Abstract
BACKGROUND
The high incidence of and few identified risk factors for prostate cancer underscore the need to further evaluate markers of prostate carcinogenesis. The aim of this pilot study was to evaluate urinary estrogen metabolites as a biomarker of prostate cancer risk.
METHODS
Using a liquid chromatography-tandem mass spectrometry method, urinary concentrations of 15 estrogen metabolites were determined in 77 prostate cancer cases, 77 healthy controls, and 37 subjects who had no evidence of prostate cancer after a prostate biopsy.
RESULTS
We observed an inverse association between the urinary 16-ketoestradiol (16-KE2) and 17-epiestriol (17-epiE3)- metabolites with high estrogenic activity- and prostate cancer risk. Men in the lowest quartile of 16-KE2, had a 4.6-fold risk of prostate cancer (OR= 4.62, 95% CI =1.34–15.99), compared with those in the highest quartile.
CONCLUSIONS
We observed modest differences in estrogen metabolite concentrations between prostate cancer patients and subjects without cancer. Larger studies with both androgen and estrogen measurements are needed to confirm these results to clarify further whether estrogen metabolites are independent biomarkers for prostate cancer risk and whether androgen/estrogen imbalance influences prostate cancer risk.
Keywords: prostate cancer, estrogen metabolites, benign prostatic hyperplasia, case-control study
INTRODUCTION
Prostate cancer is the most common non-skin cancer and the second leading cause of cancer death among men in the United States. The etiology of the disease is not well understood; the only established risk factors are age, family history of prostate cancer, ethnicity, and, more recently, variants in the 8q24 regions from genome wide association studies [1]. The prostate gland is an androgen-dependent organ [2] but it has been suggested that estrogens and their receptors (ERα and ERβ) may also be involved in the growth of the prostate [3–6]. Some estrogens and their metabolites bind to ERα or ERβ selectively [7]. ERα has been associated with the etiology of benign prostatic hyperplasia (BPH) and with cancer progression [8]. Data on ERβ signaling suggest a protective role; ERβ has an anti-proliferative function in the prostate and there is frequent loss of this receptor in prostate cancer [9;10].
The effects of estrogens on the prostate are mediated by systemic endocrine pathways that involve the pituitary gland or locally within the prostate gland via conversion of testosterone to 17β-estradiol (E2) by aromatase [2]. Although biologically estrogens may play a role in prostate cancer, data from epidemiological studies are limited and conflicting [1;11–16]. Recently, a large collaborative analysis of 18 prospective studies on serum hormones and prostate cancer risk found no associations [17]. Most of these studies investigated estrone and estradiol only, and have not examined the entire spectrum of estrogen metabolites.
The two main pathways for metabolism of estrogens are 16α-hydroxylation and 2-hydroxylation. The major estrogen metabolites excreted in urine are the parent estrone (E1) and 17β-estradiol (E2), 2-hydroxy products [2-hydroxyesterone (2-OHE1), 2-hydroxyestradiol (2-OHE2), 2-methoxyesterone (2-MeOE1)] and 16α-hydroxy products [estriol (E3) and 16α-hydroxyestrone (16α-OHE1)] [18] (Figure 1). The 16α-metabolites are considered the dominant biologically active metabolites, while the 2-OHE1 is less estrogenic. The ratio of 2-OHE1/16α-OHE1 has been linked to reduced risk of breast cancer [19]. In a recent prostate cancer study, participants with elevated urinary 2-OHE1/16α-OHE1 ratio (highest tertile) had a 40% non-significant reduction in prostate cancer risk when subjects with a prostate-specific antigen (PSA) value higher than 4 ng/ml were excluded from the control group [20]. PSA concentrations are closely related to advanced age and prostate size [21] and larger prostate size also correlates with estrogen concentrations [22]. A later case-control study, with a wider estrogen metabolite profile of only 14 prostate cancer cases, did not find differences in the concentration of estrogen metabolites but suggested that the oxidative pathway that leads to DNA adduct formation is more active in the prostate cancer group than in the control group [23].
Figure 1.
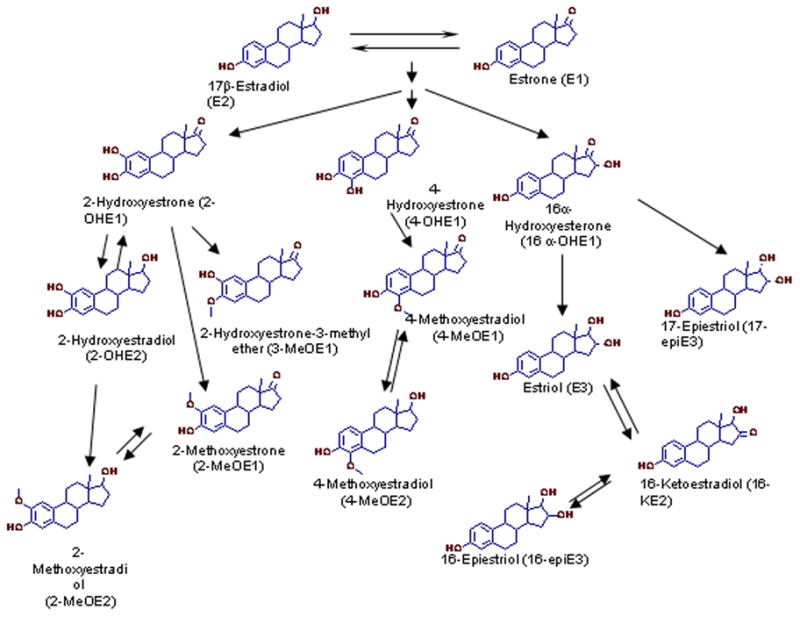
To help clarify the role of estrogen in prostate cancer etiology, we designed a study to evaluate the association of 15 urinary estrogen metabolites with prostate cancer risk by mass spectrometric isotope dilution method [24] in 77 prostate cancer cases, 77 healthy controls, and 37 men who had a biopsy but did not have cancer.
