Abstract
Preconditioning with the toll-like receptor 4 (TLR4) ligand, lipopolysaccharide, provides neuroprotection against subsequent cerebral ischemic brain injury, through a TNFα dependent process. Here we report the first evidence that another TLR, TLR9, can induce neuroprotection. We show that the TLR9 ligand (CpG ODN) can serve as a potent preconditioning stimulus and provide protection against ischemic brain injury. Our studies show that systemic administration of CpG ODN 1826 in advance of brain ischemia (middle cerebral artery occlusion; MCAO) reduces ischemic damage up to 60% in a dose and time dependent manner. We also offer evidence that CpG ODN preconditioning can provide direct protection to CNS cells as we have found marked neuroprotection in modeled ischemia in vitro. Finally, we show that CpG preconditioning significantly increases serum TNFα levels prior to MCAO and show that TNFα is required for subsequent reduction in damage, as mice lacking TNFα are not protected against ischemic injury by CpG preconditioning. Our studies demonstrate that preconditioning with a TLR9 ligand, induces neuroprotection against ischemic injury through a mechanism that shares common elements with LPS preconditioning via TLR4.
Keywords: CpG, ischemic tolerance, neuroprotection, preconditioning, TLR9
Introduction
Toll-like receptors (TLRs) are a family of pattern recognition receptors involved in the identification of, and response to foreign pathogens. To date at least 11 TLRs have been identified in mammals, and each recognize different pathogen-associated molecular patterns (PAMPs). Although the stimuli are different, the resultant signaling cascades are all mediated through toll/interleukin-1 receptor (TIR) domain containing adapters, the most prominent being MyD88, with subsequent activation of NFκB. TLRs are broadly distributed on immune cells and thus play an important role in initiation of innate and adaptive immune responses.
TLR4 was the first TLR identified in mammals and is the most widely studied of the TLRs. Endotoxin (lipopolysaccharide; LPS), a cell surface component of gram-negative bacteria, binds to TLR4 and at high levels can cause death through septic shock. An interesting counter-point is that low concentrations of LPS actually induce a protective state against a subsequent lethal dose of LPS (reviewed in West and Heagy 2002). This phenomenon, referred to as endotoxin tolerance, has been studied for over 50 years, yet the molecular mechanisms are incompletely understood. It is known, however, that many pro-inflammatory cytokines and cytotoxic mediators that are normally elicited by LPS, fail to be induced by a second exposure to LPS. Instead, new signaling proteins and anti-inflammatory pathways are increased in the setting of endotoxin tolerance (reviewed in Fan and Cook 2004; West and Heagy 2002).
More recently other TLRs have been shown to induce protection against a subsequent challenge with the same ligand, a state commonly referred to as self-tolerance. Priming of TLR2, TLR5 or TLR9 with their respective ligands induces a state of hypo-responsiveness to a subsequent challenge with their corresponding ligands (Dalpke et al 2005; Mizel and Snipes 2002; Sato et al 2000; Yeo et al 2003). This shared phenomenon is expected given the similarities in signaling pathways of the TLR family members. Interestingly, cross-tolerance (or hetero-tolerance) between two differing receptors has also been reported, as ligands for TLR2 and TLR9 induce tolerance against a subsequent challenge with LPS (Dalpke et al 2005; Lehner et al 2001; Sato et al 2000; Yeo et al 2003).
However, hetero-tolerance is not induced with all combinations of TLRs and differences in ability to induce hetero-tolerance have been reported depending on the model (Dalpke et al 2005; Dobrovolskaia et al 2003). For example, Dalpke and colleagues, although able to show hetero-tolerance between TLR2, TLR4 and TLR9 in a macrophage cell line, and a similar hetero-tolerance with TLR2 and TLR4 in an in vivo paradigm, failed to induce hetero-tolerance with TLR9 in vivo. In fact, pretreatment with unmethylated CpG oligodeoxyneucleotides (ODNs), the ligand for TLR9, actually enhanced TNFα production in vivo in response to LPS and LTA, making the system hyper-responsive (Dalpke et al 2005). These inconsistencies suggest a complicated interplay between the varying TLR signaling pathways that is more complex than a simple feedback suppression of inflammatory signals within a cell.
