Abstract
Ca2+ mobilization is central to many cellular processes, including stimulated exocytosis and cytokine production in mast cells. Using single cell stimulation by IgE-specific antigen and high speed imaging of conventional or genetically-encoded Ca2+ sensors in RBL and bone marrow-derived rat mast cells, we observe Ca2+ waves that originate most frequently from the tips of extended cell protrusions, as well as Ca2+ oscillations throughout the cell that usually follow the initiating Ca2+ wave. In contrast, antigen conjugated to the tip of a micropipette stimulates local, repetitive Ca2+ puffs at the region of cell contact. Initiating Ca2+ waves are observed in most RBL cells stimulated with soluble antigen, are sensitive to inhibitors of Ca2+ release from ER stores and to extracellular Ca2+, but they do not depend on store-operated Ca2+ entry. Knockdown of TRPC1 and TRPC3 channel proteins by shRNA reduces the sensitivity of these cells to antigen and shifts the wave initiation site from protrusions to the cell body. Our results reveal spatially encoded Ca2+ signaling in response to immunoreceptor activation that utilizes TRPC channels to specify the initiation site of the Ca2+ response.
Keywords: IgE receptors, Ca2+ puffs, Ca2+ oscillations, GCaMP2, TRPC channels
INTRODUCTION
Changes in intracellular Ca2+ play significant roles in numerous cellular responses such as secretion, gene expression, and cell migration. These cellular functions require spatial and temporal regulation of cytosolic Ca2+ (1, 2). Among these regulated events are Ca2+ puffs, waves, and regenerative oscillations that mediate localized cellular responses and support transfer of information across the cell and organelles (3).
Ca2+ waves were first characterized in Xenopus oocyte fertilization (4), and they have since been identified in excitable (5) and nonexcitable cell types, including hepatocytes (6), HeLa cells (7), and neutrophils (8). In myocytes, Ca2+ waves were shown to initiate from elementary Ca2+ events called “Ca2+ sparks” (9), and are thought to propagate through the cytosol by calcium-induced calcium release from ER stores (10). Ca2+ waves are frequently initiated by activation of plasma membrane receptors that stimulate Ca2+-dependent signaling within the cell (11). Similar mechanisms may be involved in stimulating Ca2+ puffs and maintaining the propagation of Ca2+ waves in non-excitable cells (3, 12). However, Ca2+ waves in response to immunoreceptor signaling have not been previously reported.
Ca2+ oscillations have been characterized in many cell types, including RBL-2H3 mast cells (13, 14) where they have been temporally correlated with degranulation events (15, 16). Ca2+ oscillations are sustained by store-operated Ca2+ entry (SOCE), other ion channels, as well as membrane potential (17).
Mast cells play key roles in the inflammatory process in both innate and adaptive immune responses (18). In the latter, binding of multivalent antigen to receptor-associated IgE aggregates this receptor, FcεRI, which causes mast cell activation, resulting in Ca2+ mobilization and consequent exocytotic release of mediators of allergy and inflammation (19). RBL-2H3 cells are immortalized mucosal mast cells that have been utilized for extensive biochemical and cell biological investigations of mast cell function (20-22).
In the present study, we used high-speed confocal imaging to investigate cytoplasmic Ca2+ dynamics activated via FcεRI in RBL cells and in rat bone marrow-derived mast cells (BMMCs), which are also mucosal in character (23). We find that Ca2+ responses to soluble antigen initiate in the form of a wave that begins most frequently at the tip of an extended cell protrusion and propagates throughout the entire cell in several seconds. In contrast, localized delivery of antigen attached to the tip of a micropipette results in repetitive, localized Ca2+ puffs that infrequently develop into propagated waves. Our results provide evidence that Ca2+ wave initiation from extended protrusions depends on Ca2+ influx via TRPC channels leading to the onset of SOCE-dependent Ca2+ oscillations and mast cell activation.
MATERIALS AND METHODS
cDNA plasmids
The GCaMP2 construct (24) was provided by Dr. M. Kotlikoff, Cornell University College of Veterinary Medicine. shRNA plasmids targeting TRPC channels (TRPC1, TRPC3, TRPC5, TRPC7 and GFP control) were characterized in RBL cells as previously described (25).
Chemicals and reagents
Fluo4AM and Fluo5FAM were purchased from Invitrogen/Molecular Probes (Eugene, OR). U73122, D-sphingosine, thapsigargin, A23187, 2-aminoethyldiphenyl borate (2-APB), and GdCl3 were from Sigma-Aldrich (St. Louis, MO). N,N’-dimethylsphingosine (DM-sphingosine) was from Avanti Polar Lipids (Alabaster, AL).
Cells
RBL-2H3 cells (26) were maintained in monolayer culture in Minimum Essential Medium supplemented with 20% fetal bovine serum (Atlanta Biologicals, Norcross, GA, USA) and 10 μg/ml gentamicin sulfate. All tissue culture reagents were obtained from Gibco (Grand Island, NY, USA) unless otherwise noted. Rat BMMCs were differentiated from femur-derived stem cells of Lewis strain rats by culturing for 14-28 days in the presence of rat stem cell factor (50 ng/ml) and rat IL-3 (100ng/ml) as previously described (27). Cells were harvested 3–5 days after passage and plated overnight in MatTek coverslip dishes in complete medium for experiments with Fluo-5F AM loading. For experiments with GCaMP2 as the Ca2+ indicator, 5 × 106 RBL-2H3 cells were electroporated in 0.5 ml of cold electroporation buffer (137mM NaCl, 2.7mM KCl, 1mM MgCl2, 1mg/ml glucose, 20mM Hepes, pH=7.4) with 5-8 μg of plasmid DNA at 280V and 950μF using Gene Pulser X (Bio-Rad), then plated onto MatTek dishes (~500,000 cells/plate). Cells were sensitized with 0.5 μg/ml anti-2,4-dinitrophenyl (DNP) IgE (28) during overnight cell culture incubation. Under these conditions, the cell transfection efficiency with GCaMP2 is typically 20-30%.
For experiments with TRPC shRNA constructs, plasmids containing these sequences (Origene Technologies) were co-electroporated with that for GCaMP2 using 20 μg of TRPC shRNA plasmid and 8 μg of GCaMP2 plasmid for 5 × 106 cells. No effect of these plasmids on cell viability was detected.
For characterization of rat BMMCs, differentiated cells were labeled with 2 μg/ml Alexa488-IgE (29) for 2 hr at 37°C, then washed and fixed in 4% (w/v) p-formaldehyde and 0.1% (w/v) glutaraldehye for 20 min at room temperature. Fixed cells were further labeled in 10 mg/ml BSA/PBS/0.01% NaN3 with 3 μg/ml monoclonal anti-α-galactosyl GD1b ganglioside AA4 (30), followed by 10 μg/ml Alexa555-goat anti-muIgG1 (Invitrogen Corp.). Washed, labeled cells were imaged as previously described (31).
Live cell calcium imaging
24 hours after transfection, cells were washed with buffered salt solution (BSS: 135mM NaCl, 5mM KCl, 1.8mM CaCl2, 1mM MgCl2, 1 mg/ml glucose, 20mM Hepes, pH=7.2-7.4, 1mg/ml BSA) and imaged using a heated (37°C) 63X (NA=1.4) oil immersion objective on a Zeiss Meta 510 confocal microscope. GCaMP2, Fluo-4 or Fluo-5F were excited using the 488 nm line of a krypton/argon laser and viewed with a 505–530 band-pass filter. In experiments using Fluo-4 AM or Fluo-5F AM, cells were labeled by incubating with 0.5 μM (RBL cells) or 2.5 μM (rat BMMCs) of the indicator in BSS in the presence of 2.5 mM sulfinpyrazone for 30 min in 37°C (5 min for rat BMMCs), then washed and imaged in BSS.
Cells expressing GCaMP2 or loaded with Fluo dyes were approached with a ~5 μm diameter pulled glass capillary, typically positioned within 100 microns from the cell and pre-filled with stimulating solution. Cells were imaged at 10-30 Hz while applying a puff of 10 seconds/5psi from the pipette. Pressure and duration of stimulation was controlled by a Picospritzer III (FMI Medical Instruments, GmbH). Pharmacological reagents were added to the dishes just prior to initiating data collection (see Results). For some experiments, DNP-BSA (Ag) or unmodified BSA was covalently coupled to the micropipette surface using chemical treatments and procedure previously described for covalent attachment of proteins to patterned surfaces (32).
