Abstract
Background
We have reported that compared with glucose-sweetened beverages, consuming fructose-sweetened beverages with meals results in lower 24-h circulating glucose, insulin and leptin concentrations, and elevated triacylglycerol (TG). However, pure fructose and glucose are not commonly used as sweeteners. High fructose corn syrup (HFCS) has replaced sucrose as the predominant sweetener in beverages in the U.S.
Objective
We compared the metabolic/endocrine effects of HFCS with sucrose, and in a subset of subjects with pure fructose and glucose.
Design
34 men and women consumed 3 isocaloric meals with either sucrose- or HFCS-sweetened beverages, and blood samples were collected over 24 hours. Eight of the male subjects were also studied when fructose- or glucose-sweetened beverages were consumed.
Results
In 34 subjects, 24-h glucose, insulin, leptin, ghrelin and TG profiles were similar between days that sucrose or HFCS were consumed. Postprandial TG excursions after HFCS or sucrose were larger in men than women. In the men in whom the effects of 4 sweeteners were compared, the 24-h glucose and insulin responses induced by HFCS and sucrose were intermediate between the lower responses during consumption of fructose and the higher responses during glucose. Unexpectedly, postprandial TG profiles after HFCS or sucrose were not intermediate, but comparably high as after pure fructose.
Conclusions
Sucrose and HFCS do not have substantially different short-term endocrine/metabolic effects. In male subjects, short-term consumption of sucrose and HFCS resulted in postprandial TG responses comparable to those induced by fructose.
Keywords: glucose, fructose, high fructose corn syrup, sucrose, insulin, leptin, ghrelin, triacylglycerol, free fatty acids, postprandial hypertriacylglycerolemia, humans
Introduction
It has been suggested that the consumption of fructose, which is consumed mainly in the form of HFCS and sucrose, may be a contributing factor to the increased incidence of obesity and metabolic syndrome (1, 2). The current estimate for the mean intake of added sugars by Americans is 15.8% of energy. However this value is based on consumption data from the 1994-1996 Continuing Survey of Food Intakes by Individuals (CSFII) (3), and recent reports suggest that energy intake from sugar-sweetened beverages alone approaches or exceeds 15% of calories in several population groups (4-8). The large standard deviations in several of these reports suggest that at least 16% of the studied populations were consuming greater than two times the mean intake, and likely well over 25% of daily energy requirements from sugar-sweetened beverages (5, 7, 8). Based on these reports it is reasonable to estimate that the percentage of energy consumed as sugar from both beverages and solid food is greater than 20% in a significant portion of the U.S. population.
We have reported that consuming fructose-sweetened beverages with meals results in lower 24-h circulating glucose, insulin and leptin concentrations, and decreased postprandial suppression of plasma ghrelin levels when compared with consumption of glucose-sweetened beverages (9). We and others have reported that consuming fructose-sweetened beverages increases postprandial triacylglycerol (TG) concentration compared with glucose-sweetened beverage (9-11), and that these responses are more pronounced in men compared with women (10, 11), and in overweight/obese subjects compared with normal weight subjects (11). Since insulin, leptin, and possibly ghrelin function as key signals to the CNS in the long-term regulation of energy balance, prolonged consumption of diets high in energy from fructose could lead to increased caloric intake and contribute to weight gain and obesity (2). The sustained elevation of plasma TG concentrations after fructose ingestion suggests that chronic over-consumption of fructose could also contribute to atherogenesis and cardiovascular disease (12, 13). However, pure fructose and pure glucose are not commonly employed as sweeteners. Until a few decades ago, most foods and beverages in the U.S. were sweetened with the disaccharide sucrose, which is composed of 50% glucose and 50% fructose. In 1970 an enzymatic process to convert corn sugar (composed of glucose) into high fructose corn syrup (HFCS) was developed. Since then, HFCS, mainly in the form containing 55% fructose and 45% glucose (HFCS-55), has replaced sucrose as the predominant sweetener used in soft drinks and represents approximately 40% of sweeteners added to foods consumed in the U.S. (14).
There are currently few studies comparing the metabolic and endocrine effects of HFCS with sucrose (15, 16), and to our knowledge, there are no studies comparing the effects of consuming HFCS with pure fructose or glucose. Therefore the objectives of this study were to compare the metabolic and endocrine effects of consuming HFCS- and sucrose-sweetened beverages, and to determine if responses are affected by gender and adiposity. A third objective was to compare the effects of consuming HFCS- and sucrose-sweetened beverages with the consumption of beverages sweetened with fructose or glucose.
Design
Circulating glucose, insulin, leptin, ghrelin, triacylglycerol (TG), and free fatty acid (FFA) concentrations were measured in 34 subjects over a 24-h period on 2 separate days during which they consumed three isocaloric, mixed nutrient meals accompanied by either sucrose-sweetened or HFCS-sweetened beverages. In a subset of 8 male subjects, these variables were also measured on 2 additional days during which these subjects consumed the same isocaloric meals accompanied by beverages sweetened with 100% fructose or 100% glucose.
Subjects
Thirty-four subjects (18 men and 16 women) with an age range of 20–50 yr (Mean: 34.7 ± 1.7y) participated in the study. Participants were recruited through newspaper advertisements and underwent a telephone interview, a complete blood count, and a serum biochemistry panel to assess eligibility. Respondents with anemia, hepatic or renal disease, diabetes mellitus, fasting serum TG levels >400mg/dL, hypertension, eating disorders, or who had surgery for weight loss were excluded from the study. Individuals who smoked, or who took thyroid, lipid-lowering, glucose-lowering, anti-hypertensive, anti-depressant, or weight loss medications, or who were pregnant or lactating were also excluded from participating. The Institutional Review Board of University of California, Davis approved the experimental protocol, and subjects provided informed consent to participate in the study.
Experimental Protocol
Each subject participated in two experimental trials conducted in random order. The experimental days were spaced 1 month apart, and each required an overnight stay in the University of California, General Clinical Research Center (GCRC). During each experimental day the subjects consumed identical meals based on calculated energy requirements (as described below) that included beverages sweetened with either HFCS or sucrose. The 18 male subjects were invited to extend participation in the study, and of these, a subset of 8 men completed 2 additional study days during which they consumed the identical meals accompanied by beverages sweetened with either 100% fructose or glucose. Participation in the additional fructose and glucose trials was limited to male subjects due to budgetary constraints.
Percent body fat was determined by measurement of bioelectrical impedance (BIA) (Tanita 310GS Body Composition Analyzer, Tanita Corp, Tokyo, Japan). BIA measurements of body fat in healthy adults has been shown to correlate well with body fat measurements by duel-energy X-ray absoptiometry (DXA) (17, 18). As there is no consensus on the percent body fat standards for overweight and obesity (19), we used a statistical approach of dividing subjects of each gender into two equal groups based on ranking by percent body fat. Therefore, the men and women were divided into two groups with lower and higher body adiposity based on a percent body fat lesser or greater than 22% and 32%, respectively.
The subjects were instructed to maintain their normal dietary intake and level of physical activity during the interval between the GCRC studies. Following a 12-h overnight fast, the subjects checked into the GCRC at 0700 h. A physical examination was conducted by the study physician, and an intravenous catheter was inserted and kept patent with a slow saline infusion. Blood sampling commenced at 0800 h and continued for 24 hours. Thirty-six blood samples were collected for substrate and hormone measurements over the 24-h sampling period during which the subjects consumed three standardized meals. Each meal was accompanied by a sucrose-, HFCS-, glucose-, or fructose-sweetened beverage.