In addition to the phenomenon of cross-tolerance that exists among the different TLRs, tolerance against ischemic injury can be induced by LPS in various organs such as heart, brain and kidney (Heemann et al 2000; Rowland et al 1997; Tasaki et al 1997). Although the mechanism of protection in these models is even less well understood, the paradigm appears similar in that a small inflammatory response is initiated that mitigates the subsequent damaging inflammatory response associated with the secondary stimuli. In the case of brain ischemia, a systemic low dose of LPS delivered, at least 1day but not longer than 7 days, prior to stroke reduces the ischemic injury (Bordet et al 2000; Rosenzweig et al 2004; Tasaki et al 1997; Toyoda et al 2000). A critical role for TNFα has been shown by us (Rosenzweig et al 2007) and others (Tasaki et al 1997) wherein LPS-induced tolerance to ischemia fails to occur in the absence of TNFα.
Similarities among the known TLR signaling pathways and their shared ability to induce hetero-tolerance between certain members of the TLR family has lead us to hypothesize that other TLR ligands may also provide neuroprotection against ischemic brain injury. Further, we postulated that TNFα may play a central role in conferring protection. To test our hypothesis we examined the protective potential of CpG ODN 1826, a mouse specific TLR9 ligand. We chose to examine TLR9 because, similar to TLR4, it is coupled to the signaling adapter, MyD88. In addition, activation of TLR9 by CpG ODNs increases serum TNFα levels in mice within 6 hrs of administration (Vasilakos et al 2000; Vollmer et al 2004). TLR4 and TLR9 display a similar cell type distribution as both are expressed by multiple systemic immune cell types (Applequist et al 2002; Zarember and Godowski 2002), and on cells of the central nervous system (McKimmie and Fazakerley 2005; Olson and Miller 2004; Tang et al 2007). CpG ODNs are currently approved for human trials as vaccines and cancer therapies (reviewed in Krieg 2006), which makes them particularly well-suited for therapeutic development for use in stroke neuroprotection. Here we report that ligand activation of TLR9 induces neuronal protection against brain ischemia. We show that neuroprotection is time and dose dependent. In addition, we report that TNFα plays an essential role in CpG ODN-induced ischemic tolerance, just as it does in LPS-induced tolerance to ischemic brain injury. These data are the first to indicate that TLR9 is a target for the induction of tolerance against ischemic injury in the brain.
Materials and Methods
Mice
C57Bl/6 mice (male, 8 to 10 weeks) were obtained from Jackson Laboratories (West Sacramento, California, USA). TNFα knock-out mice (B6.129S-Tnftm1Gkl/J), were also obtained from Jackson Laboratories. This strain is backcrossed at least 5 generations to C57Bl/6 at Jackson Laboratories. All mice are housed in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. The animal protocols met National Institutes of Health guidelines with the approval of the Oregon Health and Science University Institutional Animal Care and Use Committee.
Reagents
ODN1826 (tcc atg acg ttc ctg acg tt), a mouse-specific phosphothioate CpG-ODN ligand for TLR9, and ODN2088 (tcc tgg cgg gga agt), a mouse-specific TLR9 signaling inhibitor (Gursel et al 2003; Stunz et al 2002), were obtained from Invivogen. Invivogen has confirmed the specificity of ODN1826 for mouse TLR9 by testing against cells transfected with the other TLR family members (personal communication). In addition, endotoxin levels were determined to be negligible (<0.125EU/mg). A control ODN (Invivogen; tcc atg agc ttc ctg agc tt) was used which contained the same sequence as 1826 but the CpG dinucelotides have been replaced by GpC dinucelotides (shown in bold). Therefore, it does not stimulate TLR9.
