Abstract
The BTBR T+tf/J inbred mouse strain displays a variety of persistent phenotypic alterations similar to those exhibited in autism spectrum disorders. The unique genetic background of the BTBR strain is thought to underlie its lack of reciprocal social interactions, elevated repetitive self-directed grooming and restricted exploratory behaviors. In order to clarify the existence, range and mechanisms of abnormal repetitive behaviors within BTBR mice, we performed detailed analyses of the microstructure of self-grooming patterns and noted increased overall grooming, higher percentages of interruptions in grooming bouts and a concomitant decrease in the proportion of incorrect sequence transitions compared to C57BL/6J inbred mice. Analyses of active phase home cage behavior also revealed an increase in stereotypic bar-biting behavior in the BTBR strain relative to B6 mice. Finally, in a novel object investigation task, BTBR mice exhibited greater baseline preference for specific unfamiliar objects as well as more patterned sequences of sequential investigations of those items. These results suggest that the repetitive, stereotyped behavior patterns of BTBR mice are relatively pervasive and reflect both motor and cognitive mechanisms. Furthermore, other pre-clinical mouse models of autism spectrum disorders may benefit from these more detailed analyses of stereotypic behavior.
Keywords: BTBR, autism, repetitive behavior, stereotypy, restricted interests, bar-biting, self-grooming
Introduction
Repetitive, stereotyped behaviors are often exhibited by laboratory rodents (Garner & Mason, 2002). While these behaviors are commonly considered to be indicative of barren housing conditions, aberrant responses to environmental stressors, or inability to complete goal-directed actions and coping strategies (Lewis et al, 2007; Rushen et al, 1993; Wiedenmayer, 1997), they are useful in modeling the stereotyped behaviors of human psychiatric disorders (Berridge et al, 2005; Eilam et al, 2006; Garner and Mason, 2002; Hart et al, 2010). Autism spectrum disorder (ASD) diagnosis includes stereotyped interests or behaviors in addition to impaired social behavior (APA, 2000).
Existing mouse models of ASD include qualitative and quantitative analyses of perseverative motor behaviors, particularly self-directed behaviors and restricted object exploration. The BTBR T+tf/J (BTBR) inbred mouse strain displays a variety of physical and behavioral abnormalities that constitute reliable face validity for modeling ASD (McFarlane et al, 2008). In addition to profound, and well-replicated reductions in social approach and social communication, BTBR mice exhibit elevations of repetitive self-grooming behavior in their home cage, clean novel cages, and within semi-natural environments (McFarlane et al, 2008; Pobbe et al, 2010; Yang et al, 2007a,b, 2009). Despite the regularity with which the repetitive behavior phenotype is displayed in the BTBR mouse, few experimental studies have been performed to characterize its causes, development, or function.
Although BTBR mice show deficits in all domains of ASD symptoms, alternative explanations for the presence of specific behaviors should be explored. For instance, the patterned hair loss of BTBR mice (Lyon, 1956) may account for elevations in repetitive self-grooming; additional or alternative analyses of stereotypy may be necessary to avoid these potential confounds. Other spontaneous cage stereotypies such as back-flipping, jumping, bar-biting, and cage top twirling have been characterized in laboratory mice. These behaviors are often analyzed in models of neurodegeneration, psychostimulant sensitivity, and aberrant striatal systems (e.g. Hart et al, 2010; Ishiguro et al, 2007; Kuczenski et al, 1991; Lewis et al, 2007; Thornburg & Moore, 1975; Würbel et al, 1996) or by researchers concerned with the animal welfare (Mason, 1991). Although a great deal of investigation has been performed to elucidate the neurobiology of abnormal repetitive behavior (see Rapp & Vollmer, 2005 and Langen et al, In Press for recent reviews) few, if any, laboratories are investigating these behaviors in animal models of ASD.
Distinctions in “lower-order” motor stereotypies (e.g. movements, self-injurious behavior, repetitive object manipulation) from “higher-order,” or cognitive stereotypies which include, among other things, aversion to change (Lewis et al, 2007; Moy et al, 2008) may be an important distinction in assessing candidate models of ASD. In a hole board task, BTBR mice display inflexibility in exploratory behavior and fail to shift exploration away from a familiar bedding stimulus to a palatable food odor (Moy et al, 2008). These results suggest that the BTBR mouse displays both aberrant motor and cognitive stereotypy, but the range of potential abnormal behaviors present in this mouse strain has not been exhaustively studied. Herein, we provide detailed analyses of the grooming microstructure and quantified a bar-biting stereotypy in BTBR mice compared to C57BL/6J (B6) mice. We also report results of a new task to measure restricted object exploration in BTBR versus B6 mice.