Data analysis
Images of individual cells are representative from multiple experiments. Off line image analysis was carried out using Zeiss LSM image analysis software (Carl Zeiss, Inc) and ImageJ (NIH). Changes in fluorescence (F) were normalized by the initial fluorescence (F0) and are expressed as ΔF.
Unless stated otherwise, cells that displayed any type of Ca2+ elevation including Ca2+ puffs upon stimulation were counted as responding cells. Wave occurrence was calculated as the percentage of cells with a detectable Ca2+ wave, compared to the total number of responding cells in a given experimental condition. The lag time before Ca2+ wave initiation was measured from the initiation of the pulse of Ag or other stimulus. Statistical analysis of Ca2+ oscillations was carried out by counting and averaging the oscillatory peaks during the first 2 minutes of stimulation, including the initiating wave. Wave propagation velocity was measured as the time (number of frames x time per frame) for spreading of the wave-front across a cell of measured dimension. Unless otherwise stated, all data are presented as mean ± SEM. For each of the experiments, the number of cells analyzed (n) is presented in the text. Data were processed and plotted using Origin 8 (OriginLab) and Excel (Microsoft Corp.). Statistical comparisons between experiments were preformed using Students t-test.
RESULTS
Ca2+ mobilization events in individual RBL mast cells
To observe the earliest steps in the responses of RBL mast cells to stimulation of IgE receptors by multivalent antigen, we delivered a short (10 sec) pulse of antigen (Ag) via a micropipette placed within 100 μm of the cell while imaging changes in cytosolic Ca2+ (Figure 1A). For most experiments, we used the genetically encoded Ca2+ indicator, GCaMP2, which has a moderate Kd (~350 nM) with rapidly reversible kinetics (33). GCaMP2 is brighter than wild type GFP, maintains a 5-fold dynamic range, displays very low pH sensitivity and is fully functional at 37°C (24, 33).
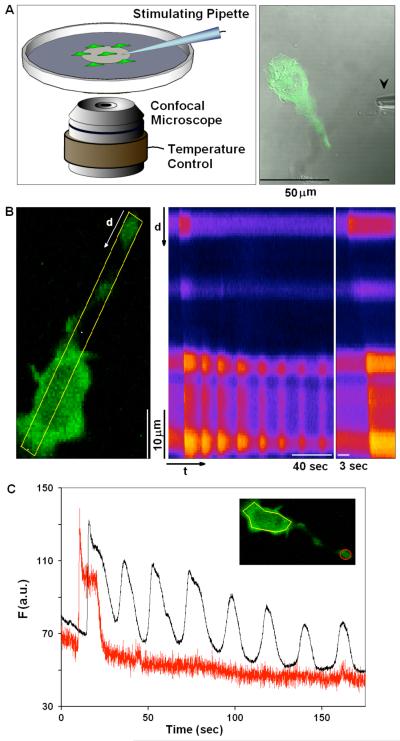
Figure 1. Visualization of Ca2+ dynamics in individual RBL mast cells.
A) Cells were plated at low density on MatTek dishes, and individual cells were stimulated using a pulled capillary (arrow; 0.5-1 μm diameter), filled with stimulating solution (as specified for each experiment) that was puffed at the cell (5psi/10sec pulse) from a distance within 100 μm. B) Image of RBL cell expressing GCaMP2 and sensitized with anti-DNP IgE, stimulated with a puff of 1.7 μg/ml Ag (DNP-BSA). Middle panel shows time line analysis of the stimulated cell and changes in Ca2+ concentration occurring over time in the 7 pixel wide line segment defined in the left panel. Changes in Ca2+ concentration are indicated by relative changes in brightness, where brighter colors represent higher Ca2+ concentration. Right panel expands early time points of the time line analysis and highlights our general observation that Ca2+ elevation initiates in cellular protrusions and propagates through the cell body. C) Traces of GCaMP2 intensity changes over time as calculated for the same cell imaged in B (inset). Black and red traces represent the changes in Ca2+ concentration in cell body (yellow ROI) or cell protrusion (red ROI), respectively.
Using high imaging rates of 10-30 frames per second, we observe two major types of spatially and temporally resolved Ca2+ mobilization dynamics in individual cells as illustrated in Figure 1B and Supplemental Movie 1. Stimulation of cells with a saturating concentration of Ag (1.7 μg/ml in the pipette) resulted in more than 90% of the cells responding with a Ca2+ wave that initiated a global Ca2+ response. This initiating Ca2+ wave followed a lag period of ~20 seconds, on average, after starting the Ag pulse (Table I). As illustrated in Figure 1B and will be discussed below, the Ca2+ wave usually initiates from the tip of an extended cell protrusion and propagates down the length of the cell body. The site of wave initiation does not detectably correlate with the location of the micropipette relative to any particular part of the cell. Ca2+ waves also initiated most frequently from the tips of extended protrusions in response to bath addition of antigen, but the lag time for wave initiation was more variable under these conditions (data not shown).
Table I.
Summary of Ca2+ Response Parameters with GCaMP2a
| Condition |
Response (% of Cells) |
Ca2+ Waves (% of Cells) |
Waves from PRTS (% of Waves) |
Oscillationsb |
wave velocity (μm/sec) |
Laq Time (sec) |
|
|---|---|---|---|---|---|---|---|
| Ag | 1700ng/ml | 96.3 | 90 | 65 | 4.5 ± 0.3 | 42.6 ± 4 | 19.4 ± 1.25 |
| 170ng/ml | 94 | 87 | 54 | 4.9 ± 0.4 | 37.3 ± 5.1 | 19.5 ± 2.1 | |
| 17ng/ml | 90.5 | 81 | 62 | 5 ± 0.4 | 18.7 ± 2.2 | 41.8 ± 4.4 | |
| 1.7ng/ml | 53.8 | 78 | 86 | 3.7 ± 0.9 | 15.9 ± 1.6 | 42.6 ± 6.5 | |
| 0[Ca2+}o | 96.8 | 89 | 31 | 1.5 ± 0.1 | 25.8 ± 4.5 | 30.2 ± 4.2 | |
| 50mM KCI | 91 | 73 | 27.2 | 5.7 ± 0.5 | 27.8 ± 4.2 | 30.2 ± 4.9 | |
| 2mM Ba2+ | 64 | 78 | 72 | 1.6 ± 0.3 | 46.9 ± 3.5 | 20.9 ± 0.8 | |
| 2mM Sr2+ | 90 | 89 | 75 | 1.7 ± 0.2 | 44.4 ± 3.6 | 19.4 ± 1.5 | |
| TG | 76.2 | 43.7 | 71 | 1.7 ± 0.2 | 30.3 ± 16.4 | 29.1 ± 4 | |
| A23187 | 73.9 | 44.8 | 30.7 | 2 ± 0.2 | 19.9 ± 5.3 | 17.9 ± 2.7 | |
| Ag | U73122 1μM | 100 | 72 | 35 | 4 ± 0.5 | 47.2 ± 11 | 14.8 ± 2.3 |
| U73122 2μM | 100 | 58 | 33 | 4.2 ± 0.4 | 47.2 ± 7.5 | 16.9 ± 2.3 | |
| U73122 2.5μM | 30 | 13 | - | 1 ± 0.2 | |||
| U73122 3.3μM | 25 | 16 | - | 1 ± 0.3 | |||
| U73122 5μM | 0 | - | - | - | |||
| 2-APB | 95.8 | 86 | 84 | 1.6 ± 0.2 | 25.9 ± 4 | 27.5 ± 2.7 | |
| sphingosine | 26.9 | 15 | 75 | 4.4 ± 0.8 | 27.8 ± 4.7 | 49.8 ± 5.7 | |
| DM-sphingosine | 62.1 | 15 | 33 | 2.8 ± 0.5 | 41.2 ± 9 | 29.3 ± 6.3 | |
| Nifedipine | 91 | 93 | 43 | 4.6 ± 0.3 | 26.8 ± 3.8 | 12.8 ± 0.8 | |
| 1μM Gd3+ | 80 | 87 | 69 | 3.7 ± 0.7 | 40.1 ± 6.2 | 22.7 ± 2.9 | |
| 10μM Gd3+ | 80.8 | 89 | 50 | 1.8 ± 0.1 | 21.4 ± 3 | 20.8 ± 2.3 | |
Unless otherwise stated Ag was used at 1700ng/ml; number of cells in each experiment was 16-80 as specified in text or figure legends
During first two minutes of response
Data are mean ± SEM
In most cells the Ca2+ wave is followed by a second manifestation of calcium dynamics – regenerative Ca2+ oscillations that occur throughout the cell body as previously described for these cells (13, 14, 16). Although not seen in the cell shown in Figure 1B, Ca2+ oscillations often extend throughout the length of the cell, including protrusions. Spatial and temporal resolution of these two processes are shown in Figure 1C as time traces for the tip of the protrusion (red trace and red region of interest (ROI) in inset) and the cell body (black trace, yellow ROI in inset) for the cell in Figure 1B. In some cells, we observed localized Ca2+ puffs prior to the initiation of waves; however, these were infrequent under conditions of optimal stimulation (see below).