Meals
The meals consisted of whole foods and were designed by a registered dietitian. The nutrient composition of the diets was determined using the Food Processor SQL, ESHA Research, Inc. (Salem, OR). Each subject ingested three meals per day. Breakfast consisted of scrambled eggs, ham, potatoes, and asparagus. Lunch consisted of chicken/tortilla soup with corn chips and cheese. Dinner consisted of lasagna with beef and a salad with lettuce, tomato, cucumber, celery, cheese and vinaigrette dressing. The energy content of the meals was based on each subject's daily energy requirement as estimated by the Mifflin equation with an activity factor of 1.3 (20). The activity factor was low because the subjects remained relatively sedentary while at the GCRC. Twenty percent of the energy requirement was consumed at breakfast, 35% at lunch, and 45% at dinner. The meals contained 30% energy from fat, 15% from protein, 30% from complex carbohydrate and 25% of energy from sugar. The 25% sugar consisted of sucrose, HFCS, glucose, or fructose in the form of a beverage. It is important to study the effects of sugars at the 25% of energy intake level. The Institute of Medicine of the National Academies in the 2002 Dietary References Intakes concluded that there was insufficient evidence to set an upper intake level for added sugars since there were not specific adverse health outcomes associated with excessive intake (21). Therefore, they suggested a maximal intake level of 25% of energy intake from added sugars. The sucrose and HFCS beverages were provided by the sponsor (PepsiCo, Inc., Purchase, NY) as 11% w/w sugar in non-caffeinated, carbonated sodas. The fructose and glucose were prepared as 11% w/w solutions in carbonated soda water, flavored with a commercial unsweetened drink mix. The subjects and GCRC study staff were blinded to the sweetener contained in the beverages. The beverages were not matched for sweetness, but as the trials were spaced approximately one month apart it not likely the subjects would have noticed a difference in the sweetness of the beverages. The only other beverage served during the 24-h study period was water. Breakfast was consumed at 0900 h, lunch at 1300 h, and dinner at 1800 h. The subjects were required to ingest all of the provided food and beverage within 20 minutes, and were observed to ensure compliance.
Blood sampling
Blood samples were drawn from the catheters at 30-min intervals around periods of meal ingestion and during the predicted nocturnal rise of plasma leptin concentrations (i.e. 5 hours after the evening meal) and at hourly intervals at other times (22). After the three baseline samples, which were collected at 0800, 0830, and 0900 hours before ingestion of the first meal, a total of 33 additional samples were collected 30–60 min apart. Each sample collection involved the removal of 1 ml of blood to clear the catheter tubing, followed by a 5mL collection into blood collection tubes containing EDTA. Samples were then centrifuged, aliquoted, and stored at -80 °C until assayed.
Assays and data analysis
Plasma glucose and lactate concentrations were measured with an automatic analyzer (YSI 2300 StatPlus Glucose Analyzer, Yellow Springs Instruments, Yellow Springs, OH). Insulin and leptin concentration were measured by radioimmunoassay (RIA) (Linco Research, Inc., St Charles, MO). Total ghrelin concentrations were measured in unextracted plasma with an RIA (Phoenix Peptide, Phoenix, AZ). TG concentrations were measured with an automatic analyzer using maunufacturer's reagents (PolyChem Analyzer, PolyMedCo, Inc., Cortlandt Manor, NY). FFAs were measured with enzymatic colormetric reagents (Wako Chemicals, Richmond, VA). Fasting lipid concentrations were measured at baseline and at 0800h the following morning, and apolipoprotein B100 (ApoB) concentrations were measured at baseline and at 2200h with an automatic analyzer (PolyChem Analyzer).
The area under the curve (AUC) was calculated for glucose, insulin, ghrelin, FFA and TG with a data spreadsheet program (Microsoft Excel; Microsoft, Redmond, WA) using the trapezoidal method. The mean of the three baseline values was determined, and net AUC was calculated by subtracting the areas below baseline from AUC values above baseline. AUCs for glucose, insulin, ghrelin, FFA and TG are expressed as units per 23 hours above each subject's fasting baseline levels because the three samples during the first hour determined the baseline levels. The nadirs for plasma leptin were determined as the two lowest consecutive morning values before 1200 h as previously described (22). The AUCs for leptin are therefore expressed as units above each subject's nadir over 24 h. Data from the 34 subjects were analyzed with the statistical software programs SPSS 15.0 (SPSS Inc. Chicago, IL) using the general linear model for repeated measures that included a sugar × adiposity group × gender interaction. Age was also included in the analysis with subjects grouped by 20-29, 30-39 and 40-50 years. Time was included as a within subject factor to assess change from fasting levels in lipid concentrations. When the general linear model results indicated the presence of a significant sugar effect, or gender or body adiposity interaction, paired and unpaired t tests were performed within group, or between men and women or between adiposity groups. Differences between the responses to the four sugars in the subset of 8 male subjects were assessed with GraphPad Prism v.4.03 (San Diego, CA, USA) using repeated measures 1-factor ANOVA, and post-tests were performed using Tukey's test for multiple comparisons.
Results
Baseline measures
The age, body weight (BW), percent body fat and body mass index (BMI) of the subjects are presented in Table 1. Body weight and baseline fasting plasma concentrations of glucose, hormones, and lipids are shown in Table 2 for the sucrose and HFCS study days. Fasting TG and apoB concentrations were lower in women (-14.1±6.5 and -12.1 ± 4.2 mg/dl respectively; p<0.05, Paired t test) and higher in men (12.0±5.1 and 6.9 ± 3.4 mg/dl respectively; p<0.05, Paired t test) on the day of the sucrose feeding compared with the day of the HFCS feeding. There were no differences in baseline concentrations of the other measured parameters on the two study days.
TABLE 1.
Age, body weight and adiposity of study participants
| Women <32% BF | Women >32% BF | Men <22% BF | Men >22% BF | Men 4-Sugar Subset | |
|---|---|---|---|---|---|
| n | 8 | 8 | 9 | 9 | 7 |
| Age (y) | 29.1 ± 3.6 | 39 ± 3 | 38 ± 4 | 31 ± 3 | 37.1 ± 4.3 |
| BW (kg)+++ | 60.2 ± 1.7 | 76.4 ± 4.0** | 73.7 ± 3.0 | 88.5 ± 3.6** | 82.0 ± 5.2 |
| Body Fat (%)+++ | 25.7 ± 1.4 | 39.2 ± 1.7*** | 18.3 ± 1.1 | 25.1 ± 1.1*** | 21.0 ± 2.5 |
| BMI (kg/m2)+++ | 21.9 ± 0.6 | 28.9 ± 1.6*** | 24.3 ± 0.5 | 28.0 ± 0.7*** | 26.1 ± 1.0 |
Mean ± SEM
Within gender groups, subjects ranked by %body fat and divided into 2 equal groups.
General linear model - Univariate with gender and adiposity group as fixed factors.
p<0.0001 for differences among the 4 gender/weight groups Within gender unpaired t tests for adiposity group differences:
p<0.01
p<0.001
TABLE 2.