Materials and Methods
Animals and Housing
Subjects were male C57Bl/6J (B6) and BTBR T+ tf/J (BTBR) mice bred from Jackson Laboratory (Bar Harbor, ME) stock. Mice were weaned at post-natal day 25 and housed 4–5 per same sex groups in standard polypropylene mouse cages measuring 26.5 × 17 × 11.5 (H) cm. Two independent cohorts of mice were tested. Non-naïve, individually housed mice of both strains were assessed for two forms of motor stereotypies. These mice were individually housed 10 days prior to behavioral experiments and were tested between 17 and 20 weeks of age; the mice had been previously tested in social behavior tasks (Pobbe et al, 2010) three weeks prior to motor stereotypy analysis. These mice remained individually housed in order to prevent aggression associated with re-grouping male mice and to allow recording of cage-related stereotypy of individual mice. An additional cohort of socially-housed, naïve 20 to 25 week old B6 and BTBR mice was assessed for cognitive stereotypy. All animals were maintained under a 12:12 hour light-dark schedule (lights on at 06:00) with constant access to standard rodent chow and tap water. All procedures were performed according to protocols approved by the University of Hawaii’s Laboratory Animal Services (LAS) in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Behavioral Testing
Grooming Analysis
Mice were individually assessed for grooming microstructure in a clear Plexiglas chamber measuring 14 × 7 × 30 (H) cm (Fig 1) under normal fluorescent lighting conditions. Standard lighting was utilized to ensure video quality, and the dimensions of this arena were chosen to limit the locomotion of the animal but not physically restrain it. An aluminum lid that permitted air circulation was placed over the top of the chamber to prevent escape. Mice were relocated to the behavior room at least 30 minutes prior to grooming analysis and remained in their home cages until testing which was conducted between 10:00 and 16:00 hours. The grooming chamber was disinfected with 70% ethanol and paper towels and dried completely between subjects. Two digital cameras were used to collect video from the frontal and side aspect so that the mouse’s grooming behavior was always visible. Videotapes were scored using Observer software (Noldus Information Technology, Wageningen, The Netherlands) for the frequency and duration of paw licking, head washing, body grooming, leg licking, and tail/genital grooming. At the end of the 30 minute session, the mice were removed, and the number of hairs left on the bottom surface was manually counted for each individual. To further characterize the progressive nature of the grooming microstructure, we applied a previously validated syntactical grooming analysis (Kalueff et al, 2007). The variables listed in Table 1 were calculated to provide an interpretation of the disruptions and incorrect transfers within and between grooming bouts for each strain.
Figure 1.
Analysis chamber for high quality video tape collection and scoring of self-grooming microstructure.
Table I.
Grooming variables
| Bouts | At least one episode of any category of grooming, or an uninterrupted sequence of grooming types. Bouts are divided by at least 6 seconds of inactivity or by an activity other than grooming |
| Interrupted Bouts | A grooming bout that is interrupted by less than 6 seconds |
| % Interrupted Bouts | The proportion of grooming bouts with interruptions (Interrupted bouts divided by total bouts) |
| Transitions | The total number of transfers between grooming types |
| Incorrect Transitions | Transfers between grooming types which do not follow the cephalo-caudal progression (0-No Grooming, 1-Paw Licking, 2-Head Wash, 3-Body Groom, 4-Leg Licking, 5-Tail/Genital) |
| % Incorrect Transitions | The proportion of transitions that are incorrect (Incorrect transitions divided by total transitions) |
Bar-Biting
Twenty-one days following grooming analysis, the same mice were placed in the behavior room at 16:00 hours and remained within their individual home cages. The micro-isolator top of each cage was removed, leaving the wire tops, feed, and water bottles in place. The room was then left undisturbed. A video camera located at an inclined angle to the tops of two adjacent cages permitted clear visualization of the biting of the wire mesh cage tops. This enabled collection of video from four sets of two cages simultaneously. DVD recorders were programmed to collect video from 18:00 to 22:00 hours during the beginning of the active, dark period under infrared illumination. Videotapes were then time-sampled for the presence or absence of bar-biting during a 60 second scan every 10 minutes for the entire duration. Bar-biting was only scored when mice clearly placed their mouth over a bar on the cage lid for a minimum of one second without any attention, sniffing, or oral manipulation of food occurring during the bout of the behavior. Singly- housed animals are required for this analysis to determine individual frequencies.