We used Fluo4 and Fluo5F to confirm our main results for these Ca2+ dynamics. Fluo4 and GCaMP2 have similar affinities for Ca2+, but the fluorescence response kinetics are approximately an order of magnitude slower for the GFP-based sensor. As shown in Supplemental Figure S1, despite this slower response time for GCaMP2, Ca2+ wave velocities measured using GCaMP2 were significantly higher than those measured with Fluo4, indicating that GCaMP2 kinetics are not a limiting factor in our measurements. The percentage of cells exhibiting Ca2+ waves detected by Fluo4 is smaller, and the observed waves initiate after a longer lag time and exhibit slower propagation velocities. Interestingly, fewer Ca2+ oscillations were detected with both Fluo4 and Fluo5F compared to GCaMP2, possibly due to Ca2+ buffering effects of these organic dyes, which are difficult to load into cells at controlled concentrations. Overall, we found GCaMP2 to be most useful for our experiments.
Although there are cell-to-cell differences in appearance and characteristics of Ag-stimulated Ca2+ waves and subsequent oscillations in the RBL cells, we found that several characteristics are highly consistent for a particular concentration of Ag. These include the lag time to initiation of the first Ca2+ peak, the wave velocity, and the frequency (number observed in 2 minutes) of the oscillations (Table I).
Cellular protrusions are the primary initiation sites for Ca2+ wave responses to Ag stimulation in RBL cells
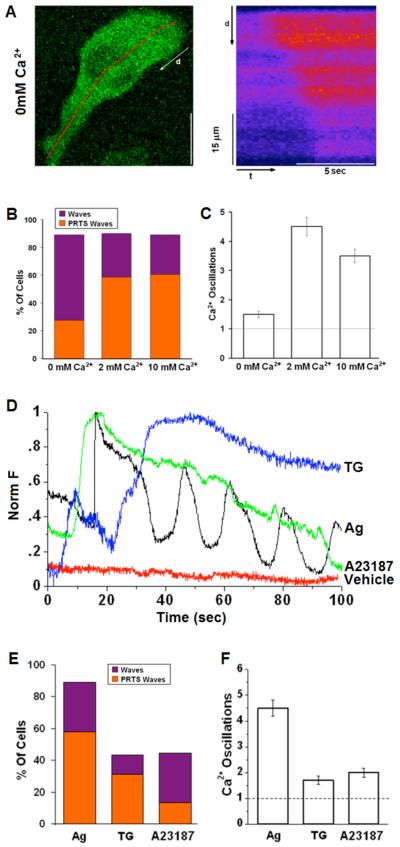
As summarized in Figure 2B, ~90% of the cells responded to Ag (1.7 μg/ml) with a propagating Ca2+ wave, whereas for the remaining ~5-10% of the responding cells we detected a non-directional, global elevation in cytoplasmic Ca2+. Although stimulated influx of extracellular Ca2+ is required for cellular degranulation, we observed wave initiation with similar frequency in the presence and absence of extracellular Ca2+, suggesting that Ca2+ influx is not necessary for Ca2+ wave generation and propagation. However, a clear difference in the spatial localization of wave initiation is observed, depending on the presence or absence of extracellular Ca2+. As illustrated in Figure 2A and Supplemental Movie 2, and summarized in Figure 2B, wave initiation in the absence of extracellular Ca2+ occurs most frequently at a site in the cell body, with less frequent (<30%) initiation in elongated protrusions. This contrasts with extracellular Ca2+ at 2-10mM when the majority of waves stimulated by Ag initiate near the tips of these protrusions (Fig. 1 and Fig. 2B). Similarly, reducing the driving force for Ca2+ influx by depolarizing the cells with addition of 50 mM KCl prior to Ag stimulation also decreases the incidence of waves initiating from elongated protrusions to <30% (Table I). These results suggest that initiation of Ca2+ waves from elongated protrusions depends on Ca2+ influx.
Figure 2. Dependence of stimulated Ca2+ waves and oscillations on extracellular Ca2+and stimulus.
A) Representative cell sensitized with IgE and stimulated with 1.7μg/ml Ag in the absence of extracellular Ca2+. Right panel shows time line analysis for early time points and indicated segment in left panel image. B) Histogram showing percentage of cells responding to stimulus with Ca2+ waves as averaged over multiple experiments for 0mM Ca2+ (n=29), 2mM Ca2+ (n=80); 10mM Ca2+ (n=28). Bar height shows total percentage of waves; orange portion represents the percentage of waves originating in protrusions (PRTS). C) Average number of oscillations within 2 min of stimulation under specified conditions. D) Representative traces of cells sensitized with anti-DNP IgE and stimulated with puff from pipette containing 1.7 μg/ml DNP-BSA (Ag), 1μM thapsigargin (TG), 20μM A23187 or BSS buffer only (vehicle). E) Percentage of cells responding to stimulus with Ca2+ waves averaged over multiple experiments and represented as in (B) with indicated conditions: Ag (n=80); TG (n=16); A23187 (n=29). F) Average number of oscillations within 2 min of stimulation under specified conditions. The first oscillation corresponds to the originating Ca2+ wave (dotted line). Error bars correspond to standard error of the mean (SEM).
Extended protrusions are common morphological features of RBL mast cells that are readily observed at low cell densities. They develop several hours after cell attachment and define an axis of cell migration (J. Lee and D. Holowka, unpublished results). Similar protrusions are frequently observed with mucosal mast cells imaged in vivo (for examples see: (34-37).
We found that substitution of extracellular Ca2+ with equimolar concentrations of Ba2+ or Sr2+ prior to stimulation did not markedly alter either the percentage of cells with Ag-stimulated waves or initiation from elongated protrusions (Table I). The velocity of wave propagation was reduced by ~40% in the absence of extracellular Ca2+, whereas substitution of Ca2+ by Ba2+, which is not taken up into stores by SERCA pumps (38), does not alter wave velocity (Table I). These results are consistent with wave initiation from protrusions by a Ca2+ influx component and propagation by Ca2+-dependent Ca2+ release from intracellular stores (10, 39). Interestingly, we find that the lag time for wave appearance following Ag stimulation was longer in the absence of extracellular Ca2+ (Table I), but was not changed when Ca2+ was substituted with Ba2+ or Sr2+. This delay could be attributed to a reduced “ignition” capacity of the calcium response in the absence of a Ca2+ influx, and is further discussed below.
In addition to the observed effects on Ca2+ waves, the absence of extracellular Ca2+ substantially decreases the frequency of subsequent Ca2+ oscillations (Figure 2C), as expected from the dependence of these oscillations on sustained, store-operated Ca2+ influx (17). Substitution of Ba2+ or Sr2+ for extracellular Ca2+ markedly reduces the frequency of oscillations (Table I), consistent with the high selectivity of CRAC channels to Ca2+ over Ba2+ or Sr2+ (40, 41).
We compared the dynamic features of Ag-stimulated Ca2+ responses in RBL cells to those observed upon stimulation with either the SERCA inhibitor thapsigargin (TG) or the Ca2+ ionophore, A23187. As illustrated by individual time traces in Figure 2D and summarized in figure 2F, the oscillatory dynamics of Ag responses are largely absent from responses to TG and A23187. For TG at 1μM in the pipette, a transient increase in cytoplasmic Ca2+ was typically observed, followed by a more sustained increase that depends on extracellular Ca2+. As summarized in Figures 2E, Ca2+ waves are less frequently observed with TG stimulation, but, when observed, waves usually initiate from extended protrusions. For stimulation by A23187 at 20 μM in the pipette, Ca2+ responses initiate as a wave in only about 40% of the cells, and this occurs very infrequently from extended protrusions (Figure 2E). Furthermore, when the response to A23187 does initiate as a Ca2+ wave, the wave velocity is substantially slower than observed in cells stimulated by high concentration of Ag or TG (see Table I). Similar to TG, A23187-mediated elevation in cytoplasmic Ca2+ does not cause oscillatory behavior in the majority of the stimulated cells (Figures 2D and F).