Baseline body weight and fasting plasma concentrations of glucose, hormones, and lipids on each study day
| All | Women <32% BF | Women >32% BF | Men <22% BF | Men >22% BF | ||
|---|---|---|---|---|---|---|
| n | 34 | 8 | 8 | 9 | 9 | |
| Body Weight (kg) | Sucrose | 75.2 ± 2.4 | 60.1 ± 1.7 | 76.1 ± 4.2 | 74.0 ± 3.0 | 89.0 ± 3.5 |
| HFCS | 74.9 ± 2.3 | 60.2 ± 1.7 | 76.6 ± 3.8 | 73.4 ± 2.9 | 88.0 ± 3.6 | |
| Glucose (mg/dl) | Sucrose | 88.0 ± 1.4 | 82.5 ± 1.4 | 86.4 ± 2.3 | 89.5 ± 2.3 | 92.9 ± 3.7 |
| HFCS | 86.8 ± 1.4 | 81.5 ± 1.8 | 84.6 ± 2.8 | 87.7 ± 1.2 | 92.6 ± 3.3 | |
| Insulin (μU/ml) | Sucrose | 11.6 ± 1.2 | 9.5 ± 0.6 | 14.9 ± 2.7 | 7.6 ± 1.2 | 14.4 ± 3.4 |
| HFCS | 11.4 ± 1.3 | 9.2 ± 0.7 | 14.8 ± 2.9 | 7.3 ± 0.9 | 14.5 ± 3.6 | |
| Leptin (ng/ml) | Sucrose | 10.8 ± 1.6 | 8.5 ± 1.4 | 23.7 ± 3.9 | 3.6 ± 0.4 | 8.6 ± 1.3 |
| HFCS | 10.1 ± 1.4 | 8.3 ± 1.1 | 20.9 ± 2.9 | 3.5 ± 0.5 | 8.5 ± 1.5 | |
| Ghrelin (pg/ml) | Sucrose | 441.0 ± 36.4 | 613.2 ± 111.3 | 414.9 ± 60.6 | 396.7 ± 50.9 | 355.6 ± 31.9 |
| HFCS | 435.4 ± 30.6 | 590.0 ± 75.2 | 398.9 ± 66.5 | 399.8 ± 47.7 | 365.9 ± 29.9 | |
| TG1 (mg/dl) | Sucrose | 107.7 ± 10.4 | 78.2 ± 9.7 | 94.5 ± 16.0 | 106.1 ± 20.9 | 147.1 ± 25.9 |
| HFCS | 109.2 ± 9.1 | 98.9 ± 12.2 | 102.9 ± 10.24 | 92.0 ± 17.5 | 135.5 ± 23.64 | |
| FFA (mEq/l) | Sucrose | 0.34 ± 0.02 | 0.39 ± 0.06 | 0.39 ± 0.04 | 0.30 ± 0.05 | 0.30 ± 0.03 |
| HFCS | 0.37 ± 0.02 | 0.41 ± 0.05 | 0.43 ± 0.04 | 0.32 ± 0.04 | 0.32 ± 0.03 | |
| Cholesterol2 (mg/dl) | Sucrose | 176.1 ± 5.5 | 153.4 ± 6.0 | 181.8 ± 14.5 | 186.4 ± 9.0 | 180.9 ± 11.0 |
| HFCS | 176.8 ± 5.7 | 156.9 ± 4.2 | 199.4 ± 13.95 | 175.8 ± 11.0 | 175.6 ± 10.75 | |
| LDL (mg/dl) | Sucrose | 107.7 ± 4.4 | 86.1 ± 5.9 | 106.6 ± 8.8 | 120.9 ± 7.8 | 114.7 ± 8.9 |
| HFCS | 108.0 ± 5.1 | 85.9 ± 5.7 | 120.0 ± 12.8 | 113.8 ± 10.4 | 111.3 ± 8.0 | |
| HDL (mg/dl) | Sucrose | 47.0 ± 1.8 | 51.6 ± 3.9 | 56.1 ± 3.2 | 44.3 ± 2.2 | 37.3 ± 1.2 |
| HFCS | 47.4 ± 1.9 | 52.0 ± 3.7 | 58.4 ± 3.8 | 44.0 ± 1.7 | 37.1 ± 1.6 | |
| ApoB3 (mg/dl) | Sucrose | 76.4 ± 4.4 | 56.3 ± 4.9 | 72.3 ± 8.9 | 95.1 ± 6.2 | 79.4 ± 9.6 |
| HFCS | 77.6 ± 4.3 | 60.6 ± 5.3 | 88.5 ± 9.34 | 87.0 ± 8.5 | 73.7 ± 8.64 |
Mean ± SEM.
General linear model for repeated measures for the interaction of sugar and gender, adiposity and age group.
Sugar p = 0.37; Sugar × Gender p < 0.001; Sugar × Age p=0.012; Sugar × Gender × weight × Age p=0.019.
Sugar p = 0.83; Sugar × Gender p = 0.038.
Sugar p = 0.76; Sugar × Gender p = 0.0034.
Significant Sugar × Gender differences tested for within gender group difference by paired t test (Female: n=16, Male: n=18; p<0.05).
Significant Sugar × Gender differences tested for within gender group difference by paired t test (Female: n=16, Male: n=18; NS).
Effects of HFCS and sucrose – 24-h profiles
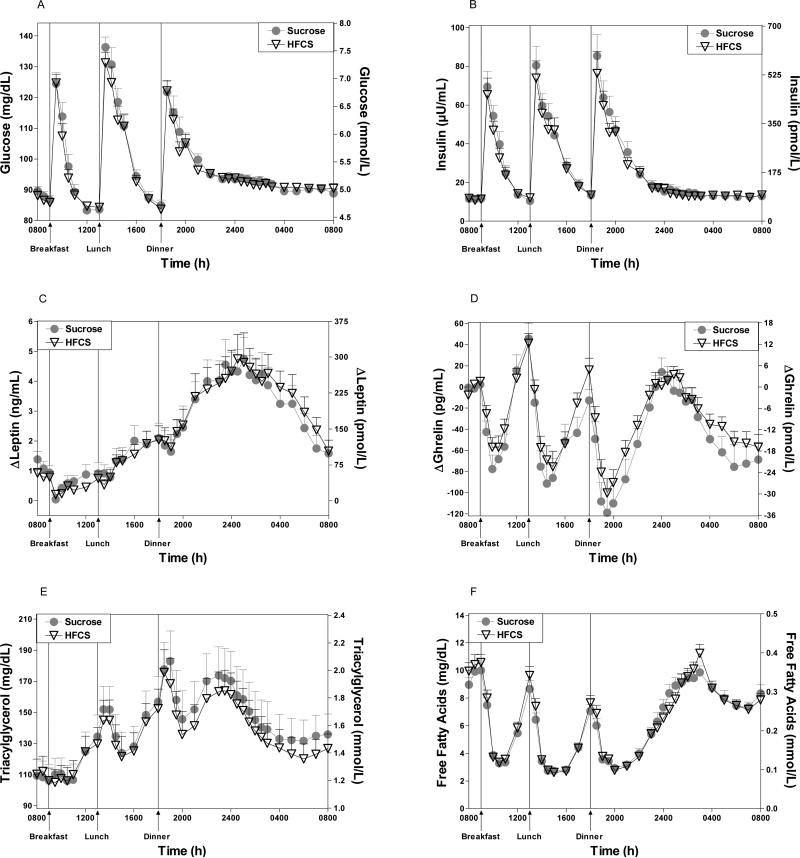
Twenty-four hour plasma profiles of glucose, leptin, ghrelin, TG, and FFA concentrations, as assessed by AUC, were not different on the days that HFCS-sweetened beverages were consumed with meals, compared to when sucrose-sweetened beverages were consumed (Figures 1A-F, Table 3). The insulin AUC was slightly, but significantly, increased by consumption of sucrose-sweetened beverages compared with HFCS-sweetened beverages. This difference was significant in subjects younger than 35 years (p<0.01; n=17), but was not significant in subjects older than 35 years (p=0.5; n=17).