Oxygen glucose deprivation in vitro
Primary mouse mixed cortical cultures were prepared from E15-E17 mouse fetuses. Cortices were dissected and dissociated with Trypsin-EDTA (Gibco) and plated at a density of 1 × 106 cells/ml onto coverslips coated with poly-L-Ornithine (15mg/L). Cells were cultured in Neurobasal media (containing 4.5g/L glucose; supplemented with Glutamax and B27-AO; Gibco) for 5 days prior to each experiment. Cultures consisted of ~60% neurons (range 53–66%) as determined by staining for NeuN (Chemicon), with less than 5% astrocytes (GFAP+; Sigma) and less than 5% microglia (tomato lectin+; Vector Labs). Oxygen-glucose-deprivation (OGD) was performed by removal of the culture medium and replacement with D-PBS (Gibco) followed by incubation in an anaerobic atmosphere of 85% N2, 10% CO2, 5% H2 at 37°C for 3 h. The anaerobic conditions within the chamber were monitored using an electronic oxygen/hydrogen analyzer (Coy Laboratories). OGD was terminated by replacement of the exposure medium with Neurobasal medium (containing 4.5g/L glucose; supplemented with Glutamax and B27-AO) and return of the cells to a normoxic incubator. Control plates were kept in the normoxic incubator during the OGD interval.
Cell Death Evaluation in vitro
Cell death in vitro was examined 24 hr following OGD by means of fluorescent, cell-permeable, DNA-binding dyes: propidium iodide (PI), as an indicator of cell death, and 4’,6-diamidino-2-phenylindole (DAPI), as an indicator of the total number of cells. Coverslips were incubated with PI (1.5ug/ml, Sigma) for 2 min, washed with PBS and fixed for 30 min in 10% formalin. Coverslips are mounted on slides with Vectashield mounting medium containing DAPI (Vector labs). Stained cells were visualized with a fluorescent microscope (Leica GMBH) and analyzed using Metmorph7 software (Molecular Devices Corp., Downington, PA). The number of PI and DAPI stained cells were counted in two random fields of view on each coverslip, and percent death was calculated as mean (PI)/(DAPI) × 100 per field of view. Each treatment was performed with triplicate coverslips within an experiment and the entire experiment was repeated three or more times.
Drug treatments
CpG ODN 1826 and the saline vehicle were administered by intraperitoneal (i.p.) injection in a volume of 200ul. For the dose response studies mice were injected 72 hr prior middle cerebral artery occlusion (MCAO). For the time window of protection mice were treated from 1–14 days prior to MCAO.
Surgery
Cerebral focal ischemia was induced by MCAO as published previously (Clark et al 1997). Mice were briefly induced with 3% isoflurane and maintained with 1.5–2% throughout the surgery. The middle cerebral artery (MCA) was blocked by threading silicone-coated 8-0 monofilament nylon surgical suture through the external carotid to the internal carotid, and finally blocking its bifurcation into the MCA and anterior cerebral artery. The filament was maintained for 60 min (unless otherwise noted) while the mice were maintained under anesthesia. The filament was removed, and blood flow restored. Cerebral blood flow was monitored with Laser Doppler Flowmetry (Transonic System Inc.). Temperature was maintained at 37°C±0.5°C with a rectal thermometer-controlled heating pad and lamp (Harvard Apparatus). All surgical procedures were performed under an operating stereomicroscope. After surgery mice were kept alive for 24 hr on a heating pad with access to soft food and water and were then sacrificed. We consistently have a survival rate for the MCAO procedure that exceeds 85%.
Infarct Measurement
Mice were deeply anesthetized with isoflurane, then perfused with ice-cold saline containing 2U/mL heparin. Brains were removed rapidly, placed on a tissue slicer and covered with agarose (1.5%). The olfactory bulbs were removed and the remainder of the brain was sectioned into 1-mm slices beginning from the rostral end, for a total of 7 slices. The area of infarction was visualized by incubating the sections in 1.5% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma Aldrich) in PBS for 15 min at 37°C. The sections were then transferred to 10% fomalin (Sigma Aldrich). Images of the sections were scanned, and the hemispheres and areas of infarct were measured using ImageJ software (Abramoff et al 2004). The measurements were multiplied by the section thickness and summed over the entire brain to yield volume measurements. Ischemic damage data was calculated using the indirect method to minimize error introduced from edema. % Infarct = (contralateral hemisphere volume – volume of non-infarcted tissue of the ipsilateral hemisphere)/(contralateral hemisphere volume) × 100 (Swanson et al 1990).