In summary, elevations in cytoplasmic Ca2+ caused by either ionophore or TG-induced release from stores can also initiate Ca2+ waves from discrete sites along the plasma membrane, but with lower probability. These results further indicate that cytoplasmic Ca2+ oscillations such as those initiated by Ag can be uncoupled from Ca2+ wave initiation caused by these non-receptor means of cell activation.
Characterization of the Ca2+ response in rat BMMCs
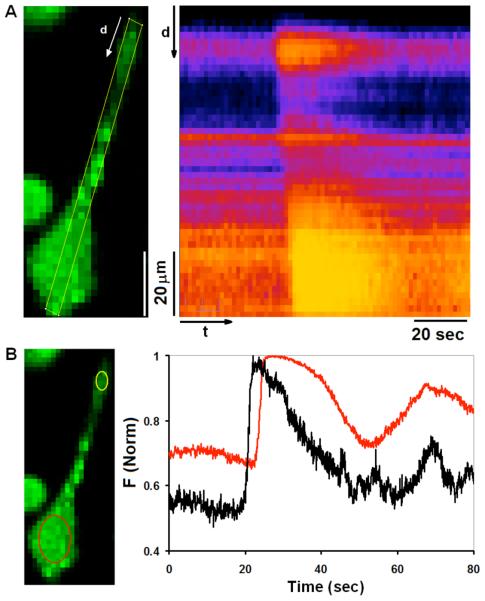
To evaluate whether the characteristics of Ca2+ responses that we observe for RBL-2H3 cells are general for mast cells, BMMCs were differentiated from Lewis rats as previously described (27). When plated overnight on glass slides in MatTek dishes, these cells attach firmly and frequently exhibit extended protrusions, similar in morphology to RBL cells. We confirmed their identity by labeling with Alexa 488-IgE and AA4 mAb that labels a mast cell specific ganglioside (42, 43; supplemental figure S4) as well as by staining granules with Alcian blue ((44); data not shown). BMMCs sensitized with IgE and labeled with Fluo-5F were stimulated as described above for RBL cells. As shown in Figure 3, we find that the majority of responding BMMCs exhibit initiating Ca2+ waves that often begin in extended protrusions, similar to Ca2+ waves observed in RBL cells. Also similar to Ca2+ responses in RBL cells, oscillations throughout the cytoplasm often follow the initial, propagating Ca2+ wave (Fig. 3b). These findings indicate that antigen-stimulated Ca2+ responses in rat BMMCs, including Ca2+ waves that initiate from specific morphological features, are similar to those characterized in cultured RBL mast cells.
Figure 3. Ca2+ dynamics in individual rat BMMCs.
A) Confocal image of a representative rat BMMC sensitized with anti-DNP IgE and loaded with Fluo5F (left panel), and time line analysis of indicated ROI for this cell stimulated at t=0 with a puff of 1.7 μg/ml DNP-BSA (right panel). Changes in Ca2+ concentration in response to stimulation are depicted, where brighter colors represent higher Ca2+ concentration. Time line analysis highlights the Ca2+ elevation initiating in the tip of the cellular protrusion and propagating through the cell body. B) Traces of Fluo5F intensity changes over time as calculated for the same cell imaged in (A). Black and red traces in right panel represent changes in Ca2+ concentration in cell body (yellow ROI) and in cell protrusion (red ROI), respectively, in the left panel.
Antigen dose dependence and spatial localization
To assess further the nature and function of the Ca2+ waves, we characterized the dose dependence of Ag-stimulated Ca2+ responses in RBL cells detected with GCaMP2 (Supplemental Figure S2). More than 90% of the cells respond to antigen doses in the range of 17-1700 ng/ml in the pipette, and more than 50% of the cells respond at the lowest dose tested, 1.7 ng/ml (Figure S2A). Ca2+ waves were observed in a similarly high percentage of the responding cells (≥80%) at all doses tested, but Ca2+ waves initiate more frequently at extended protrusions at the lowest Ag dose (Figure S2C). Wave velocity was found to be dependent on the concentration of Ag (Figure S2B), and longer lag times between antigen addition and the first Ca2+ response were observed at lower Ag doses (Figure S2D), but the frequency of Ca2+ oscillations changes little over the four orders of magnitude of Ag concentration (Figure S2E).
Ca2+ puffs are localized and transient elevations in cytoplasmic Ca2+ that may serve as a priming mechanism for wave initiation and subsequent oscillations (39, 45, 46). Stimulated Ca2+ puffs are observed less often than Ca2+ waves at higher doses of antigen, but appear more often at the lowest concentration of Ag tested (Figure S2F). Consistent with these observations, Ca2+ puffs may represent sub-threshold activation of the cells and production of insufficient amounts of IP3 to generate a propagated Ca2+ elevation and a global Ca2+ response.
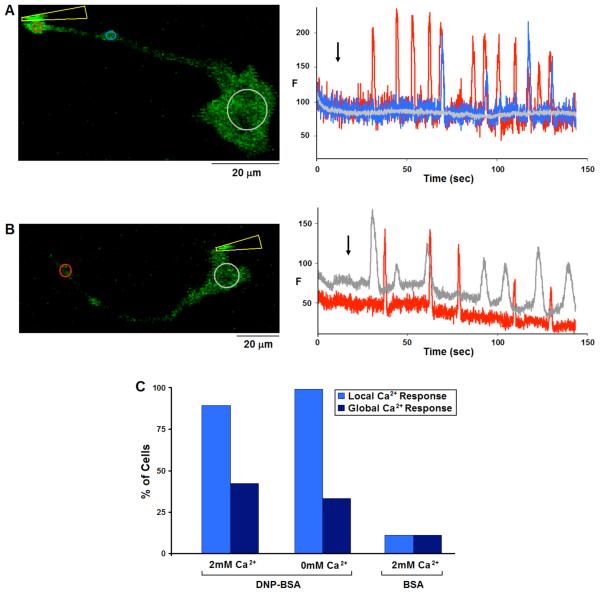
Interestingly, when antigen is covalently coupled to the tip of a micropipette and delivered locally by cell contact, we usually observe a series of stimulated Ca2+ puffs that frequently occur near the point of contact. As illustrated in Figure 4, two different responses are typical: In one case, repetitive puffs are observed at relatively regular time intervals, often over several minutes of contact, but they fail to propagate as waves or global cellular Ca2+ elevation for more than several microns (Fig. 4A). Alternatively, Ca2+ puffs initiated by local contact of the antigen-coated pipette occasionally propagate as waves that traverse the length of the cell (Fig. 4B). Under these conditions of stimulation, contact at a cell protrusion did not significantly increase the probability of wave propagation. Ca2+ puffs or waves were observed in more than 85% of cells contacted by DNP-BSA coated tips in both the presence and absence of extracellular Ca2+ (Fig. 4C) and only rarely in cells contacted by unconjugated micropipettes or those conjugated with unmodified BSA (Fig. 4C). Moreover, Ca2+ puffs were just as frequent when cells were locally stimulated with DNP-BSA conjugated micropipettes in the absence of extracellular Ca2+ (Fig. 4C). These results suggest that the localized responses are mediated by release of Ca2+ from internal stores, rather than by Ca2+ influx. Moreover, localized activation of FcεRI can stimulate spatially restricted Ca2+ responses, and, in this situation, the site of Ca2+ response initiation is determined by the location of receptor stimulation.
Figure 4. Stimulation of RBL cells by contact with Ag-coated micropipette.