Figure 1.
Plasma glucose (1A), insulin (1B), TG (1E), FFA (1F) concentrations during a 24-h period (0800–0800 h) in 34 women and men consuming HFCS- or sucrose-sweetened beverages with each meal. Change of plasma leptin (1C) over the morning nadir and ghrelin concentrations (1D) from mean baseline levels (0800–0900 h) during a 24-h period (0800–0800 h) in 34 women and men consuming HFCS- or sucrose-sweetened beverages with each meal. Data shown as Mean ± SEM.
TABLE 3.
24-h AUC of endocrine/metabolic variables during consumption of sucrose- and HFCS-sweetened beverages with meals
| All | Women <32% BF | Women >32% BF | Men <22% BF | Men >22% BF | ||
|---|---|---|---|---|---|---|
| AUC | n | 34 | 8 | 8 | 9 | 9 |
| Glucose (mg/dl*23hr) | Sucrose | 222.2 ± 27.2 | 278.7 ± 41.1 | 243.0 ± 55.6 | 209.6 ± 41.7 | 166.2 ± 72.6 |
| HFCS | 226.8 ± 25.2 | 299.6 ± 40.4 | 276.0 ± 46.5 | 210.5 ± 52.4 | 134.6 ± 47.0 | |
| Insulin1 (μU/ml*23hr) | Sucrose | 381.1 ± 48.9 | 321.2 ± 64.5 | 544.3 ± 164.4 | 237.7 ± 31.6 | 432.6 ± 75.7 |
| HFCS | 344.4 ± 43.3 | 255.0 ± 42.9 | 515.9 ± 148.9 | 221.1 ± 25.3 | 394.8 ± 59.5 | |
| Leptin2 (ng/ml*24hr) | Sucrose | 57.0 ± 10.4 | 56.4 ± 18.0 | 120.1 ± 28.2 | 19.2 ± 3.9 | 39.3 ± 11.34 |
| HFCS | 57.2 ± 9.5 | 61.3 ± 13.8 | 117.6 ± 24.4 | 18.6 ± 5.0 | 38.5 ± 11.25 | |
| Ghrelin (pg/ml*23hr) | Sucrose | -933.6 ± 234.8 | -1,677.9 ± 786.1 | -841.1 ± 294.2 | -685.9 ± 350.1 | -602.0 ± 323.9 |
| HFCS | -897.2 ± 188.1 | -1,331.7 ± 358.4 | -547.2 ± 496.6 | -946.2 ± 341.2 | -773.2 ± 326.7 | |
| TG3 (mg/dl*23hr) | Sucrose | 793.3 ± 135.8 | 359.5 ± 153.1 | 398.7 ± 145.3 | 1,017.3 ± 344.5 | 1,305.8 ± 238.44 |
| HFCS | 611.1 ± 132.1 | -29.2 ± 184.2 | 315.2 ± 257.2 | 978.2 ± 177.4 | 1,076.2 ± 251.85 | |
| FFA2 (mEq/l*23hr) | Sucrose | -3.0 ± 0.4 | -3.7 ± 1.1 | -3.8 ± 0.9 | -2.4 ± 0.6 | -2.2 ± 0.36 |
| HFCS | -3.5 ± 0.4 | -4.3 ± 1.1 | -4.7 ± 0.8 | -2.3 ± 0.5 | -2.8 ± 0.34 |
Mean ± SEM
General linear model for repeated measures for the interaction of sugar and gender, adiposity and age group.
Sugar p = 0.016; Sugar × Age p < 0.001.
Sugar × Age interaction for insulin tested for within age group difference by paired t test (cutoff 35 years; n=17/group).
Subjects ± 35 years: Mean insulin AUC = 398.5±53.4 μU/ml*23hr sucrose, 310.6±39.4 μU/ml*23hr HFCS; p=0.0011.
Subjects ± 35 years: Mean insulin AUC = 363.6±83.5 μU/ml*23hr sucrose, 378.2±77.7 μU/ml*23hr HFCS; p=0.51.
All other Sugar × between subject factors p > 0.05.
Gender difference by general linear model (p<0.05).
Gender difference by general linear model (p<0.01).
Variables with significant gender differences tested for between gender group difference by unpaired t test (Female: n=16, Male: n=18; p<0.01).
Variables with significant gender differences tested for between gender group difference by unpaired t test (Female: n=16, Male: n=18; p<0.001).
Variables with significant gender differences tested for between gender group difference by unpaired t test (Female: n=16, Male: n=18; NS).
All other between subject factors and 2- and 3-way interactions p > 0.05.
Effects of HFCS and sucrose – Changes of fasting lipid concentrations
There were no differences between the effects of consuming sucrose- or HFCS-sweetened beverages with 3 meals on changes in fasting lipid concentrations from baseline to the following morning (0800h) for plasma TG, cholesterol, LDL or HDL concentrations, and on changes between baseline and postprandial concentrations of ApoB at 2200h (Table 4). Consumption of both sucrose and HFCS significantly increased fasting TG concentrations the following morning (Sucrose: +28.3 ± 5.4 mg/dl, p<0.001; HFCS +18.9 ± 4.5 mg/dl, p<0.001) and decreased postprandial ApoB concentrations at 2200h (Sucrose: -5.8 ± 1.3 mg/dl, p<0.001; HFCS –6.0 ± 1.9 mg/dl, p<0.01).
TABLE 4.
Absolute change from baseline lipid concentrations after consumption of sucrose- and HFCS-sweetened beverages with meals. TG, Cholesterol, LDL, HDL: Concentration at 0800h the following day minus baseline concentration. ApoB: Concentration at 2200h (4 hours after dinner) minus baseline concentration.
| All Subjects | Women <32% BF | Women >32% BF | Men <22% BF | Men >22% BF | ||
|---|---|---|---|---|---|---|
| n | 34 | 8 | 8 | 9 | 9 | |
| TG1 (mg/dl) | Sucrose | 28.3 ± 5.4 | 15.2 ± 4.1 | 29.5 ± 9.5 | 19.0 ± 9.6 | 48.3 ± 14.1 |
| HFCS | 18.9 ± 4.5 | -2.8 ± 7.1 | 16.8 ± 6.6 | 36.6 ± 9.5 | 22.3 ± 8.1 | |
| Cholesterol (mg/dl) | Sucrose | 2.4 ± 2.8 | -5.3 ± 6.0 | 2.9 ± 4.5 | -1.1 ± 6.3 | 12.1 ± 4.7 |
| HFCS | 1.1 ± 2.5 | -7.5 ± 7.1 | 5.8 ± 5.3 | 3.2 ± 3.2 | 2.3 ± 3.9 | |
| LDL (mg/dl) | Sucrose | -2.9 ± 1.9 | -5.1 ± 4.1 | -4.0 ± 3.1 | -3.6 ± 5.0 | 0.9 ± 2.7 |
| HFCS | -2.5 ± 1.7 | -4.4 ± 4.0 | 0.8 ± 3.7 | -3.7 ± 2.8 | -2.4 ± 3.2 | |
| HDL (mg/dl) | Sucrose | -0.4 ± 0.8 | -3.1 ± 2.3 | 1.3 ± 1.6 | -1.4 ± 1.1 | 1.4 ± 1.0 |
| HFCS | -0.6 ± 0.8 | -3.3 ± 2.2 | 1.6 ± 1.7 | -0.9 ± 1.2 | 0.1 ± 1.3 | |
| ApoB2 (mg/dl) | Sucrose | -5.8 ± 1.3 | -5.9 ± 1.1 | -3.75 | -10.7 ± 3.7 | -2.8 ± 2.2 |
| HFCS | -6.0 ± 1.9 | -10.5 ± 4.4 | -5.25 | -5.9 ± 2.6 | -2.8 ± 3.5 |
Mean ± SEM.