Quantification of Serum TNFα
Blood was taken from mice (cardiac puncture) and allowed to clot for 2 hr at room temperature. The blood was centrifuged (2000 rpm, 20 min) and the clear serum was removed and stored at −80°C until analyzed. Serum TNFα was measured using an ELISA available commercially from R&D Systems (Minneapolis, MN, USA). The assay sensitivity is ~5.1 pg/ml. All samples were run in duplicate.
Statistical Analysis
All statistical analyses were performed using Prism (Graphpad). Mean differences were analyzed using one-way ANOVA with Bonferroni’s post hoc test. Data are represented as mean ± standard error of the mean (SEM) and differences were considered statistically significant when p<0.05.
Results
CpG ODN preconditions against ischemia-induced neuronal cell death in vitro
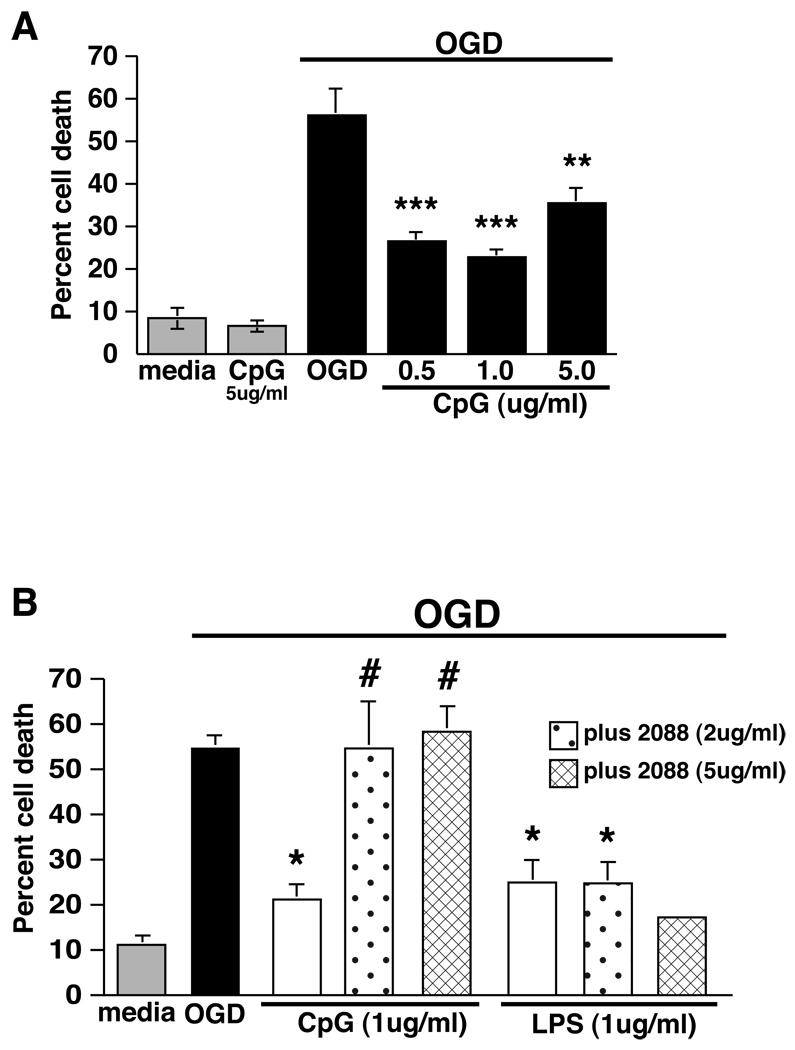
We tested whether the TLR9 ligand, CpG ODN, would induce tolerance to ischemic cell death in an in vitro model of ischemia, oxygen-glucose-deprivation (OGD). Mouse mixed cortical cultures subjected to 3 hr of OGD showed 56% cell death compared to untreated control cultures. Cultures preconditioned by exposure to CpG ODN 1826 (0.5–5.0 ug/ml) for 24 hr prior to OGD showed a dose-dependent reduction in cell death (Fig. 1A). A dose of 1ug/ml CpG ODN produced maximal protection, which resulted in a 60% reduction in cell death following exposure to OGD. No significant cell death was detected in cultures treated with CpG ODN alone (Fig. 1A). Our findings show that pretreatment with CpG ODN provides significant protection from cell death induced by exposure to modeled ischemia (OGD) and suggests that TLR9 is a new target for preconditioning against ischemic neuronal injury.
