Abstract
Major depression is a growing problem worldwide with variation in symptoms and response to treatment. Individual differences in response to stress may contribute to such observed individual variation in behavior and pathology. Therefore, we investigated depressive-like behavior following exposure to repeated social defeat in a rat model of individual differences in response to novelty. Rats are known to exhibit either high locomotor activity and sustained exploration (high responders, HR) or low activity with minimal exploration (low responders, LR) in a novel environment. We measured anhedonia using the sucrose preference test in HR and LR rats following exposure to social defeat stress or in basal, non-defeated conditions. We then compared histone acetylation in the hippocampus in HR and LR defeat and non-defeated rats and measured mRNA levels of histone deacetylases (HDAC) 3, 4, 5, and CREB binding protein (CBP). We found that basally, HR rats consumed more sucrose solution than LR rats, but reduced consumption after exposure to defeat. LR rats’ preference was unaffected by social defeat. We found that HR rats had higher levels of histone acetylation on H3K14 and H2B than LR rats in non-stress conditions. Following defeat, this acetylation pattern changed differentially, with HR rats decreasing acetylation of H3K14 and H2B and LR’s increasing acetylation of H3K14. Acetylation on histone H4 decreased following defeat with no individual variation. Basal differences in CBP expression levels may underlie the observed acetylation pattern; however we found no significant effects of defeat in levels of HDACs 3, 4, 5 in the hippocampus.
Keywords: Social Defeat, Individual differences, Response to novelty, Histones, Histone deacetylases, Creb binding protein
Introduction
Major depression is a devastating illness characterized primarily by depressed mood and loss of interest in previously pleasurable pursuits (anhedonia) (DSM IV-TR 2000). Depression is a global problem, rated as the fourth leading contributor to disease burden in the world, with an estimated 121 million people classified as clinically depressed in 2000 (Moussavi et al., 2007). This situation is particularly acute not only because much of depression goes undiagnosed and untreated, but also because a significant proportion of depression is treatment resistant for reasons that have yet to be elucidated. While we do not have direct animal models of psychiatric disorders, we do have the tools to study in animals some of the variables that represent components of these human disorders, including emotional responsiveness and reactivity to stress.
The most common stressors in humans are of a psychological or social nature (Bjorkqvist 2001; Kessler 1997; Kessler et al. 1985) and therefore, using social conflict between members of the same species to generate stress has an obvious advantage over animal models that require aversive physical stimuli. Chronic social defeat in particular has the advantage of ecological and ethological validity (Tidey and Miczek 1997). We used a model of social conflict called social defeat, where a subordinate male rat replaces the female rat in the home cage of an aggressive dominant male.
This stressor, unlike many environmental stressors, does not result in habituation upon repeated presentation, and so it generates persistent emotional stress (Tidey and Miczek 1997). When flight is barred, the intruder will assume a submissive supine posture and emit loud and frequent ultrasonic distress calls (Blanchard and Blanchard 1977). Even after a brief encounter with aggressive rats or mice, intruders exhibit elevated glucocorticoid activity, tachychardia and hyperthermia that take many hours to recover (Schurman 1980; Tornatzky and Miczek 1993). Chronic social defeat also induces some long-term changes. These include decreased locomotor and exploratory activity (Koolhaas et al. 1997; Meerlo et al. 1996); reduced aggression and sexual behaviour (Meerlo et al. 1996); increased submissive behavior and anxiety (Ruis et al. 1999). Chronically defeated rats show reduced mobility in a forced swim test, and reduced preference for sweet sucrose solution (anhedonia) as well (Rygula et al. 2005). Reduced locomotor and exploratory activity implies a deficit in motivation while reduced mobility in the forced swim test indicates behavioral despair, a characteristic of depressive disorders. Decreased sucrose preference may indicate desensitization of the brain reward circuitry. Taken together, these findings suggest that chronic social defeat in rats is an appropriate model for depressive disorders.