General linear model for repeated measures for the interaction of sugar and gender, adiposity and age group.
Sugar p = 0.43; Time p < 0.0001; Sugar × Time p = 0.23.
Sugar p = 0.81; Time p < 0.0001; Sugar × Time p = 0.89.
All Sugar × Time × Between Subject Factors p > 0.05.
Effects of HFCS- and sucrose-sweetened beverages in men and women, and adiposity groups
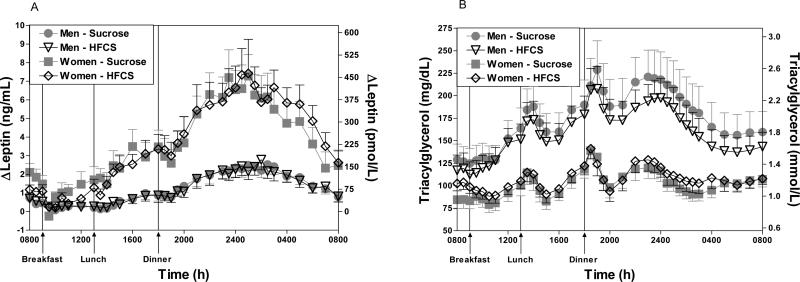
The 24-h AUCs of glucose, insulin, leptin, ghrelin, TG or FFA were not significantly different between the lower and higher adiposity groups. The 24-h leptin AUC in women was approximately 3 times higher than in men (p<0.01, Unpaired t test) during consumption of both HFCS and sucrose (Table 3, Figure 2A). There was a marked gender difference in 24-h TG AUC, with a mean AUC during sucrose consumption 3 times higher (p<0.01) and during HFCS consumption 7 times higher (p<0.001, Unpaired t test) in men than in women (Table 3, Figure 2B). The AUC for FFA was less suppressed (i.e., less negative) in men compared with women (Table 3), with the effect being statistically significant during HFCS consumption (Men: -2.6± 0.3mmol/L•23h; Women: -4.5± 0.6mmol/L•23h, p<0.05), but not during sucrose consumption (Men: -2.3±0.3 mmol/L•23h; Women: -3.7±0.7 mmol/L•23h, NS, Unpaired t test).
Figure 2.
Change of plasma leptin concentrations (2A) over the morning nadir during a 24-h period (0800–0800 h) in 16 women and 18 men consuming HFCS- or sucrose-sweetened beverages with each meal. Plasma TG concentrations (2B) during a 24-h period (0800–0800 h) in 16 women and 18 men consuming HFCS- or sucrose-sweetened beverages with each meal. Data shown as Mean ± SEM.
Effects of HFCS-, sucrose-, fructose- and glucose-sweetened beverages
One of the male subjects who participated in four 24-h blood collections while consuming sucrose, HFCS, fructose or glucose had mild hypertriacylglycerolmia (Fasting [TG] = 196 mg/dl), therefore this subject's results were not included in the final analyses. Figure S1 under “Supplemental data” in the current online issue at www.ajcn.org presents the 24-h profile graphs from this subject. The characteristics of the 7 male subjects with normal fasting TG concentrations (< 150 mg/dl – U.S. National Cholesterol Education Program Guideline) are presented in Table 1. There were no significant differences in body weight or baseline concentrations of any of the measured parameters across the four study days (Table 5).
TABLE 5.
Baseline body weight and plasma concentrations of glucose, hormones, and lipids on each study day in 7 men
| Beverage | Body Weight (kg) | Glucose (mg/dl) | Insulin (μU/ml) | Leptin (ng/ml) | Ghrelin (pg/ml) | TG (mg/dl) | FFA (mEq/l) |
|---|---|---|---|---|---|---|---|
| Glucose | 82.4 ± 5.5 | 92.0 ± 1.5 | 9.2 ± 1.3 | 5.4 ± 1.1 | 316.6 ± 76.3 | 91.4 ± 11.0 | 0.32 ± 0.04 |
| Fructose | 81.3 ± 5.8 | 93.7 ± 2.1 | 10.2 ± 2.1 | 5.5 ± 1.3 | 316.9 ± 73.3 | 102.0 ± 13.6 | 0.34 ± 0.04 |
| Sucrose | 82.0 ± 5.6 | 93.5 ± 2.4 | 10.5 ± 1.5 | 4.8 ± 1.0 | 312.6 ± 53.6 | 96.7 ± 9.0 | 0.26 ± 0.04 |
| HFCS | 81.5 ± 5.6 | 90.3 ± 2.4 | 9.0 ± 1.7 | 4.8 ± 1.3 | 306.3 ± 52.4 | 87.4 ± 9.0 | 0.29 ± 0.04 |
| Repeated measures 1-factor ANOVA | p = 0.50 | p = 0.39 | p = 0.56 | p = 0.56 | p = 0.98 | p = 0.47 | p = 0.10 |
Mean ± SEM
There were no significant differences in baseline body weight and plasma concentrations of glucose, hormones, and lipids on the 4 study days.