A and B) Representative cells expressing GCaMP2, sensitized with IgE and stimulated with DNP-BSA conjugated micropipettes. A) Micropipette (indicated by yellow arrowhead) contacting the cell at the tip of a protrusion elicits a train of spatially restricted Ca2+ puffs, each traveling no more than 30μm along the protrusion. B) Contact stimulation at the cell body results repetitive Ca2+ puffs in the cell body that sometimes propagate as a wave to the protrusion. Left panels show images with ROIs defined; Right panels show Ca2+ concentration changes in ROIs of corresponding color. Black arrow indicates initiation of contact between micropipette and cell. C) Histogram showing percentage of cells responding with local Ca2+ puffs only (light blue) or more global Ca2+ elevation (dark blue) due to contact with DNP-BSA-conjugated micropipettes in the presence (n=28) or absence (n=18) of extracellular Ca2+, or with unmodified BSA-conjugated micropipettes (n=18).
These results show that the localized Ca2+responses can be initiated by localized receptor stimulation, and these responses are apparently independent of Ca2+ influx. Overall, these results show that Ca2+ responses to soluble Ag begin most frequently as Ca2+ waves that are propagated from initiation sites near the tips of extended protrusions, whereas those stimulated by locally applied micropipette-conjugated antigen are initiated at the site of stimulation, and are more commonly manifested as repetitive Ca2+ puffs that less frequently evolve into Ca2+ waves.
Pharmacologic characterization of Ca2+ dynamics
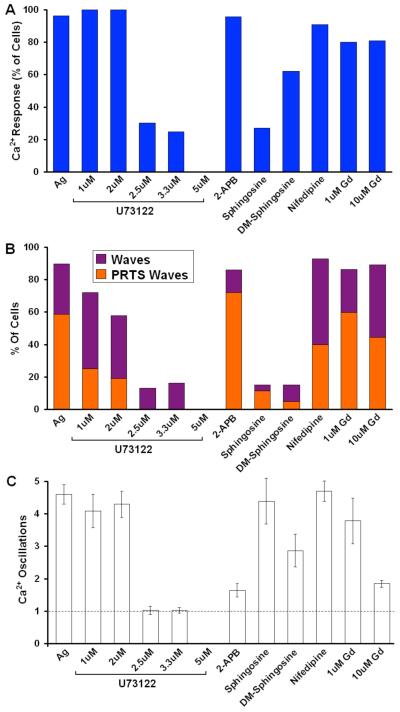
In initial efforts to identify molecular components of Ag-stimulated waves and oscillations, we investigated the effects of several different inhibitors of IP3 production and modulators of Ca2+ influx. As summarized in figure 5 and in Table I, the phospholipase C (PLC) inhibitor, U73122, reduces the percentage of cells showing Ca2+ responses with a sharp dose dependence that is half maximal between 2 and 2.5 μM. U73122 also reduces the percentage of cells with an initiating Ca2+ wave with a similar dose-dependence, suggesting that Ag-stimulated Ca2+ waves depend on IP3 and/or diacylglycerol production (Figure 5). In this dose range, U73122 is relatively selective for PLC isoforms in these cells, but at higher doses other proteins are also affected by this maleimide derivative (47). As summarized in Figure 5, Ag-stimulated Ca2+ oscillations are inhibited with a similar dose-dependence, consistent with IP3-dependent intracellular Ca2+ increase as a necessary precursor to SOCE (48).
Figure 5. Effects of inhibitors on intracellular Ca2+ waves and oscillations.
A) Percentage of cells from multiple experiments showing a measurable Ca2+ response to stimulation by 1.7 μg/ml Ag in the presence of the following reagents in the extracellular buffer: 1-5 μM U73122 (n>18 each), 20mM 2-APB (n=23), 8μM D-sphingosine (n=26); 8μM DM-sphingosine (n=31), 10μM nifedipine (n=15), 1 or 10 μM GdCl3 (Gd3+; n>20 each) B) Percentage of cells responding to stimulus with Ca2+ waves as averaged over multiple experiments with indicated conditions. Bar height shows total % waves; orange portion represents % waves originating in protrusions. C) Average number of oscillations within 2 min of stimulation under specified conditions. The first oscillation corresponds to the originating Ca2+ wave (dotted line). Error bars correspond to SEM.
2-APB was initially described as a non-competitive antagonist of IP3 receptor (IP3R)-mediated Ca2+ release by microsomal preparations (49), and it was subsequently shown to inhibit SOCE in T cells at concentrations in the range of 10-50μM (50). In RBL cells, 2-APB at concentrations up to 40μM has no effect on Ag-stimulated Ca2+ release from stores in the absence of extracellular Ca2+, indicating that it fails to inhibit IP3R-mediated Ca2+ release from stores in these cells (D. Holowka, unpublished results). As summarized in Figure 5, addition of 20μM 2-APB prior to Ag stimulation does not significantly reduce the percentage of responding cells (Figure 5A), or the percentage of cells that exhibit wave initiation (Figure 5B). In contrast, 20μM 2-APB strongly inhibits Ca2+ oscillations subsequent to wave initiation (Figure 5C), consistent with its inhibitory effect on SOCE that contributes to Ca2+ oscillations in these cells (51-53)
A previous study by Penner and colleagues (54) identified both D-sphingosine and N,N-dimethylsphingosine (DMS) as effective inhibitors of IP3 and TG-elicited Ca2+ release-activated Ca2+ (CRAC) channel activation in RBL cells. As summarized in Figure 5B and Table I, we found that 8 μM D-sphingosine or 8 μM DMS strongly inhibits Ca2+ wave initiation. Earlier studies suggested that DMS prevents Ca2+ responses by inhibition of sphingosine kinase (55, 56). However, this mechanism is unlikely for the inhibition we observe because D-sphingosine is a substrate of this enzyme family. In other studies, we have evidence that these basic amphiphiles inhibit Ca2+ responses by electrostatically neutralizing negatively charged phospholipids at the inner leaflet of the plasma membrane ((57); N. Smith et al., manuscript in preparation). The small percentage of cells that do respond in the presence of D-sphingosine appear to have oscillation frequencies similar to untreated cells (Figure 5C), suggesting that this aspect of SOCE is less potently inhibited than wave initiation. These results identify these sphingosine derivatives as effective inhibitors of the earliest manifestations of Ca2+ responses to Ag in these cells.
We further tested the effects of inhibitors of dihydropyridine-sensitive Ca2+ channels on Ag-stimulated Ca2+ wave initiation in RBL cells (58). As summarized in Figure 5, we observed a modest (~30%) reduction of wave initiation from extended protrusions by 10 μM nifedipine, but no effects of other L channel inhibitors such as Bay K (+) were detected (data not shown), suggesting that the effect of nifedipine observed may be due to non-specific inhibition of K+ channels resulting in cell depolarization (59, 60). To investigate further a possible contribution of CRAC conductance to wave generation, we examined the effects of Gd3+ on Ca2+ responses to Ag. Pre-addition of 10 μM Gd3+ strongly inhibits Ca2+ oscillations in response to Ag (Fig. 5C), consistent with inhibition of SOCE (61), but it causes only a small reduction in the percentage of waves initiating from extended protrusions (Figure 5B). These results are consistent with those obtained with 2-APB that indicate little or no contribution of CRAC channels to Ag-stimulated wave initiation. Because Ca2+ influx contributes to the spatial localization of Ca2+ wave initiation from protrusions (Fig. 2), these results suggest that Ca2+ channels other than CRAC or voltage gated channels contribute to this process.