The numerous behavioral responses resulting from exposure to social defeat underscore the potential for underlying neurobiological alterations. Such behavioral changes may be the products of chromatin modification in response to the experience of social defeat. Chromatin modification is a dynamic process that regulates gene expression without alteration of the DNA sequences (Crosio et al. 2003; Guan et al. 2002). This modification is primarily accomplished through modifications of histone N-terminal tails at the promoter regions of specific genes (Cheung et al. 2000a). Recent studies have found changes in modifications at specific gene promoter regions in association with social defeat (Covington et al., 2009; Wilkinson et al., 2009; Tsankova et al. 2006; Tsankova et al. 2004). One such modification is histone acetylation. Acetylation has been widely studied and it is generally accepted that hyperacetylation leads to an increase in gene expression, while hypoacetylation leads to gene silencing (Forsberg and Bresnick 2001; Ito and Adcock 2002). Acetylation of lysine 14 on histone H3 (H3K14) for instance, has been implicated in the activation of immediate-early genes in the brain (Tsankova et al. 2004). Furthermore, increased acetylation at specific promoter regions of genes such as Brain Derived Neurotrophic Factor (BDNF) has been associated with a reversal of depressive-like behavior following electroconvulsive shock therapy (ECS), while overall increased acetylation in the nucleus accumbens has been associated with depressive-like symptoms in mice (Covington et al. 2009; Tsankova et al. 2004).
To investigate individual response to social stress, we utilized an animal model of individual differences in stress responsiveness. When experimentally naïve rats are exposed to the mild stress of a novel environment, some rats, known as High responders (HR), exhibit high rates of exploratory locomotion while others, known as Low Responders (LR), exhibit low rates of locomotor activity (Hooks et al. 1992; Kabbaj and Akil 2001; Piazza et al. 1989; Pierre and Vezina 1997). The locomotor response in a novel environment not only predicts subsequent behavioral responses to drugs such as amphetamine and cocaine (Hooks et al. 1992; Hooks et al. 1991a; b; Kabbaj and Akil 2001; Piazza et al. 2000; Pierre and Vezina 1997), but also predicts anxiety-related behavior in these animals (Dellu et al. 1996; Kabbaj et al. 2000). HR and LR rats also exhibit different behaviors in the forced swim test and respond differently to fluoxetine treatment with HR rats being more prone to depression-like symptoms (Taghzouti et al. 1999). As individuals vary in their response to stress and depression, we were interested in the individual HR and LR response to social defeat.
Recent studies have linked epigenetic modifications in the hippocampus with the reversal of depressive-like behaviors such as anhedonia (Jiang et al., 2010) and behavioral despair (Schroeder et al., 2007). Therefore, we investigated depressive-like behavior in HR and LR rats following exposure to repeated social defeat with the sucrose preference test. We then examined changes in histone modifications using triton-acid-urea (TAU) and SDS-PAGE gels in HR and LR rats under basal conditions and following repeated social defeat and quantified changes in gene expression for several genes relevant to histone acetylation and potentially depression.
Materials and Methods
In these experiments, a total of one hundred male Sprague-Dawley rats were used. Subjects were housed 2 per cage in clear Plexiglas cages (19 in. × 10.5 in. × 8 in.) and were kept in a 12 h light cycle (lights on at 7am) with food and water provided ad libitum. All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Florida State University. Fifty-two male Sprague-Dawley rats were used in experiment 1, thirty-six male Sprague-Dawley rats were used for experiment 2, and thirty-two male Sprague-Dawley rats were used for experiment 3. All animals were ordered from Charles River Laboratories (Wilmington, MA) at 250–300g. Rats were allowed to habituate to the colony room for three days at which point they were handled, weighed, and numbered on their tails. Additionally, twenty-four vasectomized male Long-Evans rats weighing 325–350 g were pair-housed with twenty-four female Long-Evans rats weighing 200–225 g. These Long-Evans males served as the resident attackers in social defeat and were chosen for consistent aggressive behavior.
After habituation, animals’ locomotor response to novelty was tested in circular activity chambers (Med Associates Inc., St. Albans, Vermont) for one hour to determine their HR/LR phenotypes. Four photo-beam sensors at equal distances recorded each rats’ crossings between adjacent quadrants. Photo-beam breaks were recorded and a locomotor score and was assigned to each rat as previously described (Dietz et al., 2005). Rats were assigned to HR and LR groups based on the position of their locomotor score relative to the median of a normal population of an extensive number of rats (n = 730). The rats that scored in the range of 33% above the median were classified as HR rats (mean = 292.7 ± 6.9 S.E.) and the rats that scored 33% below the median were classified as LR rats (mean = 139.6 ± 4.4 S.E.).