METHODS
Study Population
The 77 prostate cancer cases were recruited between 2004 and 2007 at the Georgetown University Medical Center, Departments of Urology and Radiation Oncology and the Veterans Administration (VA) Hospital, Department of Urology, Washington DC. All prostate cancer cases were confirmed by biopsy and enrolled prior to initiation of treatment. Forty cases (52%) presented with Gleason score 6, and 24 (31%) with Gleason score 7 and higher. Information on Gleason score was not available for 13 cases (17%). By design, the study had two control groups. Biopsy controls (n=37) were patients who were confirmed prostate cancer free at biopsy. These controls typically had BPH (n=27) or other urologic conditions that were likely responsible for their elevated PSA. Healthy controls (n=77) were prostate disease free patients and visitors not related to prostate cases. These participants accompanied other patients (n=10), visited for routine checkups, non-prostate issues (e.g. testicular pain, erectile dysfunction, bladder stone analysis) (n=11); or participated in the National Lung Screening Trial (NLST; a multicenter study of lung cancer risk sponsored by the NCI) (n=52) [25]. Eligible subjects had to be able to speak English well enough to be interviewed and have no prior history of cancer. After informed consent, cases and controls received a structured, in-person interview assessing prior medical history, tobacco and alcohol use, current medications, family medical history and socioeconomic characteristics. All participants provided a urine sample at enrollment and the time of urine collection was recorded. Samples were frozen immediately at −80 °C until analysis; all analyses were carried out at second thaw. Protocols were approved by the Georgetown University Institutional Review Board.
Sample handling and Estrogen Metabolite Measurements
All urine samples were handled, as described previously, by laboratory personnel blinded to the case-control status [24]. Briefly, estrogen metabolites were measured in 0.5 ml of urine using a liquid chromatography-mass spectrometry method quantifying the absolute abundance of the following 15 urinary estrogen metabolites: 16-ketoestradiol (16-KE2), estriol (E3), 16α-hydroxyestrone (16α-OHE1), 16-epiestriol (16-epiE3), 17-epiestriol (17-epiE3), 2-hydroxyestrone-3-methyl ether (3-MeOE1), 2-methoxyestrone (2-MeOE1), 4-methoxyestrone (4-MeOE1), 2-methoxyestradiol (2-MeOE2), estrone (E1), 4-methoxyestradiol (4-MeOE2), 17β-estradiol (E2), 2-hydroxyestrone (2-OHE1), 2-hydroxyestradiol (2-OHE2), 4-hydroxyestrone (4-OHE1) (Figure 1). The measurement represents total estrogen concentrations (glucuronidated + sulfated + unconjugated) and all the estrogen values were corrected for urinary creatinine concentration. There was insufficient urine volume for 5 participants (1 case, 3 healthy controls, 2 biopsy controls) to assay for creatinine; accordingly the estrogen results obtained for these subjects were excluded from the analysis. The laboratory assays were done in batches of 60 samples including the following: 14 calibration samples (2 at each concentration), 6 quality control samples (2 at each concentration), and 40 participant samples. The quantification limit of the assay for the estrogen metabolites was 2 pg as described previously [24]. Accuracy of the assay, calculated as the measured percentage of a weighed amount of an estrogen metabolite added to urine samples, was 96–108 %. Precision, including the hydrolysis, extraction and derivatization steps was less than 5% relative standard deviation (RSD) for samples prepared in the same batch and less than 12% RSD for samples prepared in separate batches [24]. The assay is sufficiently sensitive and reproducible to quantify the estrogen metabolites in the urine of men; the range of estrogen metabolite concentrations among men is large relative to assay variability [26].
Statistical Analysis
The Chi-square goodness-of-fit or Student t test were used to examine the distributions of age, smoking status, presence of BPH and other parameters of interest among cases and controls. Smoking status was stratified into three categories: never smokers, individuals who smoked less than 100 cigarettes in their life; former smokers, individuals who had smoked more than 100 cigarettes and have not smoked for a year or longer; and current smokers. Positive family history of prostate cancer was defined as having a father, brother, uncle or cousin diagnosed with prostate cancer. Information on presence of BPH was self reported based on whether the subject responded positive to any of the following questions: “Have you ever been diagnosed with enlarged prostate? Do you wake up 2 times or more every night to urinate?” Presence of BPH diagnosis as described above was in 100% concordance with presence of BPH based on pathology reports. However, as there were no medical records available for 35% of the study population, primarily the healthy control group, we chose to define BPH based on the criteria described above.
Non-parametric statistics were used to compare case and control groups with respect to urinary estrogen metabolite concentrations. Median values as well as 25th and 75th percentile values are presented; medians proved to be better descriptors of the data compared to means due to the non normal distribution of the measurements. Multivariate analysis of variance (MANOVA) was used to identify interactions among estrogen metabolites and their association to the case control status while adjusting for age. The p-values were obtained using 1,000 permutations. Spearman correlation was used to estimate correlations among estrogen metabolites and/or PSA. For risk estimations only one estrogen metabolite was included in each logistic regression model at each analytical session to calculate odds ratios (OR) for estrogen metabolite concentrations and prostate cancer risk, adjusted for age, race, time of urine collection, smoking status and the presence of BPH. To denote high versus low estrogen concentrations, the median concentration for each estrogen metabolite in the healthy control group was used as a cutoff. Urine collection time was introduced to the model as a dichotomous variable using 12 pm as a cutoff; this cutoff would capture subjects before and/or after a main meal which may directly alter estrogen concentrations via insulin dependent pathways [27]. All P values were two-sided. All analyses were performed using SAS software, version 9 (SAS Institute Inc., Cary, NC).
