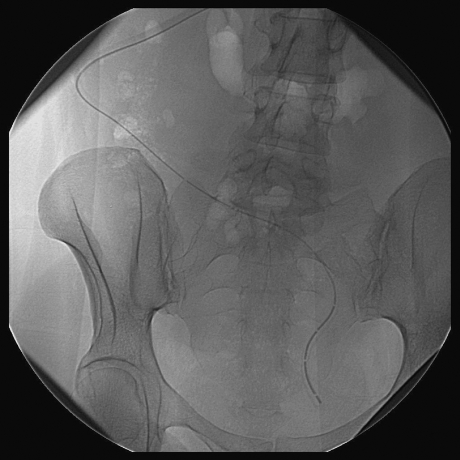
The technique for image-guided placement of the Pleurx in the abdomen is the same as that described in the chest, with the access site chosen so the catheter will course inferomedially into the lower abdomen and pelvis (Fig. 5). Placing the site of entry above the umbilicus may help prevent leakage of ascitic fluid along the tract. Creation of a C-shaped tunnel may also be useful in some patients. Patients should be instructed to drain the catheter frequently enough to avoid the development of tense ascites, usually one to two times per week.
Figure 5.
Fluoroscopic image demonstrates good position of an abdominal Pleurx catheter, which follows a smooth S-shaped course into the lower abdomen and pelvis.
In a retrospective study comparing 40 patients with abdominal Pleurx catheters and 67 patients who underwent repeated LVPs, complication rates were similar and satisfaction rates with the Pleurx catheter were high, leading the authors to recommend either procedure for management of malignant ascites. The complications reported with the abdominal Pleurx in this study included peritonitis in one patient, gross leakage into the subcutaneous tissues in a morbidly obese patient, and development of loculations in one patient.16 In another series, 10 abdominal Pleurx catheters were placed without procedural complications or catheter-related infections. One catheter malfunction in this series was treated with 2 mg of tissue plasminogen activator (alteplase; Genentech, South San Francisco, CA) yielding excellent secondary patency.24
The use of implanted subcutaneous ports for the management of both cirrhotic and malignant ascites has been described.25,26 In a recent prospective study, 28 peritoneal ports were placed in 27 patients, 22 of whom had refractory malignant ascites. The long-term patency rate was 100%, and the long-term clinical success rate was 96%. One minor immediate complication, a small hematoma, and two major delayed complications, persistent leakage from the port site and bacterial peritonitis, occurred.26
Peritoneovenous devices, such as Denver (Denver Biomedical, Golden, CO) and LeVEEN shunts (BD, Franklin Lakes, NJ), were designed to permit reinfusion of ascitic fluid into the central venous system, thereby limiting the protein loss associated with frequent LVP. Although historically a surgical procedure, placement is easily accomplished in the interventional suite.27,28,29 The complications reported after surgical peritoneovenous shunting include disseminated intravascular coagulopathy, sepsis, tumor seeding, ARDS, and variceal bleeding with shunt failure rates as high as 36% and 30-day mortality rates of 43%.14,16,26 The outcomes reported in the interventional literature are more encouraging than these, possibly because of better patient selection and extremely close monitoring of patients for the first 24 hours after the procedure, but the number of patients in each series is very small.27,28,29
Peritoneogastric and Peritoneourinary Drainage
Although peritoneogastric and peritoneourinary drainage routes are described, these have not proven to be viable alternatives for the management of malignant ascites. Placement of a Denver shunt in the abdomen that was then connected to a Cope-loop gastrostomy tube was technically successful in five patients, but all of the shunts failed within 2 weeks because of mechanical problems.30 A peritoneourinary drainage device, consisting of one catheter in the peritoneal cavity, a Jackson-Pratt reservoir, and a second catheter in the urinary bladder, was successfully placed in six patients, five of whom reported high satisfaction with the device. This pilot study was limited by design problems with the device that required six revisions in three patients and by a lack of long-term follow-up because patient survival ranged from 2 to 21 weeks.31
Transjugular Intrahepatic Portosystemic Shunt
Refractory ascites may develop in patients with portal hypertension in the setting of extensive primary or metastatic liver disease. Although the presence of liver lesions has been considered a relative contraindication to placement of a TIPS, the successful creation of a TIPS through hepatic neoplasms has been described. In a retrospective review of nine patients, seven with primary hepatic malignancy and two with metastatic disease, a TIPS was created across tumor with no immediate procedural complications and comparable outcomes to nontumor patients with the same Child-Pugh classification. Five of these patients had intractable ascites, three of whom experienced subjective improvement following TIPS. The TIPS in this series did have a higher rate of premature occlusion than expected, possibly related to factors released into the bloodstream following unavoidable trauma to the tumor, but covered stents were not available for use in all of the patients.32
CONCLUSION
Percutaneous therapy is becoming the treatment of choice for malignant fluid collections in the chest and abdomen. The percutaneous options for management include thoracentesis, paracentesis, and placement of nontunneled and tunneled drainage catheters. All of these treatments are minimally invasive, well tolerated by patients, and highly effective when used appropriately.