Repetitive Novel Object Contact Task
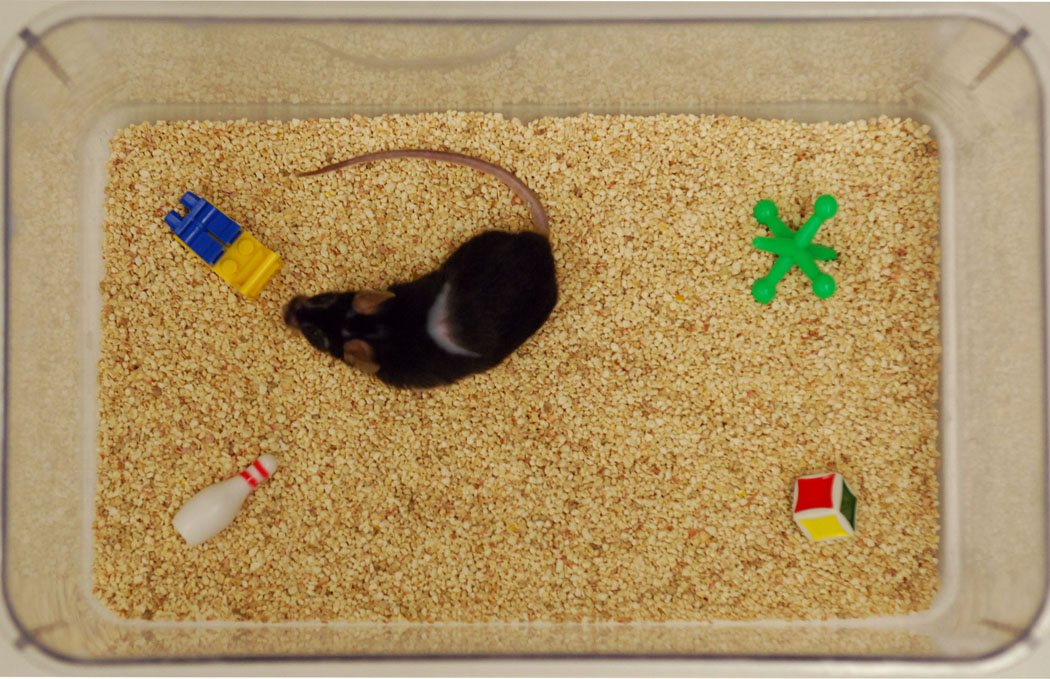
Additional groups of B6 and BTBR mice were assessed for the frequency of repetitive contacts with novel objects. On the first day, subjects were transported to the experimental room and left undisturbed for 30 min prior to habituation. The experimental room was illuminated with a dim red light (40 lux) in an attempt to reduce anxiety and the tests were conducted between 10:00 to 16:00 hours. To further reduce anxiety, each mouse was previously habituated to a standard polypropylene cage, 26.5 × 17 × 11.5 (H) cm, with the floor covered by a layer of sawdust bedding (1 cm), during a 20 minutes session on the day prior to testing under the same lighting condition. The cage was covered by a polypropylene micro-isolator lid, 29 × 18 × 10 (H) cm, with the filter element removed which allowed the recording of subject behavior from an upper view while preventing escape. On the following day, mice were then individually placed within an identical clean cage containing fresh sawdust bedding as well as four novel objects located approximately 4 cm from each of the four corners (Fig 2). The objects were four distinct small children’s toys: a Lego® piece (3 cm length), a jack (4 cm length), a dice (1.5 cm length), and a bowling pin (3.5 cm length) which were all made out of high-density plastic to prevent chewing. Each mouse was then able to investigate the environment and objects during a 10 minute session. A video camera mounted above the cage was used to record the test. The arrangement of objects was identical for all subject mice, and each object was thoroughly cleaned with 20% ethanol and dried between trials.
Figure 2.
BTBR mouse in a standard mouse cage with four novel objects for the repetitive novel object contact task. The micro isolator lid has been removed for the photograph.