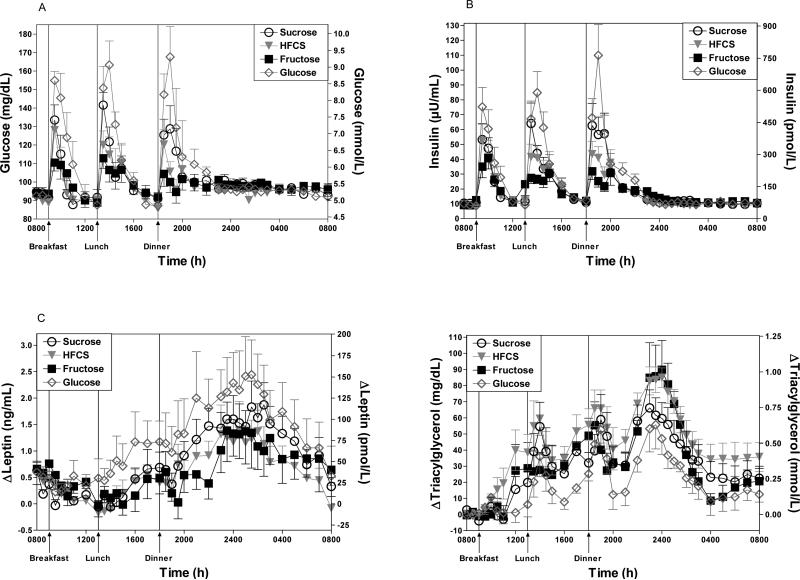
The effects of the four sugars on 24-h AUC were significantly different for glucose (p = 0.010), insulin (p<0.0001) and TG (p = 0.007) by repeated measure 1-factor ANOVA, while there were no differences in the AUCs for leptin, ghrelin or FFA (Figures 3A-D, Table 6). Consumption of fructose-sweetened beverages resulted in glucose and insulin AUCs that were significantly lower than the AUCs induced by glucose consumption (p<0.01, p<0.001 glucose and insulin respectively, Tukey's post test). As expected consumption of the HFCS- and sucrose-sweetened beverages resulted in glucose and insulin AUCs that tended to be intermediate between the higher AUCs induced by glucose consumption and the lower AUCs induced by fructose. However, the glucose AUCs were lower during consumption of sucrose and HFCS than would be expected based on the relative glucose concentrations of the four beverages. The 24-h AUCs for plasma glucose concentrations averaged 76% of the expected AUC (mean of the glucose AUCs during fructose and glucose consumption) during sucrose consumption, and 81% of the expected AUC (0.55 X fructose mean + 0.45 X glucose mean) during HFCS consumption. Accordingly, the insulin AUCs during sucrose and HFCS consumption were also lower than expected (84% of the expected the value for sucrose; 83% of expected for HFCS). The 24-h postprandial TG profiles on the days the subjects consumed HFCS or sucrose were not intermediate between those of glucose and fructose as expected, but were comparable to the magnitude of the profile following consumption of 100% fructose beverages (Figure 3D). The TG 24-h AUC during HFCS consumption was significantly higher than the AUC during glucose consumption (p<0.01, Tukey's post tests) (Table 6).
Figure 3.
Plasma glucose (3A) and insulin (3B) concentrations during a 24-h period (0800–0800 h) in 7 men consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal. Change of plasma leptin concentration (3C) over the morning nadir and plasma TG (3D) concentrations from mean baseline levels (0800–0900 h) during a 24-h period (0800–0800 h) in 7 men consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal. Data shown are Mean ± SEM.
TABLE 6.
24-h AUC of endocrine/metabolic variables during consumption of sucrose-, HFCS-, fructose-, and glucose-sweetened beverages with meals in 7 men
| Beverage | Glucose (mg/dl•23h) | Insulin (μU/ml•23h) | Leptin (ng/ml•24h) | Ghrelin (pg/ml•23h) | TG (mg/dl•23h) | FFA (mEq/l•23h) |
|---|---|---|---|---|---|---|
| Glucose | 344.6 ± 73.7 | 399.6 ± 58.5 | 29.7 ± 10.1 | -874.1 ± 614.1 | 458.3 ± 89.1 | -1.8 ± 0.6 |
| Fructose | 113.8 ± 53.7* | 181.8 ± 20.0* | 14.9 ± 6.7 | -1,120.8 ± 634.1 | 751.2 ± 150.2 | -2.7 ± 0.5 |
| Sucrose | 175.1 ± 48.9 | 242.7 ± 33.2* | 19.2 ± 5.2 | -570.0 ± 311.4 | 738.7 ± 170.7 | -1.6 ± 0.5 |
| HFCS | 177.7 ± 79.0 | 230.6 ± 42.9* | 13.5 ± 3.7 | -640.2 ± 422.2 | 1,043.5 ± 132.0* | -2.0 ± 0.4 |
| Repeated measures 1-factor ANOVA | p = 0.010 | p < 0.0001 | p = 0.081 | p = 0.27 | p = 0.0065 | p = 0.31 |
Mean ± SEM.
p <0.05
p <0.01
p <0.001 compared to glucose (reference) using the Tukey's Multiple Comparison Test.
Consumption of all four sugar-sweetened beverages with meals also resulted in significant increases in the fasting TG concentrations the following morning (0800h), but there were no differences among the effects of the four sugars. Similarly, all four sugars decreased postprandial ApoB concentrations (2200h), however, the sugar x time interaction was not statistically significant (Table 7).
TABLE 7.
Absolute change from baseline lipid concentrations after consumption of glucose-, fructose-, sucrose- and HFCS-sweetened beverages with meals. TG: Concentration at 0800h the following day minus baseline concentration. ApoB: Concentration at 2200h (4 hours after dinner) minus baseline concentration.
| Beverages n=7 | TG (mg/dl)1 | ApoB (mg/dl)2 |
|---|---|---|
| Glucose | 12.7 ± 4.5 | -3.3 ± 1.3 |
| Fructose | 20.9 ± 7.4 | -7.6 ± 2.7 |
| Sucrose | 22.4 ± 7.0 | -6.4 ± 2.7 |
| HFCS | 35.7 ± 8.4 | -12.6 ± 4.0 |
Mean ± SEM
General linear model for repeated measures.
Sugar: p = 0.87; Time: p < 0.0001; Sugar × Time: p = 0.21
Sugar: p = 0.95; Time: p < 0.0001; Sugar × Time: p = 0.22
Discussion
Effects of HFCS and Sucrose
The 24-h postprandial glucose, leptin, and ghrelin profiles during the consumption of HFCS-sweetened beverages were not significantly different from those when sucrose-sweetened beverages were consumed. These results are similar to those of Melanson et al, who investigated sucrose and HFCS consumption at 30% of energy by 30 normal weight women (16). Our results extend their findings to men and to overweight and obese subjects. The insulin AUC was slightly, but significantly, increased during consumption of sucrose- compared with HFCS-sweetened beverages. This increase is not unexpected given that sucrose contains 10% more glucose than HFCS, however Melanson et al did not report a similar increase in their study (16). Consumption of HFCS and sucrose also resulted in similar 24-h TG and FFA AUCs, variables that were not examined in the study by Melanson.
Fasting plasma TG concentrations at 0800 hours the following morning were increased compared to baseline levels after both sucrose and HFCS consumption. In the subset of 7 men, consumption of all four sugars resulted in significant increases of fasting TG the following morning. These increases in fasting TG induced by 24-h exposure to sugar-sweetened beverages may be transitory. We and other investigators have reported that long term consumption (≥2 weeks) of fructose at 20-25% of energy did not increase fasting TG concentrations in healthy subjects (23), in older overweight and obese subjects (24, 25), in hyperinsulinemic female subjects (26), and in patients with type 2 diabetes (27, 28) and hypertriacylglycerolemia (29). However, in other studies, 2 or more weeks of fructose consumption at 15-20% of energy has increased fasting TG concentrations in healthy (10, 30-32) and hyperinsulinemic male subjects (32, 33), and in subjects with type 2 diabetes (34). The reasons underlying these conflicting results are not clear. Interestingly, postprandial concentrations of ApoB, measured 4 hours after dinner, were significantly decreased compared with baseline levels following consumption of HFCS or sucrose in the 34 subjects, and following consumption of all four sugars in the subset of 8 male subjects. These data also contrast with results from our two long-term studies in which consumption of fructose-sweetened beverages for 10 weeks increased both fasting and postprandial ApoB concentrations, whereas consuming glucose-sweetened beverages did not (24, 25). These differences in the effects of short-term and long-term sugar exposure on ApoB and fasting TG concentrations demonstrate the need for long term studies with both fasting and postprandial measurements to determine the metabolic effects of prolonged daily consumption of sugar-sweetened beverages.