Figure 1. CpG protects primary mixed cortical cultures from OGD-induced cell death through TLR9.
A) Mixed cortical cultures were stimulated with increasing doses of CpG 1826 (0.5–5 ug/ml) 24 hr prior to 3 hr OGD. Cell death was assessed 24 hr following OGD by propidium iodide (PI) staining. For all experiments, values are mean ± SEM, **p<0.01, ***p<0.001 versus media-treated OGD controls, n=5 individually repeated experiments. CpG 1826 treatment alone at the highest dose (5ug/ml) did not result in increased cell death over media alone (grey bar). B) Mixed cortical cultures were stimulated with either CpG or the TLR4 agonist LPS (1ug/ml) in the presence or absence of the TLR9 antagonist ODN 2088 (2 or 5 ug/ml) 24 hr prior to 3 hr OGD. Cell death was assessed 24 hr following OGD by PI staining. Values are mean ± SEM, *p<0.05 vs media-treated OGD controls, #p<0.05 vs CpG-treated OGD; n=2–4 individually repeated experiments, except for LPS +5ug/ml ODN 2088 which represents a single experiment.
We used a TLR9 specific antagonist (ODN 2088), to confirm that protection was induced specifically via TLR9 signaling (Gursel et al 2003; Stunz et al 2002). Mixed cortical cultures were preconditioned with CpG ODN 1826 (1ug/ml) in the presence or absence of the TLR9 antagonist, ODN 2088 (2 or 5 ug/ml) for 24 hr prior to OGD (3 hr). ODN 2088 abolished the protective effect of CpG 1826 at both doses, but failed to inhibit the protective effect of LPS (1ug/ml; Sigma L-2880), a TLR4 agonist that we have shown induces tolerance to ischemia in vitro (Rosenzweig et al 2007) (Fig. 1B). These data, suggest that CpG 1826 signals through TLR9 to induce protection against OGD.
Preconditioning with CpG reduces ischemic damage in an in vivo model of stroke
We next examined the protective potential of CpG ODN treatment in a mouse model of stroke. Mice were preconditioned with varying doses of CpG ODN 1826 (5–40 ug; i.p.) 72 hr prior to MCAO. Twenty-four hours following MCAO mice were sacrificed and infarct damage was determined. Pretreatment with CpG ODN reduced the infarct size significantly at doses of 20 and 40ug (56.5 and 57.5% reduction respectively; Fig. 2). Thus, CpG ODN delivered systemically to mice preconditions the brain in a dose-dependent manner leading to marked tolerance to ischemic brain injury. To confirm that the protection was specific to the CpG ODN, we tested a control ODN which contained the same sequence as 1826 but the CpG dinucelotides were replaced by GpC dinucelotides. No significant protection was observed in mice preconditioned with 20 ug of the control ODN (data not shown). The reduction in infarct size reported here at 24 hr post MCAO remains evident in mice sacrificed 72 hr post MCAO (unpublished observation).
Figure 2. Preconditioning with CpG reduces infarct size in a mouse model of focal ischemia.
C57BL/6 (males, 6–10/dose) received various doses of CpG 1826 (5–40ug; i.p.) 72 hr prior to ischemic challenge (60 min MCAO). Infarct volume was determined 24 hrs following MCAO by TTC staining. Values are group means ± SEM; *p<0.05 comparison to saline controls by one way ANOVA followed by Bonferroni’s multiple comparison test.
Preconditioning time window of CpG induced neuroprotection
LPS preconditioning induces ischemic neuroprotection in the brain by 1 day—an effect that last for at least 7 days but is lost by 14 days following treatment (Rosenzweig et al 2007). To determine the time window of neuroprotection induced by CpG ODN 1826, we administered CpG ODNs (20 ug; i.p.) 1, 3, 7 and 14 days prior to MCAO. We found pretreatment with CpG ODNs induced significant neuroprotection within 1 day (46% reduction in infarct volume)— a neuroprotective effect that remains evident at 3 days (61% reduction) but diminished by 7 days. By 14 days post administration, the protective effect is completely abolished in the CpG treated mice (Fig. 3). Thus, CpG preconditioning provides a time window of neuroprotection that lasts ~1 week and is lost by 2 weeks following administration. This time window of protection mirrors that seen with systemic administration of LPS.