RESULTS
Selected characteristics of study participants are presented in Table I. Cases were well matched to controls by age. The population was primarily of Caucasian race. Healthy controls were more likely to be smokers compared to both cases and biopsy controls (p=0.01), possibly due to inclusion of participants from the NLST. Cases did not differ from controls in frequency of diabetes diagnosis or in how often they reported using non steroidal anti-inflammatory drugs (NSAIDs). Healthy controls were less likely to be diagnosed with BPH compared to both cases (p=0.02) and biopsy controls (p<0.01). Cases reported a positive family history of prostate cancer more often than healthy controls (p=0.04). Socioeconomic characteristics of cases and controls as judged by household income were comparable among the groups. 75% of cases and same percentage of biopsy controls had abnormally high PSA (>4 ng/ml) while only 8% of healthy controls belonged to the high PSA category.
Table I.
Descriptive Characteristics of Study participants
| Cases (n=77) | Controls |
|||||
|---|---|---|---|---|---|---|
| Healthy (n=77) | PH | Biopsy (n=37) | PB | PH/B | ||
| Age years (SD) | 63.71 (8.27) | 63.40 (6.81) | 0.80 | 62.89 (8.60) | 0.59 | 0.74 |
| Race n (%) | ||||||
| Caucasian | 63 (84) | 71 (92) | 27 (75) | |||
| African American | 10 (13) | 4 (5) | 5 (14) | |||
| Other | 2 (3) | 2 (3) | 0.22 | 4 (11) | 0.18 | 0.04 |
| Mean BMI (SD) | 24.39 (2.59) | 25.28 (3.05) | 0.08 | 24.80 (3.13) | 0.55 | 0.49 |
| Smoking Status | ||||||
| Never | 30 (43) | 5 (7) | 16 (52) | |||
| Former | 38 (54) | 42 (56) | 11 (35) | |||
| Current | 2 (3) | 28 (37) | <0.01 | 4 (13) | 0.10 | <0.01 |
| BPH diagnosis | ||||||
| Yes | 49(71) | 39 (51) | 27 (82) | |||
| No | 20 (29) | 37 (49) | 0.02 | 6 (18) | 0.24 | <0.01 |
| PSA | ||||||
| < 4 | 19 (25) | 70 (92) | 9 (24) | |||
| >= 4 | 58 (75) | 6 (8) | 0.01 | 28 (75) | 0.15 | 0.01 |
| Family history of prostate cancer | ||||||
| Yes | 23 (35) | 15 (20) | 8 (28) | |||
| No | 42 (65) | 61 (80) | 0.04 | 21 (72) | 0.45 | 0.38 |
| Household income | ||||||
| < 100K | 27 (40) | 29 (39) | 16 (47) | |||
| >=100K | 40 (60) | 46 (61) | 0.84 | 18 (53) | 0.52 | 0.41 |
BMI=Body Mass Index
BPH =benign prostatic hyperplasia
PSA=prostate specific antigen (ng/ml)
Family history of prostate cancer=defined as having any blood relative diagnosed with prostate cancer
PH: comparison between cases and healthy controls
PB: comparison between cases and biopsy controls
PH/B: comparison between healthy and biopsy controls
Results showed that in all groups, the ranking of the estrogen metabolites based on median concentration in urine was similar. 4-OHE1 ranked higher in abundance among cases than in either of the control groups however the median concentration was comparable among the 3 groups studied (Table II). Concentrations of E3, E1, 16-KE2, 2-OHE1 and E2 were the highest among all groups, accounting for approximately 60–70% of the total urinary estrogen metabolites with E3 being the dominant estrogen in all study groups. The methoxy estrogen 4-MeOE2, on the other hand, was the least abundant metabolite detected in the urine of all the groups followed by other methoxy estrogens. Results from MANOVA with Pillai’s trace statistics showed that, the age adjusted interactions among estrogen metabolites were comparable among the 3 subject groups (data not shown). Univariate analysis showed that 16-KE2 and 17-epiE3 were significantly lower in cases compared to healthy controls. 17-epiE3 was also found to be lower among biopsy controls compared to healthy controls (p=0.01). There was no significant correlation between either 17-epiE3 or 16-KE2 and PSA; 16-KE2 and 17-epiE3 were highly correlated (r=0.75, p<0.0001). The 2-OHE1/16α-OHE1 ratio was comparable among groups as was the total amount of estrogen metabolites found in urine. Interestingly, biopsy controls tended to have more protein in their urine (8.5mg/dL) compared to the healthy control (7.0 mg/dL) and prostate cancer group (7.0 mg/dL).
Table II.