Thoracentesis and paracentesis are low-risk procedures that can provide diagnostic information as well as immediate relief from symptoms related to large effusions or ascites. Although recurrent fluid collections can be managed with repeat procedures, malignant fluid tends to reaccumulate rapidly. If frequent thoracentesis or paracentesis becomes necessary, placement of either a nontunneled or tunneled drainage catheter can provide more durable palliation of symptoms.
The type of drainage catheter should be selected based on the patient's prognosis and functional status. Nontunneled drainage catheters are associated with an increased risk of infection when used long term and should be reserved for patients with a very short life expectancy. Placement of a tunneled drainage catheter, such as a Pleurx catheter, should be considered in patients who have a good symptomatic response following thoracentesis or paracentesis, have a reasonably good prognosis, and have the ability to care for the catheter as required.
A growing body of literature supports the safety and efficacy of the Pleurx tunneled catheter specifically. The Pleurx was designed and first approved by the FDA for use in the chest, but it is also effective and cleared for use in the abdomen. The Pleurx reliably provides symptomatic relief in > 80 to 90% of patients with a low rate of complications. Most importantly, the Pleurx catheter has a high rate of patient satisfaction.
REFERENCES
- Pollak J S, Burdge C M, Rosenblatt M, et al. Treatment of malignant pleural effusions with tunneled long-term drainage catheters. J Vasc Interv Radiol. 2001;12:201–208. doi: 10.1016/s1051-0443(07)61826-0. [DOI] [PubMed] [Google Scholar]
- Pollak J S. Malignant pleural effusions: treatment with tunneled long-term drainage catheters. Curr Opin Pulm Med. 2002;8:302–307. doi: 10.1097/00063198-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Erasmus J J, Goodman Philip C, Patz Edward F., Jr Management of malignant pleural effusions and pneumothorax. Radiol Clin North Am. 2000;38:375–383. doi: 10.1016/s0033-8389(05)70168-8. [DOI] [PubMed] [Google Scholar]
- Saffran L, Ost D E, Fein A M, Schiff M J. Outpatient pleurodesis of malignant pleural effusions using a small-bore pigtail catheter. Chest. 2000;118:417–421. doi: 10.1378/chest.118.2.417. [DOI] [PubMed] [Google Scholar]
- Dresler C M, Olak J, Herndon J E, II, et al. Phase III intergroup study of talc poudrage vs. talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Sedrakyah A, Browne J, Swift S, Treasure T. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829–838. doi: 10.1016/j.ejcts.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Musani A I, Haas A R, Seijo L, Wilby M, Sterman D H. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71:559–566. doi: 10.1159/000081755. [DOI] [PubMed] [Google Scholar]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129:362–368. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- Ohm C, Park D, Vogen M, et al. Use of an indwelling pleural catheter compared with thorascopic talc pleurodesis in the management of malignant pleural effusions. Am Surg. 2003;69:198–202. [PubMed] [Google Scholar]
- Putnam J B, Jr, Light R W, Rodriguez R M, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992–1999. [PubMed] [Google Scholar]
- Pien G W, Gant M J, Washam C L, Sterman D H. Use of an implantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest. 2001;119:1641–1646. doi: 10.1378/chest.119.6.1641. [DOI] [PubMed] [Google Scholar]
- Rosenberg S M. Palliation of malignant ascites. Gastroenterol Clin North Am. 2006;35:189–199. doi: 10.1016/j.gtc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Brooks R A, Herzog T J. Long-term semi-permanent catheter use for the palliation of malignant ascites. Gynecol Oncol. 2006;101:360–362. doi: 10.1016/j.ygyno.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Grabau C M, Crago S F, Hoff L K, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484–488. doi: 10.1002/hep.20317. [DOI] [PubMed] [Google Scholar]