Figure 3. Time window of CpG preconditioning.
C57Bl/6 mice received an injection of saline (4 mice/time point) or CpG (20ug; i.p.) 1, 3, 7 or 14 days (6 mice/time point) prior to 60 min MCAO. Infarct volume was determined 24 hr following MCAO by TTC staining. No statistical difference was observed between the saline groups, thus they were combined for analysis. Values are group means ± SEM; ***p<0.001 comparison to saline controls by one way ANOVA followed by Bonferroni’s multiple comparison test.
TNFα is required for CpG induced neuroprotection
Previous work in the model of LPS preconditioning against brain ischemia has demonstrated that the presence of TNFα plays an essential role in conferring neuroprotection (Rosenzweig et al 2007; Tasaki et al 1997). We postulated the CpG ODN preconditioning via TLR9 may have a similar requirement for the presence of TNFα. We first tested whether CpG ODN administration increased serum levels of TNFα. We found significant increases in TNFα levels in the serum as early as 1 hr post-injection (400 pg/ml compared to vehicle treated mice which were below the level of detection; Fig. 4). The timing of the increase in TNFα was similar to what we have previously reported with LPS preconditioning (Rosenzweig et al 2007), however the magnitude is significantly less than that observed for preconditioning levels of LPS (3092 pg/ml). As with LPS, the response diminishes quickly and returns to baseline by 72 hr.
Figure 4. Serum TNF-α levels significantly increased 1-hour post CpG treatment.
Mice (n=4/time point) were administered CpG 1826 (20 ug; i.p.) and blood was collected at 1, 3, 24 or 72 hrs post injection. Blood was allowed to clot for 2 hr at room temperature and the serum collected. TNFα levels (pg/ml of blood) were measured with a TNFα ELISA (R&D Systems). Values are group means ± SEM; ***p < 0.001 by two way ANOVA followed by Bonferroni’s multiple comparison test. LPS (5 ug; i.p.) treated mice were included in the same experiment for comparison.
We investigated whether TNFα is required for CpG preconditioning by testing the neuroprotective effect of CpG ODN treatment in TNFα knock-out mice (B6.129S-Tnftm1Gkl/J; TNFα−/−). We administered CpG ODN 1826 to TNFα−/− and control mice (C57Bl/6; TNFα+/+) 72 hr prior to 40 min MCAO. Control mice pretreated with CpG showed a significant reduction in ischemic injury (46% reduction) as expected. In contrast, TNFα−/− mice pretreated with CpG did not demonstrate any reduction in infarct size (saline treated = 31.9±7.7% vs CpG treated = 29.3±6.8%; Fig. 5). These data suggest that TNFα plays an essential role in mediating CpG-induced neuroprotection against ischemic injury.
Figure 5. TNF induction is required for CpG preconditioning.
Control (C57Bl/6; TNFα+/+) or TNFα knockout (B6.129S-Tnftm1Gkl/J; TNFα−/−) mice were treated with CpG 1826 (20 ug; i.p.) at 72 hr prior to 40 min MCAO and infarcts were assessed 24 hr post MCAO. Values are mean + SEM, *p<0.05 versus saline treatment, n = 4–6 mice/group.
Discussion
We report the first evidence that a TLR9 ligand (CpG ODN) can serve as a potent preconditioning stimulus and provide protection against ischemic brain injury. This finding indicates that TLR9, in addition to TLR4, can induce preconditioning in the brain. Our studies show that systemic administration of CpG ODN 1826 in advance of brain ischemia reduces ischemic damage in a dose and time dependent manner. We offer evidence that CpG ODN preconditioning can provide direct protection to CNS cells as we have found marked neuroprotection in modeled ischemia in vitro. In addition, using our in vitro model we show that CpG ODN specifically acts through TLR9 to induce neuroprotection. Finally, our studies support a critical role for TNFα in CpG-induced neuroprotection. This latter observation suggests that the mechanism of neuroprotection between LPS and CpG preconditioning share common elements.