Concentrations and Ranks of Urinary Estrogens.
| Cases (N=76) |
Healthy Controls (N=74) |
Biopsy Controls (N=35) |
PH/B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 25th, 75th P | Rank | Median | 25th, 75th P | Rank | PH | Median | 25th, 75th P | Rank | PB | ||
| EM | ||||||||||||
| E1 | 1.17 | 0.82, 1.78 | 2 | 1.06 | 0.73, 1.84 | 3 | 0.88 | 1.36 | 0.83, 2.52 | 2 | 0.20 | 0.15 |
| E2 | 0.68 | 0.41, 1.36 | 5 | 0.58 | 0.39, 0.99 | 6 | 0.22 | 0.59 | 0.41, 1.08 | 5 | 0.68 | 0.57 |
| Catechol Estrogens | ||||||||||||
| 2-OHE1 | 0.77 | 0.43, 1.47 | 4 | 1.04 | 0.60, 1.82 | 4 | 0.13 | 0.82 | 0.29, 1.55 | 4 | 0.72 | 0.12 |
| 2-OHE2 | 0.20 | 0.11, 0.39 | 11 | 0.24 | 0.13, 0.39 | 10 | 0.31 | 0.18 | 0.11, 0.34 | 10 | 0.76 | 0.23 |
| 4-OHE1 | 0.26 | 0.13, 1.23 | 9 | 0.23 | 0.16, 0.45 | 11 | 0.56 | 0.17 | 0.11, 0.41 | 11 | 0.15 | 0.23 |
| 16α pathway | ||||||||||||
| 16α-OHE1 | 0.47 | 0.22, 1.15 | 6 | 0.63 | 0.30, 1.03 | 5 | 0.30 | 0.57 | 0.22, 1.11 | 6 | 0.82 | 0.68 |
| 17-epiE3 | 0.11 | 0.07, 0.21 | 12 | 0.16 | 0.10, 0.29 | 12 | 0.05 | 0.10 | 0.08, 1.16 | 12 | 0.44 | 0.01 |
| E3 | 2.17 | 1.32, 4.91 | 1 | 2.50 | 1.51, 2.50 | 1 | 0.29 | 2.87 | 1.36, 17.89 | 1 | 0.18 | 0.56 |
| 16-KE2 | 1.00 | 0.61, 1.61 | 3 | 1.16 | 0.84, 2.19 | 2 | 0.03 | 0.98 | 0.46, 2.17 | 3 | 0.97 | 0.12 |
| 16-epiE3 | 0.35 | 0.24, 0.55 | 7 | 0.41 | 0.27, 0.60 | 7 | 0.20 | 0.32 | 0.21, 0.49 | 7 | 0.58 | 0.10 |
| Methoxy Estrogens | ||||||||||||
| 2-MeOE1 | 0.26 | 0.18, 0.49 | 8 | 0.35 | 0.23, 0.58 | 8 | 0.22 | 0.27 | 0.18, 0.42 | 8 | 0.73 | 0.12 |
| 2-MeOE2 | 0.07 | 0.05, 0.13 | 14 | 0.08 | 0.04, 0.19 | 14 | 0.56 | 0.07 | 0.05, 0.16 | 14 | 0.95 | 0.61 |
| 3-MeOE1 | 0.20 | 0.11, 0.40 | 10 | 0.24 | 0.11, 0.56 | 9 | 0.58 | 0.24 | 0.13, 0.57 | 9 | 0.31 | 0.64 |
| 4-MeOE1 | 0.08 | 0.05, 0.15 | 13 | 0.09 | 0.04, 0.18 | 13 | 0.44 | 0.09 | 0.05, 1.13 | 13 | 0.96 | 0.47 |
| 4-MeOE2 | 0.06 | 0.04, 0.11 | 15 | 0.08 | 0.04, 0.13 | 15 | 0.45 | 0.07 | 0.04, 0.12 | 15 | 0.44 | 0.84 |
| Total | 10.75 | 6.59, 19.58 | 11.65 | 7.52, 18.58 | 0.41 | 15.23 | 9.11, 28.94 | 0.10 | 0.16 | |||
| 2-OHE1/16α-OHE1 | 1.71 | 0.55, 3.45 | 1.46 | 0.55, 3.07 | 0.87 | 1.39 | 0.45, 3.47 | 0.73 | 0.78 | |||
| Creatinine (mg/dL) | 102.5 | 62.0, 158.2 | 104.1 | 63.0, 138.6 | 0.89 | 98.6 | 59.6, 152.1 | 0.94 | 0.81 | |||
| Total protein (mg/dL) | 7.0 | 5.0, 13.0 | 7.0 | 5.0, 11.0 | 0.54 | 8.5 | 5.0, 22.0 | 0.11 | 0.04 | |||
Estrogen metabolite values are denoted as pg estrogen metabolite normalized by creatinine (mg/dL). Rank is based on estrogen metabolite concentration
There was insufficient urine volume for 5 participants (1 case, 3 healthy controls, 2 biopsy controls) to assay for creatinine, therefore estrogen metabolite measurements are not presented for these subjects.
E1 concentration was below the detection limit for 1 healthy control, 2-OHE1 for 2 cases and 4 healthy controls, 2-OHE2 for 1 case and 1 biopsy control, 4-OHE1 for 2 cases and 2 healthy controls, 2-MeOHE1 for 1 case and 1 biopsy control, 4-MeOE2 for 3 cases, 1 healthy and 2 biopsy controls
%= median percentage of individual estrogen compared to total estrogen
25th, 75th P = 25th, 75th Percentiles
PH: comparison between cases and healthy controls
PB: comparison between cases and biopsy controls
PH/B: comparison between healthy and biopsy controls
Significantly different comparisons based on Wicoxon Rank Sum test are bolded.