Effects of gender
The higher leptin AUC in women compared to men was not unexpected as gender differences in leptin are well established (35, 36). Men had higher postprandial TG responses than women and this gender difference has been previously demonstrated by us in 24-h study of overweight and obese men and pre-menopausal women (11). Bantle et al reported that men had increased postprandial TG levels during consumption of a 6-week, 17% of energy fructose diet, but this was not observed in women (10). Thus, available data suggest that men are more susceptible than women to the effects of sugars containing fructose to increase plasma TG concentrations. Whether this gender difference exists in older male compared with post-menopausal female subjects remains to be determined.
Effects of fructose, glucose, sucrose, and HFCS
As expected, the responses of circulating glucose and insulin induced by consumption of sucrose and HFCS were relative to their glucose and fructose contents, and thus were intermediate to the larger responses induced by glucose and the lower responses after fructose. We had expected that the TG profiles would be directly related to the fructose content of the beverages consumed. We and Bantle et al have previously demonstrated that fructose consumption increases postprandial TG compared with glucose consumption (9-11, 25). A likely mechanism is increased hepatic de novo lipogenesis (DNL), which has been reported to increase markedly during fructose ingestion compared to glucose ingestion (37).
In the 7 male subjects who participated in the comparison of all 4 sugars, the 24-h postprandial TG responses to HFCS and sucrose were not intermediate between those induced by fructose and glucose. Sucrose and HFCS resulted in postprandial TG responses that were comparable to pure fructose alone, and consumption of HFCS-sweetened beverages significantly increased 24-h TG AUCs compared with glucose.
It is possible the mechanism by which sucrose and HFCS increase postprandial TG comparably to pure fructose may involve fructose-stimulated hepatic glucose uptake. Low dose IV infusion of fructose has been reported to increase portal uptake of glucose in dogs (38), and oral fructose has been shown to lower peripheral plasma glucose responses to oral glucose ingestion in humans (39). Data from the present study suggest that additional glucose may have been taken up and metabolized in the liver when it was consumed concurrently with fructose as the consumption of sucrose and HFCS resulted in 24-h glucose and insulin AUCs that were less than would be expected based on their glucose and fructose content, and the AUCs measured during consumption of the beverages sweetened with 100% glucose or fructose.
Activation of sterol receptor element binding protein-1c (SREBP-1c), the major transcriptional regulator of fatty acid synthesis, may also contribute to the larger than expected postprandial TG responses during sucrose and HFCS consumption. Matsuzaka et al (40) have reported that glucose, fructose and sucrose increased hepatic SREBP-1c mRNA expression independently of insulin in streptozotocin diabetic mice. Interestingly, they found that the timing of the SREBP-1c upregulation varied following sugar feeding. Glucose and sucrose feeding induced maximal expression of SREBP-1c within 6 hours (4- to 7-fold increase), while maximal expression following fructose feeding occurred at 12 hours (5-fold increase) (40).
Results from our two studies comparing the long-term (10 week) effects of fructose and glucose consumption demonstrate that the marked postprandial hypertriacylglycerolemia induced by fructose consumption is not a transitory response (24, 25). Similar studies are needed to determine if the postprandial hypertriacylglycerolemia induced by HFCS and sucrose consumption is also maintained with more prolonged exposure. There is growing evidence to link postprandial lipemia with proatherogenic conditions (12, 13, 41-44). Two recent publications provide clinical evidence to support the association between elevated concentrations of postprandial TG and increased risk of cardiovascular disease. In a prospective cohort study of 7587 women and 6394 men followed for 26 years, elevated nonfasting TG levels were associated with increased risk of myocardial infarction, ischemic heart disease and death (13). In the Women's Health Study, 26,509 women were followed for 11 years (12). Nonfasting TG levels, but not fasting levels, were associated with incident cardiovascular events, independent of traditional cardiac risk factors, levels of other lipids and markers of insulin resistance (12). In both of these studies there was a significant linear relationship between increased nonfasting TG and increased hazard ratio for all outcomes (12, 13).
Conclusions
While consumption of sucrose compared with HFCS-sweetened beverages induced a small increase in the 24-h insulin AUC in 34 subjects, the effects of sucrose and HFCS on 24-h circulating glucose, leptin, and ghrelin concentrations were not otherwise different. Thus, it appears that sucrose and HFCS do not have substantially different short-term effects on endocrine signals involved in body weight regulation. Consumption of HFCS beverages also did not increase postprandial TG levels to a greater extent than that observed during consumption of sucrose-sweetened beverages.
Comparison of the effects of glucose, fructose, sucrose and HFCS beverages within the same male subjects demonstrated that postprandial glucose and insulin responses were intermediate between the lower responses induced by pure fructose and the larger responses induced by pure glucose. Unexpectedly, the effects of short-term consumption of HFCS and sucrose on postprandial TG levels were not intermediate to those of fructose and glucose, but comparable to fructose alone. Studies to determine whether these high postprandial TG levels are sustained during long-term consumption of sucrose and HFCS are needed. Additional studies in women and in subjects with and without components of the metabolic syndrome, as well as dose-response studies, are needed to more fully understand the metabolic effects of fructose-containing sugars.
Supplementary Material
Figure S1: Plasma glucose (S1A) and insulin (S1B) concentrations during a 24-h period (0800–0800 h) in hypertriacylglycerolemic male subject (fasting [TG] = 198 mg/dl) consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal. Change of plasma leptin concentration (S1C) over the morning nadir and plasma TG (S1D) concentrations from mean baseline levels (0800–0900 h) during a 24-h period (0800–0800 h) in hypertriacylglycerolemic male subject consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal.
Acknowledgments
The authors thank James Graham, Marinelle Nuñez, Theresa Tonjes, and Nicole Mullen and the nursing staff at GCRC for their excellent technical and nursing support. We also thank Janet Peerson for her expert advice on the statistical analysis of the data, and Dr. Dean Ornish for his contribution to initiation of the study.
This research was supported in part with funding from Pepsico, Inc., Purchase NY.
The project also received support from the UC Davis Clinical and Translational Science Center (Grant Number UL1 RR024146) and the US Department of Agriculture. Dr. Havel's laboratory also receives support from NIH grants R01 HL075675, 5R21AT2500, 1R21AT002993, 1R21AT003545 and the American Diabetes Association.
Footnotes
Publisher's Disclaimer: “This is an un-copyedited author manuscript that has been accepted for publication in The American Journal of Clinical Nutrition, copyright American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://www.ajcn.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.”