Previous studies have demonstrated that LPS, acting through TLR4 can induce cross-tolerance and provide neuroprotection against ischemic injury in the brain. We have posited that heterologous tolerance induction against brain ischemia extends beyond TLR4 to other TLRs. We demonstrate here that cross-tolerance by TLR4 is not unique to this particular TLR as we show that a TLR9 agonist, CpG ODN, also induces cross-tolerance against an ischemic insult.
It is known that TLR4 couples to both MyD88 and TRIF dependent pathways and that the MyD88 cascade culminates in NFκB-mediated induction of pro-inflammatory cytokines (i.e. TNFα, IL6, IL1β). TNFα, which is required for TLR4 induced tolerance against ischemic injury (Rosenzweig et al 2007; Tasaki et al 1997), may be induced via the MyD88 cascade. We hypothesize that signaling through TLR9 would also induce tolerance via a MyD88 dependent mechanism as TLR9 signals exclusively through MyD88 with no evidence for TRIF dependent signaling (Horng et al 2001; Schnare et al 2000). Studies to explore this possibility are in progress in our laboratory and should provide important information broadly regarding the molecular mechanisms underlying tolerance to injury.
Thus far, the known ligands for TLR9 include bacterial DNA containing unmethylated CpG motifs, certain double-stranded DNA viruses and synthetic CpG ODNs such as CpG-ODN 1826 used in the studies described here (Bauer et al 2001; Hemmi et al 2000; Krug et al 2004). Synthetic CpG ODNs have been shown to confer protection to mice against subsequent challenge from a variety of bacteria, viruses, parasites and prions (Krieg 2003). Protection against pathogen challenge typically occurred within 48 hr and lasted for several weeks (Elkins et al 1999; Krieg et al 1998)—such a time window is similar to our findings with CpG-ODN induced ischemic tolerance shown here and our recent report of LPS-induced ischemic tolerance to stroke injury (Rosenzweig et al 2007).
The data presented here provides the first evidence that in addition to tolerance (protection) against foreign pathogens, CpG ODN administration protects against a stimulus that is unrelated to a foreign pathogen, namely ischemic injury. CpG ODN-induced neuroprotection occurs following systemic administration in a mouse model of stroke. TNFα appears to be critical to the induction of ischemic tolerance by CpG preconditioning as we show here that CpG ODN administration fails to protect TNFα deficient mice against ischemic brain injury. In wild type mice, systemic administration of CpG increased TNFα in the plasma, which suggests that the actions of TNF may occur in the periphery. Further support for this lies in the observation by Nawashiro et al that systemic administration of TNFα itself preconditions against stroke injury (Nawashiro et al 1997). In addition, LPS preconditioning against ischemic brain injury is abolished by systemic blockade of TNFα using TNF-binding protein (Tasaki et al 1997). Thus, CpG ODN preconditioning in vivo very likely provides neuroprotection through a TNFα dependent mechanism similar to that seen with LPS preconditioning.
We also report that CpG induces neuroprotection when directly applied to mixed cortical cultures in vitro which are subsequently subjected to oxygen-glucose deprivation. The mechanism of this more direct route of CpG interaction in the CNS is still unclear. These cultures contain neurons, astrocytes and microglia. Until very recently, astrocytes and microglia, but not neurons were known to express TLRs, including TLR9 (Bowman et al 2003; Olson and Miller 2004). Neurons have also been reported to express TLRs, although the extent to which they are targets of cell signaling is not yet clear. (Tang et al 2007). Thus, CpG treatment may modulate the cytokine response to injury in glial cells which in turn may have a protective effect on neurons indirectly or through more direct modulation by activation of TLR9 on neurons leading to altered neuronal signaling in the setting of injury. Whether TNFα has a critical role in CpG-induced tolerance in vitro is of interest and as such, the subject of future studies in our laboratory.
We have recently reported genomic evidence that preconditioning via LPS activation of TLR4 produces a tolerant state in the brain via inflammatory mediators that are neuroprotective (e.g. Type I interferons) (Stenzel-Poore et al 2007). In addition, in the setting of LPS-induced tolerance, there is a marked absence of deleterious inflammatory mediators (IL-6, MIP1α, TRAF6) generally found in ischemic brain injury. It is possible that these particular features of neuroprotection (suppressed proinflammatory mediators/increased neuroprotective cytokines) are common to preconditioning stimuli that act through TLRs.