Recorded DVDs were scored for the occurrence of investigation of each of the four toys. Investigation was defined as clear facial or vibrissae contact with or burying of the novel objects. The occurrence of repetitive contact with three and four toys and the frequency of times that mice buried each object were counted. Total frequency of contact with each of the four toys, and the total number of burying episodes were also calculated. In order to determine if there was a strain effect on the tendency to display preferences for particular toys, the frequencies of contact with each object were ranked in decreasing order from maximum to minimum preference (contact) values for each subject, and the frequencies were averaged by strain, and compared.
Statistical Analyses
The frequency and duration of all self grooming variables (mean frequency and duration of grooming subtypes, GAA indices, and number of hairs lost) were analyzed with unpaired t-tests to compare strain means. To analyze strain effects on the temporal pattern of bar-biting, the total number of scans in which the focal mouse was displaying the behavior were tallied for every 30 minutes and means were analyzed with a two-way repeated measures ANOVA with strain as the between subjects and time as the within-subjects factor. Additionally, the mean incidence of bar-biting for each of the strains across all 24 scans was compared with an unpaired t-test. To assess the pattern of object investigation in the toy stereotypy task, each specific toy was given an arbitrary number (1–4) and the video scored and a record created for the sequence of investigation. The total number of identical three- and four-object sequences was then identified in the coding for each subject and the means for B6 and BTBR mice compared with unpaired t-tests. However, in a series of three- or four-object investigations, visits to the same item were included provided they were interrupted by a visit to another toy. For instance, repetitive sequences of visits such as [bowling pin, jack, jack, dice] were not included; however, sequences such as [jack, bowling pin, jack, dice] were. Finally, the average ranked toy preference scores for the individuals within each strain were compared with unpaired t-tests. GraphPad Prism (v. 4) software was used for all statistical tests and graphs. All analyses were two-tailed and p-values less than or equal to 0.05 were considered significant.
Results
Repetitive Self-Grooming
BTBR mice displayed elevations in the frequencies of paw licking (t(22)=−2.503, p=0.020), head wash (t(22)=−3.193, p=0.004), body groom (t(22)=−3.429, p=0.002), leg licking (t(22)=−3.608, p=0.002), and tail/genital grooming (t(22)=−2.994, p=0.007, Fig 3a upper panel) relative to B6 mice. The durations of head wash (t(22)=−2.599, p=0.016), body groom (t(22)=−2.634, p=0.015), leg licking (t(22)=−4.239, p<0.001), and tail/genital grooming (t(22)=−2.367, p=0.027) were also significantly elevated (Fig 3a lower panel). BTBR mice also showed a significant increase in the number of hairs lost during the 30 minute self grooming session (Mean ± 1. S.E.M. B6= 2.500 ± 0.957 hairs; BTBR= 10.667 ± 1.293 hairs; t(22)=−5.076, p<0.001). None of the grooming variables significantly correlated with the number of hairs lost (data not shown).
Figure 3.
In the self-grooming analysis, BTBR mice displayed increased frequencies of all grooming subtypes (Fig. 3a upper panel) and increased duration of head wash, body groom, leg licking, and tail/genital grooming relative to B6 mice (Fig. 3a lower panel). B6 and BTBR mice showed comparable grooming bouts, and showed no significant difference in the number of interrupted bouts (Fig. 3b left panel). BTBR mice showed increased correct and incorrect transitions in grooming sub-types (Fig. 3b right panel). BTBR mice showed an increased proportion if bouts that were interrupted, and a decrease in the proportion of transitions that were incorrect (Fig. 3c) N=12/ group, * p<0.05, ** p<0.01, *** p<0.001.
Figure 3b presents the number of grooming bouts and interrupted bouts, as well as the frequency of transitions between grooming stages and the incorrect transitions in which the normal cephalo-caudal sequence of grooming was not seen (Kalueff et al, 2004, 2007). While the absolute number of bouts and interrupted bouts were comparable for the two strains, the percentage of interrupted bouts was significantly increased in the BTBR strain (t(22)=−2.725, p=0.012, Fig 3b). BTBR mice exhibited more transitions between grooming stages (t(22)=−3.589, p=0.002), and showed more incorrect transitions (t(22)=−2.134, p=0.044, Fig 3b). However, the percentage of incorrect transitions was lower in the BTBR mice (t(22)=3.489, p=0.002, Fig 3c). These results suggest that while the BTBR mice show more overall grooming, there is a disproportionate increase in the percentage of interrupted bouts. Although increased transitions between body-site stages are characteristic of BTBR mice, these transitions are more likely than those of the B6 mice to involve a rigorous pattern of repetitive, self-directed behavior.