Involvement of TRPC channels in the initiation of Ca2+ waves from protrusions
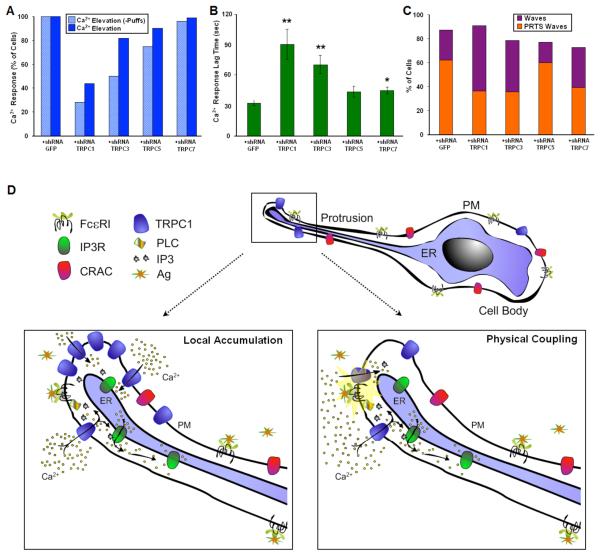
Expression of TRPC channels in RBL cells was demonstrated by Pizzo et al. (62) and more recently by Ma et al (25). TRPC channels have been identified as receptor activated Ca2+ channels, and we investigated the contribution of TRPC channels to wave initiation by IgE-FcεRI in RBL mast cells using shRNA sequences targeting TRPC1, TRPC3, TRPC5 and TRPC7 to knock down expression of these proteins (25). For these experiments, RBL cells were co-transfected with the Ca2+ indicator GCaMP2 and one of the TRPC targeting shRNA plasmids as described in Materials and Methods. We found that transfection with TRPC1 shRNA and, to a lesser extent, with TRPC3 shRNA reduces the responsiveness of the cells to a low concentration of Ag compared to cells expressing a mock shRNA sequence, and these effects are particularly notable if cells responding with Ca2+ puffs are not included in the count (Figure 6A). As shown in Supplemental Figure S3, we observe ~50% reduction in TRPC1 expression due to shRNA transfection in these cells, similar to previous results (25). This value represents a lower limit to the knockdown of TRPC1 cell expressing GCaMP2, which is co-transfected with the shRNA constructs. Under these knock down conditions, we did not observe a significant reduction in the percentage of cells responding to Ag in cells transfected with TRPC5 or TRPC7. In addition to reduction in cell responsiveness, we found that transfection with TRPC1 or TRPC3 shRNA prolongs the lag time from Ag addition to the onset of the Ca2+ response compared to cells transfected with TRPC5, TRPC7, or the control shRNA (Fig 6B). These results suggest that TRPC1 and TRPC3 play a significant role in the initiating Ca2+ wave phase of the cellular response to Ag stimulation.
Figure 6. Participation of TRPC channels in Ag stimulated initiation of the Ca2+ response.
RBL cells were co-transfected with GCaMP2 and shRNA plasmids targeting TRPC1, 3, 5, 7 or mock sequence (for each condition n>29). Cells sensitized with anti-DNP IgE were stimulated with puff from pipette containing 1.7 ng/ml DNP-BSA in BSS. A) Percentage of cells from multiple experiments showing a measurable Ca2+ response corresponding to elevation of intracellular Ca2+ cells. Dark blue bars include cells that only include Ca2+ puffs in the count, and light blue bars exclude this population. B) Average lag time before initiation of Ca2+ wave with specified shRNA. Error bars correspond to SEM. *P < 0.05, **P < 0.01 vs. cells expressing shGFP. C) Percentage of cells responding to stimulus with Ca2+ waves with indicated shRNA. Bar height shows total % waves; orange portion represents % waves originating in protrusions. D) Model for initiation of Ca2+ mobilization in extended protrusions. Ag crosslinks IgE/FcεRI complexes, activating Ca2+ influx via TRPC1 channels to cause local increase in cytoplasmic Ca2+ at the tip of a cell protrusion. This potentiates IP3-mediated Ca2+ release from the nearby ER store that propagates via Ca2+-induced Ca2+ release to generate a Ca2+ wave that initiates Ca2+ oscillations and cell activation. Two possible mechanisms for wave initiation at these protrusions are indicated.
Most notably, expression of TRPC1 and TRPC3 shRNA results in decreased frequency of Ca2+ wave initiation from cell protrusions compared to expression of mock shRNA (Figure 6C). Expression of TRPC7 shRNA also decreases the frequency of Ca2+ wave initiation from cell protrusions, even though this construct does not significantly alter the capacity of the cells to respond or the lag time of the response. Measurements of Ca2+ wave velocity show only marginal differences for cells expressing the TRPC shRNA constructs, suggesting that these channels are not involved in Ca2+ wave propagation (data not shown). These results also indicate that reduction in the capacity of cells to respond to Ag stimulation after the TRPC1 or TRPC3 shRNA expression is caused by perturbation of wave initiation of the Ca2+ response, rather than in later steps involved in propagating the Ca2+ signal, which have been shown to be inhibited by TRPC5 shRNA (25).
DISCUSSION
IgE receptor-mediated Ca2+ mobilization plays key roles in mast cell activation and consequent exocytotic release of inflammation and allergy-related mediators. Previous studies characterized dramatic Ca2+ oscillations stimulated by Ag in these cells (15) that correlate temporally to exocytotic events (13, 15). In the present study we used high-speed confocal imaging to characterize rapid, early events in stimulated Ca2+ mobilization with high spatial resolution. Our principal new finding is that the initial increase in cytoplasmic Ca2+ in response to Ag stimulation in RBL mast cells is highly spatially organized, initiating at specific regions of the cell and propagating through the cytosol as a Ca2+ wave. The existence and nature of Ca2+ waves in hematopoietic cells has been controversial, and the study characterizing high-speed, circumferential waves in neutrophils (63) could not be confirmed (64). Lee and Oliver previously described evidence for a Ca2+ influx pathway in RBL cells that precedes Ca2+ release from intracellular stores (65). The Ca2+ waves stimulated via FcεRI that we have characterized in the present study are similar in several aspects to Ca2+ waves observed in certain excitable and other non-hematopoietic cells (6, 7, 66), but they have not been previously detected in Ca responses stimulated by multichain immune recognition receptors.
In certain cell types, elementary Ca2+ events such as Ca2+ puffs can lead to initiation and propagation of a Ca2+ wave, and this has been shown to be regulated by a number of factors, including spatial organization of intracellular Ca2+ release sites (67-70). In RBL mast cells, the high incidence of wave initiation from elongated cellular protrusions suggests that these protrusions contain specialized structures that ignite the cellular response. Interestingly, the percentage of stimulated Ca2+ waves that initiate from protrusions increases at weak stimulation indicating that protrusions are particularly sensitive to Ag. A survey of the literature indicates that such protrusions are a common feature of mucosal mast cells in tissue sites (34, 36, 37, 71), consistent with an important role for these protrusions in mast cell physiology. The high sensitivity of wave initiation in protrusions that we observe could result from locally enhanced coupling of the IgE receptor and the machinery responsible for Ca2+ release from stores. We do not detect enhanced expression of FcεRI in these protrusions, but we find that initiation of Ca2+ waves in protrusions is selectively reduced in the absence of extracellular Ca2+, suggesting a facilitating Ca2+ influx pathway at these protrusions. As discussed below, our results point to Ca2+- dependent potentiation of IP3 receptors close to sites of Ca2+ influx at protrusions, rendering these receptors more sensitive and therefore more probable sites for Ca2+ wave initiation.
Ca2+ oscillations following initial waves exhibit a global, non-directional increase in Ca2+ concentration that contrasts with directional Ca2+ elevation seen for the waves. Because of this spatial distinction and the pharmacological differences, we conclude that waves and oscillations are two discrete but coupled Ca2+ events in IgE receptor-mediated signaling. We suggest that Ca2+ waves are the initiating event of mast cell activation, established by the unique spatial distribution of Ca2+ influx pathways in these cells, involving TRPC channels that may be preferentially expressed in the elongated protrusions, rendering these to be more sensitive to Ag stimulation. Subsequent to the waves are Ca2+ oscillations that are thought to result from IP3-induced release of Ca2+ from ER (72). These reoccurring Ca2+ elevations were suggested to play a role in prolonging the duration of the Ca2+ signal, enhancing its efficacy (16) and also in providing temporal encoding for downstream events such as degranulation (15). Interestingly, we could occasionally detect Ca2+ oscillations in cells that did not demonstrate a Ca2+ wave upon Ag stimulation, suggesting that the propagation of Ca2+ through the cytoplasm during the wave period is not required for the onset of Ca2+ oscillations.
In addition to Ca2+ waves and oscillations, we detect Ca2+ puffs in cells stimulated by antigen under certain conditions. Of particular interest are the repetitive Ca2+ puffs that are elicited by local application of antigen with a DNP-BSA coated micropipette, demonstrating that the molecular machinery capable of initiating local Ca2+ responses is distributed throughout the cell and is not restricted to the protrusions. Ca2+ puffs stimulated by Ag-coated micropipette contact are similarly frequent in the absence of extracellular Ca2+, indicating that the generation of spatially restricted Ca2+ elevations in these cells involves Ca2+ release from stores and does not require Ca2+ influx. However, the periodic nature of these puffs suggests a complex mechanism for their regulation that may be related to the periodic nature of more global Ca2+ oscillations elicited by soluble antigen. It will be interesting to determine whether these localized Ca2+ puffs are sufficient to elicit localize exocytosis in these cells.