To identify factors that affect estrogen metabolite concentrations we performed correlation analyses in the healthy control group. Being a current smoker was associated with a significant increase in 2-OHE1 and 2-MeOE1 and a marginal increase in the 2-OHE1/16α-OHE1 ratio (p=0.0001, 0.02, 0.07, respectively; Table III). Using a cutoff of greater than 0.2 to determine normal versus high protein-to-creatinine ratio [28], we observed that estrogen metabolites containing a methoxy group (2-MeOE1, 4-MeOE1, 2-MeOE2, 4-MeOE2) tend to be present in higher concentrations in the urine of individuals with high protein-to-creatinine ratio. In our population, there was no apparent difference in any estrogen metabolites by age category (≤60, 61–66, >66) (data not shown). Body Mass Index (BMI), use of NSAIDs, presence of diabetes, family history of prostate cancer or presence of BPH did not affect estrogen concentrations. We did not assess race due to the small number of samples from non-Caucasian subjects.
Table III.
Estrogen Concentrations by Smoking Status and Protein/Creatinine Ratio among men in the healthy control group
| Smoking Status | Protein/Creatinine Ratio* | |||||
|---|---|---|---|---|---|---|
| Never/Former (n=44) | Current (n=28) | p | Normal (n=66) | High (n=8) | p | |
|
EM | ||||||
| E1 | 1.06 (0.71–1.79) | 1.33 (0.76–2.25) | 0.36 | 1.13 (0.73–1.85) | 0.92 (0.61–1.52) | 0.48 |
| E2 | 0.60 (0.40–1.04) | 0.51 (0.36–0.95) | 0.49 | 0.55 (0.37–0.99) | 0.80 (0.67–1.14) | 0.19 |
|
Catechol Estrogens | ||||||
| 2-OHE1 | 0.77 (0.46–1.22) | 2.01 (0.88–2.40) | 0.0001 | 1.00 (0.60–1.92) | 1.10 (0.76–1.35) | 0.67 |
| 2-OHE2 | 0.21 (0.13–0.41) | 0.26 (0.16–0.37) | 0.58 | 0.22 (0.15–0.35) | 0.46 (0.12–1.09) | 0.14 |
| 4-OHE1 | 0.25 (0.16–0.59) | 0.22 (0.16–0.45) | 0.78 | 0.23 (0.16–0.44) | 0.34 (0.18–3.20) | 0.33 |
| 16α pathway | ||||||
| 16α-OHE1 | 0.65 (0.29–0.87) | 0.66 (0.36–1.33) | 0.52 | 0.62 (0.30–1.03) | 0.74 (0.32–1.40) | 0.79 |
| 17-epiE3 | 0.17 (0.10–0.32) | 0.16 (0.11–0.28) | 0.96 | 0.15 (0.10–0.28) | 0.31 (0.10–0.52) | 0.35 |
| E3 | 2.82 (1.88–5.29) | 2.26 (1.26–5.16) | 0.22 | 2.50 (1.58–5.95) | 2.08 (1.11–3.85) | 0.33 |
| 16-KE2 | 1.21 (0.92–2.39) | 1.08 (0.84–1.69) | 0.31 | 1.16 (0.84–2.19) | 1.15 (0.87–2.01) | 0.87 |
| 16-epiE3 | 0.42 (0.27–0.59) | 0.41 (0.23–0.77) | 0.85 | 0.40 (0.25–0.58) | 0.51 (0.31–0.73) | 0.44 |
|
Methoxy Estrogens | ||||||
| 2-MeOE1 | 0.33 (0.22–0.42) | 0.44 (0.27–0.66) | 0.02 | 0.33 (0.22–0.46) | 0.57 (0.38–0.69) | 0.06 |
| 2-MeOE2 | 0.07 (0.04–0.20) | 0.08 (0.04–0.59) | 0.96 | 0.07 (0.04–0.17) | 0.25 (0.11–0.46) | 0.009 |
| 3-MeOE1 | 0.21 (0.09–0.43) | 0.32 (0.14–0.80) | 0.10 | 0.22 (0.10–0.56) | 0.29 (0.18–0.55) | 0.53 |
| 4-MeOE1 | 0.10 (0.06–1.17) | 0.12 (0.04–0.26) | 0.49 | 0.09 (0.04–0.17) | 0.22 (0.11–0.49) | 0.06 |
| 4-MeOE2 | 0.07 (0.04–0.13) | 0.09 (0.04–0.12) | 0.80 | 0.07 (0.04–0.12) | 0.32 (0.07–0.37) | 0.02 |
| Total | 11.25 (7.5–17.96) | 11.95 (7.79–20.24) | 0.69 | 11.81 (7.52–18.58) | 10.26 (8.66–18.74) | 0.83 |
| 2-OHE1/16α-OHE1 | 1.19 (0.42–2.52) | 1.92 (0.87–5.33) | 0.07 | 1.29 (0.54–3.07) | 2.09 (1.04–4.25) | 0.53 |
Estrogen metabolite values are denoted as pg estrogen metabolite/mg creatinine high protein/creatinine ratio when >0.2
Significantly different comparisons based on Wicoxon Rank Sum test are bolded.
Table IV shows the association between two estrogen metabolites and prostate cancer risk. As shown, lower levels of urinary 16-KE2 were associated with an increased risk of prostate cancer risk in a dose dependent manner (p trend = 0.02), after adjustment for age, race, smoking status, presence of BPH and time of urine collection (Table IV). Men in the lowest quartile had a 4.6-fold risk of prostate cancer (OR= 4.62 95% CI=1.34–15.99), compared with those is the highest quartile. 17-epiE3 did not show a significant association in the fully adjusted model. No association was observed with any of the other estrogen metabolites examined (data not shown). Further adjustment for protein in urine did not alter the risk estimations.
Table IV.