Bar-Biting
Two-way, repeated measures ANOVA indicated a significant main effect for time (F(7,22)=14.1, p<0.0001) and for strain (F(1,22)=5.145, p=0.034, Fig 4a) indicating that bar-biting behavior changes across the period in both strains, but BTBR engage in bar-biting more than B6 mice. When collapsing across all 24 scans, BTBR mice showed higher incidences of bar-biting behavior (t(22)=2.617, p=0.012, Fig 4b).
Figure 4.
Significantly increased prevalence of stereotypic bar-biting in BTBR mice across the first four hours of the dark phase (Fig. 4a). BTBR mice also showed a higher average prevalence of bar-biting during all 24, 10 minute scans during this period (Fig. 4b). N=12/group, * p<0.05.
Repetitive Novel Object Contact Task
The frequency of object investigation did not significantly differ between the strains (Fig 5a). B6 and BTBR mice showed distinct preference for each of the four novel objects in the novel toy investigation task (Lego® t(18)= 3.073, p=0.007; jacks t(18)=−3.236, p=0.005; dice t(18)=−2.215, p=0.040; bowling pin t(18)=3.014, p=0.007, Fig 5b). When the percentage preference for each object for each mouse was ranked, and the strength of those ranks compared between strains, BTBR mice showed significantly stronger preferences for the first two ranks (t(18)=−3.331, p=0.004; t(18)=−2.276, p=0.035, respectively) and a significantly lower preference for the last ranked object compared to B6 mice (t(18)=5.415, p<0.001, Fig 5c). Finally, the number of investigations showing a specific sequential pattern of visits to three or four specific toys was significantly higher for BTBR mice (t(18)=−2.620, p=0.017; t(18)=−3.108, p=0.006, respectively, Fig 5d). These results indicate that BTBR mice show more consistency in their pattern of object exploration and stronger spontaneous object preferences than do B6 mice.
Figure 5.
BTBR and B6 mice showed comparable total numbers of visits to the novel objects (Fig. 5a). BTBR and B6 mice showed significantly different proportions of visits to each of the four novel objects (Fig. 5b). When toy preferences were ranked and ordered for each subject, then averaged across the strain and compared, it was revealed that BTBR mice show a more inequitable preference for certain objects compared to C57BL/6J mice (Fig. 5c). BTBR mice showed increased numbers of identical visits to the same order of three objects and four objects in sequence (Fig. 5d). N=10/group, * p<0.05, ** p<0.01. *** p<0.001.
Discussion
Twin studies have revealed the distinct genetic contributions to the social and repetitive behavior domains of ASD (Ronald et al, 2005, 2006) suggesting the value of separation of these two main symptom clusters in rodent models. This set of studies was directed specifically at a more complete analysis of repetitive or stereotyped behaviors in the BTBR mouse model of autism-like behaviors.
Rodents spend a vast proportion of their time grooming; 30–50% of total time has been noted in laboratory rodents (Bolles, 1960; Spruijt et al, 1992); determining a threshold between normal and disrupted grooming can be difficult. As stereotyped behaviors can be, and often are considered abnormal while many other normal behaviors (e.g. vocalizations, consummatory behavior, and grooming) form consistent, repetitive patterns illustrates the complexity of this distinction in laboratory animals (Eilam et al, 2006; Mason 1991). This is also the case when attempting to differentiate appropriate repetitive behavior from abnormal sequences in clinical populations (Lewis et al, 2007). Quantitative differences in the “content” and “spatial and temporal organization” of behaviors (Eilam et al, 2006) may be helpful in determining their classification as normal or not. Fundamental investigations of sequenced behavioral patterns (Berridge, 1989, 1990; Berridge et al, 2005; Fentress & Stilwell, 1973) and the recent analyses of Kalueff and colleagues differentiating the syntactic patterning of grooming in mice have provided new approaches to this problem. In agreement with a number of previous studies (McFarlane et al, 2008; Pobbe et al, 2010; Yang et al, 2007a,b, 2009), the BTBR mice in this study showed enhanced self-grooming, which involved, in terms of frequency, duration, or both, of each of the target regions (paws, head, body, legs, and tail/genitals).