References
- 1.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–57. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51. doi: 10.1016/S0002-8223(00)00018-3. quiz 49-50. [DOI] [PubMed] [Google Scholar]
- 4.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 5.Mundt CA, Baxter-Jones AD, Whiting SJ, Bailey DA, Faulkner RA, Mirwald RL. Relationships of activity and sugar drink intake on fat mass development in youths. Med Sci Sports Exerc. 2006;38:1245–54. doi: 10.1249/01.mss.0000227309.18902.fe. [DOI] [PubMed] [Google Scholar]
- 6.Striegel-Moore RH, Thompson D, Affenito SG, et al. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2006;148:183–7. doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 7.van der Horst K, Kremers S, Ferreira I, Singh A, Oenema A, Brug J. Perceived parenting style and practices and the consumption of sugar-sweetened beverages by adolescents. Health Educ Res. 2006 doi: 10.1093/her/cyl080. [DOI] [PubMed] [Google Scholar]
- 8.West DS, Bursac Z, Quimby D, et al. Self-reported sugar-sweetened beverage intake among college students. Obesity (Silver Spring) 2006;14:1825–31. doi: 10.1038/oby.2006.210. [DOI] [PubMed] [Google Scholar]
- 9.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 10.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–34. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 11.Teff KL, Keim NL, Townsend RR, Havel PJ. Fructose-sweetened beverages decrease circulating leptin levels and increase postprandial triglycerides in obese men and women. Diabetes. 2005;54:A385. [Google Scholar]
- 12.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. Jama. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Fields S. The fat of the land: do agricultural subsidies foster poor health? Environ Health Perspect. 2004;112:A820–3. doi: 10.1289/ehp.112-a820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forshee RA, Storey ML, Allison DB, et al. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr. 2007;47:561–82. doi: 10.1080/10408390600846457. [DOI] [PubMed] [Google Scholar]
- 16.Melanson KJ, Zukley L, Lowndes J, Nguyen V, Angelopoulos TJ, Rippe JM. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition. 2007;23:103–12. doi: 10.1016/j.nut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Jebb SA, Siervo M, Murgatroyd PR, Evans S, Fruhbeck G, Prentice AM. Validity of the leg-to-leg bioimpedance to estimate changes in body fat during weight loss and regain in overweight women: a comparison with multi-compartment models. Int J Obes (Lond) 2007;31:756–62. doi: 10.1038/sj.ijo.0803475. [DOI] [PubMed] [Google Scholar]
- 18.Sun G, French CR, Martin GR, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81:74–8. doi: 10.1093/ajcn/81.1.74. [DOI] [PubMed] [Google Scholar]
- 19.Chen YM, Ho SC, Lam SS, Chan SS. Validity of body mass index and waist circumference in the classification of obesity as compared to percent body fat in Chinese middle-aged women. Int J Obes (Lond) 2006;30:918–25. doi: 10.1038/sj.ijo.0803220. [DOI] [PubMed] [Google Scholar]
- 20.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 21.Academies IoMotN . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. National Academies Press; Washington, D.C.: 2002. [DOI] [PubMed] [Google Scholar]
- 22.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48:334–41. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 23.Swanson JE, Laine DC, Thomas W, Bantle JP. Metabolic effects of dietary fructose in healthy subjects. Am J Clin Nutr. 1992;55:851–6. doi: 10.1093/ajcn/55.4.851. [DOI] [PubMed] [Google Scholar]
- 24.Havel PJ, Elliott S, Keim NL, Rader D, Krauss R, Teff K. Short-term and long-term consmption of high fructose, but not high glucose, diets increases postprandial triglycerides and apo-lipoprotein-B in women. J Invest Med. 2003;51:S163. [Google Scholar]
- 25.Stanhope KL, Griffen S, Krauss RM, et al. Consumption of Fructose-, but not Glucose-Sweetened Beverages Produces an Atherogenic Lipid Profile in Overweight/Obese Men and Women. Diabetes. 2007:56. [Google Scholar]
- 26.Reiser S, Bickard MC, Hallfrisch J, Michaelis OEt, Prather ES. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr. 1981;111:1045–57. doi: 10.1093/jn/111.6.1045. [DOI] [PubMed] [Google Scholar]
- 27.Bantle JP, Swanson JE, Thomas W, Laine DC. Metabolic effects of dietary fructose in diabetic subjects. Diabetes Care. 1992;15:1468–76. doi: 10.2337/diacare.15.11.1468. [DOI] [PubMed] [Google Scholar]
- 28.Koivisto VA, Yki-Jarvinen H. Fructose and insulin sensitivity in patients with type 2 diabetes. J Intern Med. 1993;233:145–53. doi: 10.1111/j.1365-2796.1993.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 29.Turner JL, Bierman EL, Brunzell JD, Chait A. Effect of dietary fructose on triglyceride transport and glucoregulatory hormones in hypertriglyceridemic men. Am J Clin Nutr. 1979;32:1043–50. doi: 10.1093/ajcn/32.5.1043. [DOI] [PubMed] [Google Scholar]
- 30.Herman RH, Zakim D, Stifel FB. Effect of diet on lipid metabolism in experimental animals and man. Fed Proc. 1970;29:1302–7. [PubMed] [Google Scholar]
- 31.Le KA, Faeh D, Stettler R, et al. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–9. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 32.Reiser S, Powell AS, Scholfield DJ, Panda P, Fields M, Canary JJ. Day-long glucose, insulin, and fructose responses of hyperinsulinemic and nonhyperinsulinemic men adapted to diets containing either fructose or high-amylose cornstarch. Am J Clin Nutr. 1989;50:1008–14. doi: 10.1093/ajcn/50.5.1008. [DOI] [PubMed] [Google Scholar]
- 33.Hallfrisch J, Ellwood KC, Michaelis OEt, Reiser S, O'Dorisio TM, Prather ES. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr. 1983;113:1819–26. doi: 10.1093/jn/113.9.1819. [DOI] [PubMed] [Google Scholar]
- 34.Crapo PA, Kolterman OG, Henry RR. Metabolic consequence of two-week fructose feeding in diabetic subjects. Diabetes Care. 1986;9:111–9. doi: 10.2337/diacare.9.2.111. [DOI] [PubMed] [Google Scholar]
- 35.Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nat Med. 1996;2:949–50. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 36.Saad MF, Riad-Gabriel MG, Khan A, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–9. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz JM, Neese RA, Schakleton C, Hellerstein MK. De novo lipogenesis during fasting and oral fructose ingestion in lean and obese hyperinsulinemic subjects. Diabetes. 1993;42:A39. [Google Scholar]
- 38.Shiota M, Galassetti P, Monohan M, Neal DW, Cherrington AD. Small amounts of fructose markedly augment net hepatic glucose uptake in the conscious dog. Diabetes. 1998;47:867–73. doi: 10.2337/diabetes.47.6.867. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–8. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaka T, Shimano H, Yahagi N, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53:560–9. doi: 10.2337/diabetes.53.3.560. [DOI] [PubMed] [Google Scholar]
- 41.Hyson D, Rutledge JC, Berglund L. Postprandial lipemia and cardiovascular disease. Curr Atheroscler Rep. 2003;5:437–44. doi: 10.1007/s11883-003-0033-y. [DOI] [PubMed] [Google Scholar]
- 42.Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–55. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Miranda J, Perez-Martinez P, Marin C, Moreno JA, Gomez P, Perez-Jimenez F. Postprandial lipoprotein metabolism, genes and risk of cardiovascular disease. Curr Opin Lipidol. 2006;17:132–8. doi: 10.1097/01.mol.0000217894.85370.c2. [DOI] [PubMed] [Google Scholar]
- 44.Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. 2003;31:1066–9. doi: 10.1042/bst0311066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Plasma glucose (S1A) and insulin (S1B) concentrations during a 24-h period (0800–0800 h) in hypertriacylglycerolemic male subject (fasting [TG] = 198 mg/dl) consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal. Change of plasma leptin concentration (S1C) over the morning nadir and plasma TG (S1D) concentrations from mean baseline levels (0800–0900 h) during a 24-h period (0800–0800 h) in hypertriacylglycerolemic male subject consuming HFCS-, sucrose-, fructose- and glucose-sweetened beverages with each meal.