Odds ratios and 95% CI for prostate cancer in relation to 16-KE2 and 17-epiE3 estrogen metabolites
| Cases/Healthy controls | OR (95% CI) | |
|---|---|---|
|
16-KE2 | ||
| By Median | ||
| High (≥1.16) | 31/37 | 1.00 |
| Low (<1.16) | 45/37 | 1.97 (0.86–4.52) |
| By Quartiles | ||
| 4th (≥2.19) | 10/19 | 1.00 |
| 3rd (1.16–2.18) | 21/18 | 1.81 (0.58–5.62) |
| 2nd (0.84–1.15) | 14/21 | 1.71 (0.53–5.52) |
| 1st (<0.83) | 31/16 | 4.62 (1.34–15.99) |
| P trend | 0.02 | |
|
17-epiE3 | ||
| By Median | ||
| High (≥0.16) | 27/36 | 1.00 |
| Low (<0.16) | 49/38 | 1.34 (0.54–3.37) |
| By Quartiles | ||
| 4th (≥0.29) | 14/19 | 1.00 |
| 3rd (0.16–.28) | 13/17 | 1.10 (0.34–3.63) |
| 2nd (0.10–.16) | 19/19 | 1.28 (0.42–3.91) |
| 1st (<0.09) | 30/19 | 1.63 (0.54–4.92) |
| P trend | 0.37 | |
OR=Odds Ratio, adjusted for age, race, smoking status, presence of benign prostatic hyperplasia and time of urine collection
DISCUSSION
In this pilot study we evaluated the association of 15 urinary estrogen metabolites, quantified by LC-MS, with prostate cancer risk. We observed a suggestive trend toward decreased urinary concentration of metabolites with high estrogenic activity, namely16-KE2 and 17-epiE3, among prostate cancer patients. Our analysis confirms that smoking is a modifier of urinary estrogen levels. Although needing confirmation in larger studies, our study shows that it is possible to detect a multitude of estrogen metabolites as well as differences in estrogen metabolites in urine between prostate cancer cases and controls.
Urine contains both biologically active estrogens, which includes unconjugated parent estrogens, their phase I metabolites, and O-methylated catechol estrogens, as well as biologically inactive estrogens, which includes sulfate and/or glucuronide conjugates. Because estrogen metabolites are mostly present in urine as glucuronide or sulfate conjugates, we measured total estrogen metabolites in men which is the sum of the glucuronidated, sulfated and unconjugated forms of each estrogen metabolite [29].
Both 16-KE2 and 17-epiE3 are products of the 16α-hydroxylation pathway and derive from the biologically active 16α-OHE1 metabolite (Figure 1). One of the leading hypotheses that explains the role of estrogens in breast carcinogenesis contradicts our observations of a potential protective effect of 16-KE2 and 17-epiE3 in prostate cancer. According to the breast cancer hypothesis, 16α-hydroxylated estrogens induces breast carcinogenesis due to their much stronger hormonal and mitogenic activity as compared to the catechol estrogens [30]. However, it is possible that estrogen metabolites differentially affect different organs and thus extending results from breast to prostate may be misleading. For instance, both 16-KE2 and 17-epiE3 have shown a preferential binding capacity for ERβ [7] which was shown to have a protective role within the prostate [9]. We should also note that although 17-epiE3 retains close to 75% of the estrogenic activity of the E2, 16-KE2 has a significantly reduced binding affinity to ERα receptor and to a lesser extend to the ERβ receptor [7]. Furthermore, we cannot rule out the possibility that the observed protective effects of these estrogen metabolites could be by chance. This may be particularly true for the low abundance 17-epiE3 for which the case control differences did not persist in our adjusted multivariate analysis. In this study we did not examine the androgen to estrogen balance and its potential association with prostate cancer risk. Androgens and estrogens are transported in the circulation by the sex hormone-binding globulin (SHBG). As shown previously [31], controlling for serum SHBG levels may be necessary in order to find the relevant association between sex hormone levels and prostate cancer risk. Thus, larger studies that measure androgens as well as SHBG are needed to confirm our findings.
The 2-OHE1/16α-OHE1 ratio has been used as an index of the relative strengths of the two competing oxidative pathways in breast cancer with higher ratios having been associated with a reduced risk for the disease [32]. Our observation that smoking alters the concentrations of the less estrogenic 2-OHE1 metabolite and marginally increases the 2-OHE1/16α-OHE1 ratio in urine suggests the anti-estrogenic effects of smoking [33] and raises the issue of the role of smoking in prostate cancer development. Our finding contradicts that of Muti et al. who did not observe an association between smoking status and urinary 2-OHE1 but reported increased levels of urinary16α-OHE1 among current smokers compared to never/former smokers [20]. The different methodologies used in our study compared to that by Muti and colleagues could explain partly the contradictory findings. Additionally, while only patients with clinically apparent disease (stage B and higher) were included in the study by Muti and colleagues, most of the prostate cancer cases in the current study were of early disease stages.
In this study, the ranking of estrogens and its metabolites with respect to urinary concentrations was similar among the study groups. The estrogen metabolite 4-OHE1 ranked slightly higher among cases than the 2 control groups; however, there was no difference in the median concentration of the metabolite among the 3 groups. Given the rapid metabolic clearance of catechol estrogens, it is hard to estimate the contribution of 4-OHE1 to cancer risk. Animal studies have shown that exposure to 4-OHE1 and 4-OHE2 leads to formation of catechol estrogen quinones (CEQ) and subsequently depurinating DNA adducts, a process that is a putative tumor initiating event [34;35]. In humans, CEQ-derived DNA adducts are present in urine samples from subjects with prostate cancer in higher amounts compared to controls [23;36]. Based on this principle, low concentrations of 4-OHE1 would result in less adduct formation and would be protective against prostate cancer. However, it has also been postulated that 2-pathway catechol estrogens may actually be protective since their formation precludes 16-hydroxylation [37].