Kalueff & Tuohimaa (2004, 2005) reported that stress increases the percentage of interrupted grooming bouts and bouts that involved an incorrect sequence in the cephalo-caudal progression of grooming; the latter study found a shift in emphasis from caudal to rostral grooming associated with stress. It is notable that the BTBR mice demonstrated one aspect of this pattern, the increased percentage of interrupted bouts, but they also displayed a decreased proportion of incorrect transitions with no evidence of a shift in grooming from a caudal to rostral emphasis.
This pattern is inconsistent with regard to a stress interpretation, in line with a literature suggesting that anxiety levels in BTBR mice are higher, lower, or the same as those of B6 mice (McFarlane et al, 2008; Moy et al, 2007; Pobbe et al, In Press; Yang et al, 2009). However, the reduced percentage of incorrect transitions indicates that BTBR self grooming is more invariant than that of B6 mice- a phenotypic trait important for animal models of ASD.
A very different interpretation of the BTBR grooming increases is based on common observations that these mice, with a mutation in the tufted (tf) gene, show enhanced patterns of hair loss. If this condition is associated with pruritis, then their increased grooming may simply reflect a normal response to itching. The present finding that the numbers of hairs lost during self-grooming sessions do not significantly correlate to any grooming variables between and within the BTBR and B6 strains does not support a view that a condition associated with hair loss as well as itching can account for the elevated self-grooming phenotype. However, these findings, based in relatively few animals, cannot preclude a role for pruritis in the enhanced grooming of BTBR mice.
Bar-biting is another common stereotyped motor behavior exhibited in laboratory rodent strains (Nevison et al, 1999; Würbel et al, 1996). Casual observation of wire gnawing in the home cage of both the B6 and BTBR strains, as well as the ease of identification for scoring led us to investigate this particular behavior; we have not noted incidences of back-flipping, jumping, or circling but these have not been formally investigated. Unpublished observations on our part indicate that BTBR mice display cage-top twirling, but also note that this behavior may be more subjective in its identification relative to bar biting.
Our data indicated that B6 mice perform bar-biting, on average, in 39% of scans while BTBR mice exceed 55% percent of scans during the first four hours of the dark phase. This highly repetitive and seemingly purposeless behavior is often assessed in laboratory rodents as a welfare indicator due mostly to findings associating it with barren caging and its reduction with enrichment (Garner & Mason, 2002; Lewis et al, 2007). However, increasing evidence is being revealed which demonstrates the analogous neural circuitry (particularly those within the basal ganglia) in rodent cage stereotypies and the existing theories of the neurobiology of repetitive behavior in ASD, obsessive-compulsive spectrum, psychotic, and other psychiatric disorders (Berridge, 1989; Eilam et al, 2006; Garner & Mason, 2002; Hart et al, 2010; Langen et al, In Press; Lewis et al, 2007; Rapp & Vollmer, 2005). Other commonly occurring cage stereotypies such as jumping, twirling on the wire cage lid, back-flipping, and circling, and potentially marble burying may indeed be overlooked variables for animal models of neurodevelopmental disorders (Lewis et al, 2007; Ryan et al, 2010; Thomas et al, 2009).
The elevated motor stereotypies exhibited in BTBR mice could conceivably result from augmented stress responses within the contexts under which the behaviors are exhibited. BTBR mice appear to have altered stress responses (Benno et al, 2009; Frye & Llaneza, 2010), and both bar-biting and self-grooming behavior appear to be elicited by stress, or associated with aversive stimuli or conditions (Denmark et al, 2010; Nevison et al, 1999). This interpretation may not be contradictory to an involvement of stress in clinical autism symptoms (Lewis & Bodfish 1998), and may simply reflect the informative nature of these variables in future analyses of candidate strains irrespective of their possible etiologies.