The demonstration that ischemic tolerance in the brain occurs through TLR9, in addition to TLR4, raises the possibility that this is a conserved feature of all TLRs. Recognition that TLR9 is a new target for preconditioning broadens the range of potential antecedent therapies for brain ischemia, such as in the setting of coronary artery bypass grafting (~300,000 procedures annually) where patients are at risk of cerebral morbidity (reviewed in Newman et al 2006). Phase II clinical trials are already in progress with CpG ODNs for use in adjuvant and anticancer therapies (reviewed in Krieg 2006). Thus, CpG ODNs may offer great translational promise as a prophylactic treatment against cerebral morbidity for ‘at risk’ patients.
Acknowledgements
We acknowledge Rebecca Hillary, PhD for her outstanding technical support. This work was supported by NIH grants NS050567, NS35965.
Footnotes
Conflict of interest statement: Dr. Stenzel-Poore and Ms. Stevens are the inventors of technology used in this research. An exclusive licensing agreement exists between OHSU and Neuroprotect, Inc. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Program Oversight Council.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with Image. J. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14:1065–1074. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Deplanque D, Maboudou P, Puisieux F, Pu Q, Robin E, Martin A, Bastide M, Leys D, Lhermitte M, Dupuis B. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. J Cereb Blood Flow Metab. 2000;20:1190–1196. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Lehner MD, Hartung T, Heeg K. Differential effects of CpG-DNA in Toll-like receptor-2/-4/-9 tolerance and cross-tolerance. Immunology. 2005;116:203–212. doi: 10.1111/j.1365-2567.2005.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- Heemann U, Szabo A, Hamar P, Muller V, Witzke O, Lutz J, Philipp T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury: possible connection to an interleukin-6-dependent pathway. Am J Pathol. 2000;156:287–293. doi: 10.1016/S0002-9440(10)64729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Love-Homan L, Yi AK, Harty JT. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–835. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–125. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J Biol Chem. 2002;277:22414–22420. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Newman MF, Mathew JP, Grocott HP, Mackensen GB, Monk T, Welsh-Bohmer KA, Blumenthal JA, Laskowitz DT, Mark DB. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. doi: 10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents the cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning protects against the cytotoxic effects of TNFa after stroke: a novel role for TNFa in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600464. on line publication. [DOI] [PubMed] [Google Scholar]
- Rowland RT, Meng X, Cleveland JC, Meldrum DR, Harken AH, Brown JM. LPS-induced delayed myocardial adaptation enhances acute preconditioning to optimize postischemic cardiac function. Am J Physiol. 1997;272:H2708–H2715. doi: 10.1152/ajpheart.1997.272.6.H2708. [DOI] [PubMed] [Google Scholar]
- Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38:680–685. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- Stunz LL, Lenert P, Peckham D, Yi AK, Haxhinasto S, Chang M, Krieg AM, Ashman RF. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur J Immunol. 2002;32:1212–1222. doi: 10.1002/1521-4141(200205)32:5<1212::AID-IMMU1212>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Toyoda T, Kassell NF, Lee KS. Induction of tolerance against ischemia/reperfusion injury in the rat brain by preconditioning with the endotoxin analog diphosphoryl lipid A. J Neurosurg. 2000;92:435–441. doi: 10.3171/jns.2000.92.3.0435. [DOI] [PubMed] [Google Scholar]
- Vasilakos JP, Smith RM, Gibson SJ, Lindh JM, Pederson LK, Reiter MJ, Smith MH, Tomai MA. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Weeratna RD, Jurk M, Davis HL, Schetter C, Wullner M, Wader T, Liu M, Kritzler A, Krieg AM. Impact of modifications of heterocyclic bases in CpG dinucleotides on their immune-modulatory activity. J Leukoc Biol. 2004;76:585–593. doi: 10.1189/jlb.0104034. [DOI] [PubMed] [Google Scholar]
- West M, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J Immunol. 2003;170:1052–1061. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]