Of interest is our observation that there was higher (abnormal) protein-to-creatinine ratio in the biopsy control group compared to both the healthy and prostate cancer groups. Increased protein-to-creatinine ratio is associated with proteinuria which is subsequently associated with renal function. Urinary excretion of endogenous and exogenous compounds is determined by glomerular filtration and tubular secretion. Although we could not confirm renal abnormalities as a diagnosis for the subjects that are in the biopsy control group, our observations prompted us to investigate abnormal protein-to-creatinine ratio in relation to estrogen concentrations. Analysis among the healthy control group with signs of proteinuria revealed an association with elevated 2-and 4- methoxyestrogens (2-MeOE1, 4-MeOE1, 2-MeOE2, 4-MeOE2). Methoxyestrogens are considered as potential therapeutic agents due to their antitumor activity via induction of apoptosis and inhibition of angiogenesis [38–40]. The anti-proliferative effect of methoxyestrogens has not yet been demonstrated in relation to the prostate but it was shown that in breast cancer cells the effect can occur independently of ERα and ERβ [41]. Similar observations were made in other cells such as pancreas, leukemia and lung [42;43].
It is difficult to establish a temporal relation between urinary concentration of estrogens and prostate cancer risk in a cross-sectional study. The mean preclinical duration for prostate cancer has been estimated at least as a decade [44]. A limitation of our study is that urine samples were collected throughout the day; a 24-hour urine collection would be ideal. Although circadian variations of plasma testosterone and estrogens have been demonstrated among younger men [45], a recent study showed that testosterone concentrations in older men (mean age 60 years) are stable throughout the morning and early afternoon, declining modestly thereafter [46]. The results imply that the diurnal variation of androgens in our age group (mean age 63 years) is a minor concern. We are not aware of a similar study of estrogens but similarity of the pathways would suggest that they might follow the same trends. We adjusted for time of collection in our final model but residual confounding could bias the study towards the null.
In summary, we evaluated the association between 15 estrogen metabolites and prostate cancer risk in a small case-control study. The results show only modest differences. We observed a tendency for lower urinary concentration of the 16-KE2 and 17-epiE3, metabolites with high estrogenic activity, among prostate cancer patients. Larger studies are needed to confirm these findings that also account for androgen levels and SHBG. In addition, a longitudinal study would likely be a better design to improve the assessment of the potential long term effects of estrogen metabolites on prostatic carcinogenesis.
Acknowledgments
We wish to thank Allison Pollock and Anthony Roy Orden for the recruitment of study participants and Dr Borges, Department of Urology, Veterans Administration Medical Center, for facilitating recruitment at the VA hospital. The Clinical Molecular Epidemiology Shared Resources at the Lombardi Comprehensive Cancer Center provided services for questionnaire data entry. This project was conducted through the General Clinical Research Center at Georgetown University and supported by the National Institutes of Health National Center for Research Resources, Grant M01RR-023942. This study was supported in part by the Department of Defense Prostate Cancer Research Program grant PC081609 awarded to RG.
Reference List
- 1.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 2.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 3.Noble RL. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 1977;37:1929–1933. [PubMed] [Google Scholar]
- 4.Carruba G, Miceli MD, Comito L, Farruggio R, Sorci CM, Oliveri G, Amodio R, di FM, d’Amico D, Castagnetta LA. Multiple estrogen function in human prostate cancer cells. Ann NY Acad Sci. 1996;784:70–84. doi: 10.1111/j.1749-6632.1996.tb16229.x. [DOI] [PubMed] [Google Scholar]
- 5.Carruba G. Estrogens and mechanisms of prostate cancer progression. Ann NY Acad Sci. 2006;1089:201–217. doi: 10.1196/annals.1386.027. [DOI] [PubMed] [Google Scholar]
- 6.Leav I, Merk FB, Kwan PW, Ho SM. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate. 1989;15:23–40. doi: 10.1002/pros.2990150104. [DOI] [PubMed] [Google Scholar]
- 7.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 8.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002;193:1–5. doi: 10.1016/s0303-7207(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 10.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 11.Dorgan JF, Albanes D, Virtamo J, Heinonen OP, Chandler DW, Galmarini M, McShane LM, Barrett MJ, Tangrea J, Taylor PR. Relationships of serum androgens and estrogens to prostate cancer risk: results from a prospective study in Finland. Cancer Epidemiol Biomarkers Prev. 1998;7:1069–1074. [PubMed] [Google Scholar]
- 12.Nomura A, Heilbrun LK, Stemmermann GN, Judd HL. Prediagnostic serum hormones and the risk of prostate cancer. Cancer Res. 1988;48:3515–3517. [PubMed] [Google Scholar]
- 13.Barrett-Connor E, Garland C, McPhillips JB, Khaw KT, Wingard DL. A prospective, population-based study of androstenedione, estrogens, and prostatic cancer. Cancer Res. 1990;50:169–173. [PubMed] [Google Scholar]