Although motor stereotypies may be less common in autistic children (Borreau et al, 2009) than are cognitive, higher-order stereotypies, fewer attempts have been made to model the restricted interest and insistence on sameness that characterize the latter, in mouse models of autism-like behavior (Lewis et al, 2007; Moy et al, 2008; Silverman et al, 2010). Here, we exposed mice to four small novel objects and noted that BTBR mice showed stronger preferences for specific objects, and a stronger preference differentiation among the four objects, than did B6 mice. In addition, BTBRs showed significantly higher numbers of visits that included a repetitive sequence of 3 or 4 objects. This pattern was not mediated by higher numbers of visits overall, as these were not significantly different for the two groups. These findings indicate that the stereotypies of BTBR mice are not confined to motor behaviors, but encompass a tendency to prefer some objects over others, as well as a more repetitive pattern of sequential approaches to these objects. Repetitive investigation of sequences of objects exceeding four items, or comparisons of durations of object investigation were not performed, but may prove to be important variables for future studies. These findings are in agreement with previous reports that BTBR mice show a reluctance to investigate new stimuli over a familiar one in a hole board task (Moy et al, 2008).
The object preferences and more invariant patterning of object exploration in this study provide striking parallels to the reduced toy exploration noted in children with ASD diagnoses (Pierce & Courchesne, 2001) relating to stereotyped patterns of behavior, interests, and activities (APA, 2000). These include an “encompassing preoccupation with one or more stereotyped and restricted patterns of interest…inflexible adherence to specific, nonfunctional routines or rituals… (and) a persistent preoccupation with parts of objects (APA, 2000).” The present data indicate that BTBR mice show such “cognitive” aspects of stereotypy, in addition to enhanced display of motor sequences such as those involved in grooming and bar-biting. This suggests the potential value of incorporating object preferences and sequential object exploration analyses in an array of tasks designed to provide a more comprehensive and selective set of indices for animal models of ASD-like behavior.
These findings also suggest the possibility of a fundamental change in the way the inbred BTBR strain might be viewed, and used, in the study of autism and other ASD. In view of the many and profound social and cognitive differences between humans and mice, it is presumptuous and likely incorrect to suggest that the BTBR mouse is, in fact, autistic. However, evidence is rapidly accumulating which indicated that these mice show a host of behaviors that provide clear parallels to each of the major symptom complexes of autism. BTBR mice show deficits in social approach and reciprocal social interactions (McFarlane et al, 2008; Pobbe et al, 2010) as well as social communication impairments in ultrasonic vocalizations and scent marking (Scattoni et al, 2008; Wöhr et al, In Press). BTBR mice display repetitive self grooming behavior (McFarlane et al, 2008; Silverman et al, 2010) and resistance to change (Moy et al, 2008). The range and strength of these parallels, as well as the degree to which these effects are replicated across labs, suggest that BTBR mice may, in fact, represent aberrations in biological systems that are similar to some of those underlying ASD.
In summary, the inbred BTBR mouse strain exhibits stereotyped behavior which extends beyond dysregulated self-grooming patterns. BTBR mice exhibit elevated instances of active phase bar-biting in their home cage, as well as a patterned, and repetitive pattern of four-choice novel object exploration. The BTBR strain displays deficits in all clusters of ASD symptoms; our investigation of repetitive stereotyped behaviors suggests that these traits may be reflective of functional homologies rather than superficial parallels in restricted interests and behaviors seen in the clinical population and within this inbred model. Such phenomena might be helpful in detecting clinically-relevant endophenotypes that are collectively present in the BTBR strain, which, in turn can be applied in other candidate strains and mutants for the ultimate goal of unraveling the complex psychopathology of ASD.