Pharmacologic characterization of IgE receptor-mediated Ca2+ waves yield evidence for PLC activation in this mechanism, whereas store-operated Ca2+ entry does not appear to play an important role in this early phase of the Ca2+ response. PLCγ-dependent IP3 production is important for generating Ca2+ responses to Ag in mast cells (73), and it is possible that a specific IP3 receptor family member is involved in Ca2+ wave initiation. The greater sensitivity of IP3R-3 to Ca2+ release by IP3 (74) suggests this family member as a strong candidate for initiation of an IP3-dependent Ca2+ wave at protrusions. Ca2+ waves can be triggered by alternate means of Ca2+ mobilization, including Ca2+ ionophore and thapsigargin, but these agents cause less frequent Ca2+ waves (Table I). Furthermore, initiation of Ca2+ waves by Ca2+ ionophore occurs only infrequently at extended protrusions. Together, these results suggest that Ca2+ responses initiated by the IgE receptor are more spatially regulated than those caused by non-receptor-mediated cell activation.
The recent study by Ma et al. provided evidence that TRPC channels contribute to stimulated Ca2+ influx in RBL cells (25), suggesting their possible involvement in the Ag-stimulated Ca2+ influx pathway that initiates Ca2+ waves from extended protrusions. Our results with shRNA-mediated knockdown of TRPC proteins provide evidence that decreased expression of TRPC1, and, to lesser extent, TRPC3, reduces the responsiveness of these cells to low concentration of Ag (Figure 6A, B) and decreases the frequency of wave initiation from extended protrusions (Figure 6C). At higher concentrations of Ag, TRPC 1 channel knock down is less effective in decreasing the responsiveness of the cells (data not shown), indicating that this channel plays an important role in Ca2+ responses only under more physiological conditions of IgE receptor stimulation. Other TRPC channels may also contribute to Ca2+ wave initiation, and our failure to detect significant effects of TRPC5 knock down, or the smaller effects of TRPC3 and 7 knock down, may be limited by the efficiency of their reduction under our experimental conditions.
These results suggest that Ca2+ influx via TRPC channels, particularly TRPC1, is likely to participate in the initial steps of mast cell activation by enhancing the sensitivity of these cells to threshold Ag stimulation. Although the mechanism for this enhancement is not yet understood, it is likely that a Ca2+ influx-dependent increase in IP3R activation is involved. Two possible mechanisms are indicated in Figure 4D: In one, local accumulation of TRPC at protrusions could enhance receptor-stimulated influx at this region; Alternatively, enhanced physical coupling of the TRPC channels to IP3R (75), PLCγ (76), or FcεRI in this region could mediate local Ca2+ influx to initiate wave propagation.
Our observations that rat BMMCs exhibits similar morphologies as RBL mast cells and initiate Ca2+ responses to antigen in the form of waves that begin in extended protrusions show that these stimulated waves are a general feature of FcεRI-mediated Ca2+ signaling. Both rat BMMCs (23) and RBL mast cells (20) have marker characteristics of mucosal mast cells, and it is not yet clear whether this wave response is a specific feature of this subset of mast cells. Mouse BMMCs undergo chemotatic responses on vitronectin (77), and it may be possible to observe stimulated Ca2+ waves under these conditions of cell attachment.
Our data establish Ca2+ waves originating from a specific cellular site as a common feature of the Ca2+ response to antigen in RBL mast cells and rat BMMCs, but the physiological function of these waves and the molecular basis for their site of initiation are not completely understood. Regulated Ca2+ signaling across the cell is a fundamental mechanism for information transfer between organelles or cellular regions that conveys directional and temporal information. In this regard, we have preliminary evidence that direction of RBL cell migration correlates with the site of Ag-stimulated wave initiation (R. Cohen and J. Lee, unpublished results), suggesting a strategic localization of the Ca2+ signaling machinery to communicate directional information in these cells. Localized degranulation from extended protrusions might also be mediated by this machinery under certain physiological conditions.
In summary, we show that the response of RBL and rat BMMC to Ag stimulation initiates with a propagating Ca2+ wave, most often beginning at the tips of cellular protrusions in a Ca2+ influx-dependent manner. These waves initiate with TRPC1-dependent Ca2+ influx, then propagate by Ca2+-dependent Ca2+ release from stores, followed by extracellular Ca2+-dependent oscillations. Spatial localization of wave initiation sites to extended protrusions suggests a link between this signaling response and polarized cell motility. Our results indicate that when mast cells encounter physiological levels of antigen that are insufficient to directly activate global Ca2+ mobilization, activation of Ca2+ influx mediated by TRPC1 channels in extended cell protrusions can ignite a response that is propagated and amplified. In this manner, the initiation of a physiological cellular response is both spatially and temporally controlled.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Robert Doran for excellent technical support and Dr. Michael Kotlikoff for the GCaMP2 construct and valuable discussions. We are grateful to Lisa Blum from the laboratory of Dr. Judith Appleton and Norah Smith and Jinmin Lee for preparation and culture of rat BMMCs. We also thank Dr. Clare Fewtrell for helpful comments on the manuscript and Dr. Reuben Siraganian for purified mAb AA4.
This work was supported by NIH Grant AI022449 and by the Nanobiotechnology Center at Cornell (NSF:ECS-9876771). H-T Ma is supported by the International Program of NHLBI, NIH.
REFERENCES
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta. 2004;1742:3–7. doi: 10.1016/j.bbamcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Ridgway EB, Gilkey JC, Jaffe LF. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A. 1977;74:623–627. doi: 10.1073/pnas.74.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe L. On the conservation of fast calcium wave speeds. Cell Calcium. 2002;32:217–229. doi: 10.1016/s0143416002001574. [DOI] [PubMed] [Google Scholar]
- 6.Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- 7.Rintoul GL, Baimbridge KG. Effects of calcium buffers and calbindin-D28k upon histamine-induced calcium oscillations and calcium waves in HeLa cells. Cell Calcium. 2003;34:131–144. doi: 10.1016/s0143-4160(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 8.Hillson EJ, Hallett MB. Localised and rapid Ca2+ micro-events in human neutrophils: conventional Ca2+ puffs and global waves without peripheral-restriction or wave cycling. Cell Calcium. 2007;41:525–536. doi: 10.1016/j.ceca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie L, Bootman MD, Berridge MJ, Lipp P. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J Physiol. 2001;530:417–429. doi: 10.1111/j.1469-7793.2001.0417k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe LF. The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A. 1991;88:9883–9887. doi: 10.1073/pnas.88.21.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas AP, Bird GS, Hajnoczky G, Robb-Gaspers LD, Putney JW., Jr. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- 12.Tovey SC, de Smet P, Lipp P, Thomas D, Young KW, Missiaen L, De Smedt H, Parys JB, Berridge MJ, Thuring J, Holmes A, Bootman MD. Calcium puffs are generic InsP(3)-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J Cell Sci. 2001;114:3979–3989. doi: 10.1242/jcs.114.22.3979. [DOI] [PubMed] [Google Scholar]
- 13.Millard PJ, Gross D, Webb WW, Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988;85:1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan TA, Millard PJ, Webb WW. Imaging [Ca2+]i dynamics during signal transduction. Cell Calcium. 1990;11:145–155. doi: 10.1016/0143-4160(90)90067-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim TD, Eddlestone GT, Mahmoud SF, Kuchtey J, Fewtrell C. Correlating Ca2+ responses and secretion in individual RBL-2H3 mucosal mast cells. J Biol Chem. 1997;272:31225–31229. doi: 10.1074/jbc.272.50.31225. [DOI] [PubMed] [Google Scholar]
- 16.Narenjkar J, Marsh SJ, Assem ES. The characterization and quantification of antigen-induced Ca2+ oscillations in a rat basophilic leukaemia cell line (RBL-2H3) Cell Calcium. 1999;26:261–269. doi: 10.1054/ceca.1999.0065. [DOI] [PubMed] [Google Scholar]