- 14.Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, Henderson BE. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992;339:887–889. doi: 10.1016/0140-6736(92)90927-u. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Weiss NS, Stanczyk FZ, Lewis SK, DiTommaso D, Etzioni R, Barnett MJ, Goodman GE. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12:1410–1416. [PubMed] [Google Scholar]
- 16.Hsing AW, Comstock GW. Serological precursors of cancer: serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Biomarkers Prev. 1993;2:27–32. [PubMed] [Google Scholar]
- 17.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adlercreutz H, Fotsis T, Hockerstedt K, Hamalainen E, Bannwart C, Bloigu S, Valtonen A, Ollus A. Diet and urinary estrogen profile in premenopausal omnivorous and vegetarian women and in premenopausal women with breast cancer. J Steroid Biochem. 1989;34:527–530. doi: 10.1016/0022-4731(89)90138-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Kim SO, Lee HD, Chung BC. Estrogens and polyamines in breast cancer: their profiles and values in disease staging. Cancer Lett. 1998;133:47–56. doi: 10.1016/s0304-3835(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 20.Muti P, Westerlind K, Wu T, Grimaldi T, De BJ, III, Schunemann H, Freudenheim JL, Hill H, Carruba G, Bradlow L. Urinary estrogen metabolites and prostate cancer: a case-control study in the United States. Cancer Causes Control. 2002;13:947–955. doi: 10.1023/a:1021986811425. [DOI] [PubMed] [Google Scholar]
- 21.Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 22.Partin AW, Oesterling JE, Epstein JI, Horton R, Walsh PC. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405–409. doi: 10.1016/s0022-5347(17)38353-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Gaikwad NW, Meza J, Cavalieri EL, Muti P, Trock B, Rogan EG. Novel biomarkers for risk of prostate cancer: results from a case-control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Keefer LK, Ziegler RG, Veenstra TD. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat Protoc. 2007;2:1350–1355. doi: 10.1038/nprot.2007.176. [DOI] [PubMed] [Google Scholar]
- 25.Church TR. Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol. 2003;10:713–715. doi: 10.1016/s1076-6332(03)80095-8. [DOI] [PubMed] [Google Scholar]
- 26.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ropero AB, onso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Lemann J, Jr, Doumas BT. Proteinuria in health and disease assessed by measuring the urinary protein/creatinine ratio. Clin Chem. 1987;33:297–299. [PubMed] [Google Scholar]
- 29.Ziegler RG, Faupel-Badger JM, Sue LY, Fuhrman BJ, Falk RT, Boyd-Morin J, Henderson MK, Hoover RN, Veenstra TD, Keefer LK, Xu X. A new approach to measuring estrogen exposure and metabolism in epidemiologic studies. J Steroid Biochem Mol Biol. 2010 doi: 10.1016/j.jsbmb.2010.03.068. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fishman J, Bradlow HL, Schneider J, Anderson KE, Kappas A. Radiometric analysis of biological oxidations in man: sex differences in estradiol metabolism. Proc Natl Acad Sci USA. 1980;77:4957–4960. doi: 10.1073/pnas.77.8.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kim SO, Lee HD, Chung BC. Estrogens and polyamines in breast cancer: their profiles and values in disease staging. Cancer Lett. 1998;133:47–56. doi: 10.1016/s0304-3835(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 33.Matzkin H, Soloway MS. Cigarette smoking: a review of possible associations with benign prostatic hyperplasia and prostate cancer. Prostate. 1993;22:277–290. doi: 10.1002/pros.2990220402. [DOI] [PubMed] [Google Scholar]
- 34.Todorovic R, Devanesan P, Higginbotham S, Zhao J, Gross ML, Rogan EG, Cavalieri EL. Analysis of potential biomarkers of estrogen-initiated cancer in the urine of Syrian golden hamsters treated with 4-hydroxyestradiol. Carcinogenesis. 2001;22:905–911. doi: 10.1093/carcin/22.6.905. [DOI] [PubMed] [Google Scholar]
- 35.Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23:329–333. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- 36.Markushin Y, Gaikwad N, Zhang H, Kapke P, Rogan EG, Cavalieri EL, Trock BJ, Pavlovich C, Jankowiak R. Potential biomarker for early risk assessment of prostate cancer. Prostate. 2006;66:1565–1571. doi: 10.1002/pros.20484. [DOI] [PubMed] [Google Scholar]
- 37.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 38.Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150:S259–S265. [PubMed] [Google Scholar]
- 39.Pribluda VS, Gubish ER, Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- 40.Schumacher G, Neuhaus P. The physiological estrogen metabolite 2-methoxyestradiol reduces tumor growth and induces apoptosis in human solid tumors. J Cancer Res Clin Oncol. 2001;127:405–410. doi: 10.1007/s004320000233. [DOI] [PubMed] [Google Scholar]
- 41.Lavallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62:3691–3697. [PubMed] [Google Scholar]
- 42.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 43.Schumacher G, Kataoka M, Roth JA, Mukhopadhyay T. Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res. 1999;5:493–499. [PubMed] [Google Scholar]
- 44.Etzioni R, Cha R, Feuer EJ, Davidov O. Asymptomatic incidence and duration of prostate cancer. Am J Epidemiol. 1998;148:775–785. doi: 10.1093/oxfordjournals.aje.a009698. [DOI] [PubMed] [Google Scholar]
- 45.Leymarie P, Roger M, Castanier M, Scholler R. Circadian variations of plasma testosterone and estrogens in normal men. A study by frequent sampling. J Steroid Biochem. 1974;5:167–171. doi: 10.1016/0022-4731(74)90124-1. [DOI] [PubMed] [Google Scholar]
- 46.Crawford ED, Barqawi AB, O’Donnell C, Morgentaler A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509–513. doi: 10.1111/j.1464-410X.2007.07022.x. [DOI] [PubMed] [Google Scholar]