Acknowledgements
This study was funded by NIH grant MH081845 to RJB. Mr. Ted Murphy constructed behavioral testing arenas.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) Washington, DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- Benno R, Smirnova V, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Substantia nigra 6-OHDA lesions mimic striatopallidal disruption of syntactic grooming chains: a neural systems analysis of sequence control. Psychobiology. 1989;17:377–385. [Google Scholar]
- Berridge KC. Comparative fine structure of action: Rules of form and sequence in the grooming patterns of six rodent species. Behavior. 1990;113:21–56. [Google Scholar]
- Berridge KC, Aldridge JW, Houchard KR, Zhuang X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: a model of obsessive compulsive disorder and Tourette's. BMC Biology. 2005;3:4. doi: 10.1186/1741-7007-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC. Grooming behavior in the rat. J Comp Physiol Psychol. 1960;53:306–310. doi: 10.1037/h0045421. [DOI] [PubMed] [Google Scholar]
- Bourreau Y, Roux S, Gomot M, Barthélémy C. Repetitive and restricted behaviours (RRB) in autism: clinical evaluation. [Article in French] Encephale. 2009;35:340–346. doi: 10.1016/j.encep.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Denmark A, Tien D, Wong K, Chung A, Cachat J, Goodspeed J, Grimes C, Elegante M, Suciu C, Elkhayat S, Bartels B, Jackson A, Rosenberg M, Chung KM, Badani H, Kadri F, Roy S, Tan J, Gaikwad S, Stewart A, Zapolsky I, Gilder T, Kalueff AV. The effects of chronic social defeat stress on mouse self-grooming behavior and its patterning. Behav Brain Res. 2010;208:553–559. doi: 10.1016/j.bbr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Eilam D, Zor R, Szechtman H, Hermesh H. Rituals, stereotypy and compulsive behavior in animals and humans. Neurosci Biobehav Rev. 2006;30:456–471. doi: 10.1016/j.neubiorev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Fentress JC, Stilwell FP. Grammar of a movement sequence in inbred mice. Nature. 1973;244:52–53. doi: 10.1038/244052a0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–267. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav Brain Res. 2002;136:83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- Hart PC, Bergner CL, Dufour BD, Smolinsky AN, Egan RJ, LaPorte JL, Kalueff AV. Analysis of abnormal repetitive behaviors in experimental animal models. In: Warnick JE, Kalueff AV, editors. Translational Neuroscience in Animal Research: Advancement, Challenges, and Research Ethics. Hauppauge NY: Nova Science Publishers; 2010. pp. 71–82. [Google Scholar]
- Ishiguro A, Inagaki M, Kaga M. Stereotypic circling behavior in mice with vestibular dysfunction: asymmetrical effects of intrastriatal microinjection of a dopamine agonist. Int J Neurosci. 2007;117:1049–1064. doi: 10.1080/00207450600936874. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Grooming analysis algorithm for neurobehavioral stress research. Brain Res. 2004;13:151–158. doi: 10.1016/j.brainresprot.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in three mouse strains markedly different in anxiety and activity (129S1, BALB/c and NMRI) Behav Brain Res. 2005;160:1–10. doi: 10.1016/j.bbr.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2:2538–2544. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Kas MJ, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2010.02.004. (In Press) doi:10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Ment Retard Dev Disabil Res Rev. 1998;4:80–89. [Google Scholar]
- Lyon MF. Hereditary hair loss in the tufted mutant of the house mouse. J Hered. 1956;47:101–103. [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison CM, Hurst JL, Barnard CJ. Why do male ICR(CD-1) mice perform bar-related (stereotypic) behaviour? Behav Processes. 1999;47:95–111. doi: 10.1016/s0376-6357(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Pobbe RLH, Pearson BL, Defensor EB, Bolivar VB, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+tf/J mouse strain. Behav Brain Res. doi: 10.1016/j.bbr.2010.08.039. (In Press) doi:10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp JT, Vollmer TR. Stereotypy II: a review of neurobiological interpretations and suggestions for an integration with behavioral methods. Res Dev Disabil. 2005;26:548–564. doi: 10.1016/j.ridd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happé F, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviors characteristic of autism. Dev Sci. 2005;8:444–458. doi: 10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happé F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushen J, Lawrence AB, Terlouw EMC. The motivational basis of stereotypies. In: Lawrence AB, Rushen J, editors. Stereotypic animal behavior: fundamentals and applications to welfare. Wallingford, England, UK; Tucson, AZ, USA: CAB International; 1993. pp. 41–64. [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and the neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg JE, Moore KE. Supersensitivity to dopamine agonists following unilateral, 6-hydroxydopamine-induced striatal lesions in mice. J Pharmacol Exp Ther. 1975;192:42–49. [PubMed] [Google Scholar]
- Wiedenmayer C. Causation of the ontogenetic development of stereotypic digging in gerbils. Anim Behav. 1997;53:461–470. [Google Scholar]
- Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. doi: 10.1111/j.1601-183X.2010.00582.x. (In Press) doi:10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würbel H, Stauffacher M, von Holst D. Stereotypies in laboratory mice – quantitative and qualitative description of the ontogeny of ‘wire gnawing’ and ‘jumping’ in Zur:ICR and Zur:ICR nu. Ethology. 1996;102:371–385. [Google Scholar]
- Yang M, Scattoni ML, Zhodzishky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]