- Rosenberg S, Courtney A, Nemcek A A, Omary R A. Comparison of percutaneous management techniques for recurrent malignant ascites. J Vasc Interv Radiol. 2004;15:1129–1131. doi: 10.1097/01.RVI.0000136828.42612.B4. [DOI] [PubMed] [Google Scholar]
- Ross G J, Kessler H B, Clair M R, Gatenby R A, Hartz W H, Ross L V. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol. 1989;153:1309–1311. doi: 10.2214/ajr.153.6.1309. [DOI] [PubMed] [Google Scholar]
- Lee A, Lau T N, Yeong K Y. Indwelling catheters for the management of malignant ascites. Support Care Cancer. 2000;8:493–499. doi: 10.1007/s005200000139. [DOI] [PubMed] [Google Scholar]
- Sartori S, Nielsen I, Trevisani L, et al. Sonographically guided peritoneal catheter placement in the palliation of malignant ascites in end-stage malignancies. AJR Am J Roentgenol. 2002;179:1618–1620. doi: 10.2214/ajr.179.6.1791618. [DOI] [PubMed] [Google Scholar]
- Savader S J. Percutaneous radiologic placement of peritoneal dialysis catheters. J Vasc Interv Radiol. 1999;10:249–256. doi: 10.1016/s1051-0443(99)70026-6. [DOI] [PubMed] [Google Scholar]
- Georgiades C S, Geschwind J F. Percutaneous peritoneal dialysis catheter placement for the management of end-stage renal disease: technique and comparison with the surgical approach. Tech Vasc Interv Radiol. 2002;5:103–107. doi: 10.1053/tvir.2002.36054. [DOI] [PubMed] [Google Scholar]
- Barnett T D, Rubins J. Placement of a permanent tunneled peritoneal drainage catheter for palliation of malignant ascites: a simplified percutaneous approach. J Vasc Interv Radiol. 2002;13:379–383. doi: 10.1016/s1051-0443(07)61740-0. [DOI] [PubMed] [Google Scholar]
- O'Neill M J, Weissleder R, Gervais D A, Hahn P F, Mueller P R. Tunneled peritoneal catheter placement under sonographic and fluoroscopic guidance in the palliative treatment of malignant ascites. AJR Am J Roentgenol. 2001;177:615–618. doi: 10.2214/ajr.177.3.1770615. [DOI] [PubMed] [Google Scholar]
- Richard H M, III, Coldwell D M, Boyd-Kranis R L, et al. Pleurx tunneled catheter in the management of malignant ascites. J Vasc Interv Radiol. 2001;12:373–375. doi: 10.1016/s1051-0443(07)61919-8. [DOI] [PubMed] [Google Scholar]
- Savin M A, Kirsch M J, Romano W J, et al. Peritoneal ports for treatment of intractable ascites. J Vasc Interv Radiol. 2005;16:363–368. doi: 10.1097/01.RVI.0000147082.05392.2B. [DOI] [PubMed] [Google Scholar]
- Rosenblum D I, Geisinger M A, Newman J S, et al. Use of subcutaneous venous access ports to treat refractory ascites. J Vasc Interv Radiol. 2001;12:1343–1346. doi: 10.1016/s1051-0443(07)61561-9. [DOI] [PubMed] [Google Scholar]
- Hussain F F, Meer Z F, Lopez A J. Peritoneovenous shunt insertion for intractable ascites: a district general hospital experience. Cardiovasc Intervent Radiol. 2004;27:325–328. doi: 10.1007/s00270-004-0146-x. [DOI] [PubMed] [Google Scholar]
- Park J S, Won J Y, Park S I, et al. Percutaneous peritoneovenous shunt creation for the treatment of benign and malignant refractory ascites. J Vasc Interv Radiol. 2001;12:1445–1448. doi: 10.1016/s1051-0443(07)61707-2. [DOI] [PubMed] [Google Scholar]
- Orsi F, Francesco G R, Guido B, et al. Percutaneous peritoneovenous shunt positioning: technique and preliminary results. Eur Radiol. 2002;12:1188–1192. doi: 10.1007/s003300101049. [DOI] [PubMed] [Google Scholar]
- Lorentzen T, Sengelov L, Nolsoe C P, et al. Ultrasonically guided insertion of a peritoneo-gastric shunt in patients with malignant ascites. Acta Radiol. 1995;36:481–484. [PubMed] [Google Scholar]
- Rozenblit G N, Del Guercio L R, Rundback J H, et al. Peritoneal-urinary drainage for treatment of refractory ascites: a pilot study. J Vasc Interv Radiol. 1998;9:998–1005. doi: 10.1016/s1051-0443(98)70440-3. [DOI] [PubMed] [Google Scholar]
- Wallace Michael and Swaim Mark Transjugular intrahepatic portosystemic shunts through hepatic neoplasms. J Vasc Interv Radiol. 2003;14:501–507. doi: 10.1097/01.rvi.0000064846.87207.ab. [DOI] [PubMed] [Google Scholar]