- 17.Parekh AB. Functional consequences of activating store-operated CRAC channels. Cell Calcium. 2007;42:111–121. doi: 10.1016/j.ceca.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 20.Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, Woodbury RG. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985;82:3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong GW, Tang Y, Feyfant E, Sali A, Li L, Li Y, Huang C, Friend DS, Krilis SA, Stevens RL. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J Biol Chem. 1999;274:30784–30793. doi: 10.1074/jbc.274.43.30784. [DOI] [PubMed] [Google Scholar]
- 22.Razin E, Zhang ZC, Nechushtan H, Frenkel S, Lee YN, Arudchandran R, Rivera J. Suppression of microphthalmia transcriptional activity by its association with protein kinase C-interacting protein 1 in mast cells. J Biol Chem. 1999;274:34272–34276. doi: 10.1074/jbc.274.48.34272. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald AJ, Pick J, Bissonnette EY, Befus AD. Rat mucosal mast cells: the cultured bone marrow-derived mast cell is biochemically and functionally analogous to its counterpart in vivo. Immunology. 1998;93:533–539. doi: 10.1046/j.1365-2567.1998.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 27.Haig DM, Huntley JF, MacKellar A, Newlands GF, Inglis L, Sangha R, Cohen D, Hapel A, Galli SJ, Miller HR. Effects of stem cell factor (kit-ligand) and interleukin-3 on the growth and serine proteinase expression of rat bone-marrow-derived or serosal mast cells. Blood. 1994;83:72–83. [PubMed] [Google Scholar]
- 28.Posner RG, Lee B, Conrad DH, Holowka D, Baird B, Goldstein B. Aggregation of IgE-receptor complexes on rat basophilic leukemia cells does not change the intrinsic affinity but can alter the kinetics of the ligand-IgE interaction. Biochemistry. 1992;31:5350–5356. doi: 10.1021/bi00138a015. [DOI] [PubMed] [Google Scholar]
- 29.Gosse JA, Wagenknecht-Wiesner A, Holowka D, Baird B. Transmembrane sequences are determinants of immunoreceptor signaling. J Immunol. 2005;175:2123–2131. doi: 10.4049/jimmunol.175.4.2123. [DOI] [PubMed] [Google Scholar]
- 30.Guo NH, Her GR, Reinhold VN, Brennan MJ, Siraganian RP, Ginsburg V. Monoclonal antibody AA4, which inhibits binding of IgE to high affinity receptors on rat basophilic leukemia cells, binds to novel alpha-galactosyl derivatives of ganglioside GD1b. J Biol Chem. 1989;264:13267–13272. [PubMed] [Google Scholar]
- 31.Pierini L, Holowka D, Baird B. Fc epsilon RI-mediated association of 6-micron beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J Cell Biol. 1996;134:1427–1439. doi: 10.1083/jcb.134.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres AJ, Vasudevan L, Holowka D, Baird BA. Focal adhesion proteins connect IgE receptors to the cytoskeleton as revealed by micropatterned ligand arrays. Proc Natl Acad Sci U S A. 2008;105:17238–17244. doi: 10.1073/pnas.0802138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotlikoff MI. Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology. J Physiol. 2007;578:55–67. doi: 10.1113/jphysiol.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 35.Crivellato E, Baldini G, Basa M, Fusaroli P. The three-dimensional structure of interdigitating cells. Ital J Anat Embryol. 1993;98:243–258. [PubMed] [Google Scholar]
- 36.Demitsu T, Kiyosawa T, Kakurai M, Murata S, Yaoita H. Local injection of recombinant human stem cell factor promotes human skin mast cell survival and neurofibroma cell proliferation in the transplanted neurofibroma in nude mice. Arch Dermatol Res. 1999;291:318–324. doi: 10.1007/s004030050416. [DOI] [PubMed] [Google Scholar]
- 37.Shaikh N, Rivera J, Hewlett BR, Stead RH, Zhu FG, Marshall JS. Mast cell Fc epsilonRI expression in the rat intestinal mucosa and tongue is enhanced during Nippostrongylus brasiliensis infection and can be up-regulated by in vivo administration of IgE. J Immunol. 1997;158:3805–3812. [PubMed] [Google Scholar]
- 38.Kwan CY, Putney JW., Jr. Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990;265:678–684. [PubMed] [Google Scholar]
- 39.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 40.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 41.Bakowski D, Parekh AB. Voltage-dependent Ba2+ permeation through store-operated CRAC channels: implications for channel selectivity. Cell Calcium. 2007;42:333–339. doi: 10.1016/j.ceca.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Basciano LK, Berenstein EH, Kmak L, Siraganian RP. Monoclonal antibodies that inhibit IgE binding. J Biol Chem. 1986;261:11823–11831. [PubMed] [Google Scholar]
- 43.Jamur MC, Grodzki AC, Berenstein EH, Hamawy MM, Siraganian RP, Oliver C. Identification and characterization of undifferentiated mast cells in mouse bone marrow. Blood. 2005;105:4282–4289. doi: 10.1182/blood-2004-02-0756. [DOI] [PubMed] [Google Scholar]
- 44.Enerback L. Mast cells in rat gastrointestinal mucosa. 2 Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- 45.Parker I, Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991;246:269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- 46.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol. 1995;482(Pt 3):533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- 48.Lee HS, Park CS, Lee YM, Suk HY, Clemons TC, Choi OH. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium. 2005;38:581–592. doi: 10.1016/j.ceca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 50.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current I(CRAC) in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels. 2001;7:429–439. [PubMed] [Google Scholar]
- 53.Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW., Jr. Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 54.Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–25030. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 55.Itagaki K, Hauser CJ. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki Y, Yoshimaru T, Inoue T, Nunomura S, Ra C. The high-affinity immunoglobulin E receptor (FcepsilonRI) regulates mitochondrial calcium uptake and a dihydropyridine receptor-mediated calcium influx in mast cells: Role of the FcepsilonRIbeta chain immunoreceptor tyrosine-based activation motif. Biochem Pharmacol. 2008;75:1492–1503. doi: 10.1016/j.bcp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Lin X, Hume RI, Nuttall AL. Dihydropyridines and verapamil inhibit voltage-dependent K+ current in isolated outer hair cells of the guinea pig. Hear Res. 1995;88:36–46. doi: 10.1016/0378-5955(95)00096-m. [DOI] [PubMed] [Google Scholar]
- 60.Jensen BS, Strobaek D, Christophersen P, Jorgensen TD, Hansen C, Silahtaroglu A, Olesen SP, Ahring PK. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am J Physiol. 1998;275:C848–856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- 61.Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- 62.Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 63.Kindzelskii AL, Petty HR. Intracellular calcium waves accompany neutrophil polarization, formylmethionylleucylphenylalanine stimulation, and phagocytosis: a high speed microscopy study. J Immunol. 2003;170:64–72. doi: 10.4049/jimmunol.170.1.64. [DOI] [PubMed] [Google Scholar]
- 64.Petty HR. Letter of retraction. J Immunol. 2009;182:1770. doi: 10.4049/jimmunol.182.3.1770. [DOI] [PubMed] [Google Scholar]
- 65.Lee RJ, Oliver JM. Roles for Ca2+ stores release and two Ca2+ influx pathways in the Fc epsilon R1-activated Ca2+ responses of RBL-2H3 mast cells. Mol Biol Cell. 1995;6:825–839. doi: 10.1091/mbc.6.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499(Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marchant J, Callamaras N, Parker I. Initiation of IP(3)-mediated Ca(2+) waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marchant JS, Parker I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. EMBO J. 2001;20:65–76. doi: 10.1093/emboj/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca(2+) signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- 71.Crivellato E, Finato N, Isola M, Ribatti D, Beltrami CA. Low mast cell density in the human duodenal mucosa from chronic inflammatory duodenal bowel disorders is associated with defective villous architecture. Eur J Clin Invest. 2003;33:601–610. doi: 10.1046/j.1365-2362.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 72.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit Rev Immunol. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen R, Jou ST, Chen Y, Hoffmeyer A, Wang D. Phospholipase C gamma 2 is essential for specific functions of Fc epsilon R and Fc gamma R. J Immunol. 2002;169:6743–6752. doi: 10.4049/jimmunol.169.12.6743. [DOI] [PubMed] [Google Scholar]
- 74.Thrower EC, Hagar RE, Ehrlich BE. Regulation of Ins(1,4,5)P3 receptor isoforms by endogenous modulators. Trends Pharmacol Sci. 2001;22:580–586. doi: 10.1016/s0165-6147(00)01809-5. [DOI] [PubMed] [Google Scholar]
- 75.Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 76.van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 77.Taub D, Dastych J, Inamura N, Upton J, Kelvin D, Metcalfe D, Oppenheim J. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154:2393–2402. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.