Rats were then divided into four different groups: (a.) HR non-defeated, (b.) LR non-defeated, (c.) HR defeated, and (d.) LR defeated. Animals that were assigned to the defeat group were exposed to an aggressive male for fifteen minutes per day on four consecutive days, while non-defeated animals were left undisturbed in their home cages. In experiment 1, animals (n= 13/group) were exposed to the sucrose preference test and then sacrificed in basal conditions. In experiment two, a separate group of animals (n = 6/group) were exposedto either social defeat or control conditions and then sacrificed by decapitation 2h30 later. Their brains were immediately removed, their hippocampi rapidly dissected out, and frozen. They were then stored at −80°C until processing for detection of histone modifications. Then total histones acid extracted from ½ hippocampi from each rat was resolved on Triton-Acid-Urea (TAU) gels that separates proteins based on hydrophobicity, charge and size (Gunjan et al. 2001; to detect acetylation of H3K14, H2B and H4) or by standard SDS-PAGE (Gunjan and Verreault 2003; to detect total histone H3 for loading). In experiment 3, animals (n = 4–9/group) were exposed to repeated defeat and sacrificed by decapitation 2h30 following the fourth session of defeat. Their brains were immediately removed and frozen. The hippocampus was sectioned at 200 μm and tissue punched at 1.0mm from which total RNA was extracted and used to generate cDNA for real-time qRT-PCR.
Social Defeat Paradigm: For these experiments, we used the protocol for social defeat as previously described by our group (Hollis et al., 2010; Dietz et al. 2008; Kabbaj et al. 2001; Kabbaj et al. 2004). Briefly, the repeated social defeat paradigm consists of four consecutive encounters (for 15 minutes each) in the home cage of an aggressive Long-Evans male rat. Rats were allowed five minutes to physically interact. During this period, if the fighting became too intense or once the intruder adopted a submissive posture and was pinned for more than 3.0 seconds, the intruder rat was transferred to a wire-mesh protective cage which was then placed back in the resident’s cage for the remainder of the 15 minute period. This protective cage allowed for full visual, olfactory, and auditory exposure to the resident without unnecessary harm to the intruder. The cage was large enough for the intruders to move freely (10 x 10 x 15 cm). All intruders were placed in this protective cage only upon being successfully defeated.
Sucrose Preference Test: The sucrose preference test is a two-bottle choice paradigm performed according to the procedure outlined by Bolanos et al. 2008. This test has been used extensively in evaluating stress-induced anhedonia (Willner et al. 1987). Rats were habituated to drink water from two bottles for five days and then exposed to ascending concentrations of sucrose (.25%, 0.5% and 1%) for two days per concentration. The amount of water and sucrose solution was measured at 8:00 and 17:00 daily by experimenters blind to the treatments. The bottles were balanced between the groups once daily at 17:00. The preference for sucrose over water was used as a measure of the rats’ response to a naturally rewarding stimulus.
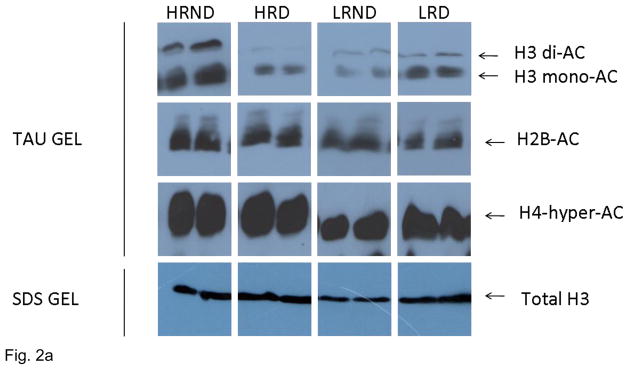
Histone isolation, separation, and detection: Total histones were acid-extracted from rat hippocampi in the presence of proteinase inhibitors. TAU polyacrylamide gel electrophoresis was employed to separate post-translationally modified forms of histone proteins, as described by Zweidler (1984). These gels contained 0.37% Triton X-100, 5% acetic acid, 8m urea, 12% acrylamide, and 0.08% bisacrylamide. Gels were soaked first in a wash solution containing 50mM acetic acid and 0.5% SDS for one hour followed by a second wash solution containing 62.5mM Tris-HCl (pH 6.8), 5% 2-mercaptoethanol, and 2% SDS for thirty minutes. Total hippocampal histones were resolved on SDS-18% polyacrylamide gels. All proteins were transferred to nitrocellulose at 70V in 20% Methanol and 10 mM 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS)-NaOH, pH 11.0 at 4°C for two hours. Membranes were soaked in 5% milk for one hour and then incubated with primary antibody (H3K14ac (1:10000), H2Bac (1:2500), H4hyperacetylayed (1:3000), or total H3; 1:1000; Millipore) overnight. Membranes were washed and incubated with a secondary antibody (1:10000; Jackson ImmunoResearch Laboratories, Inc, Westgrove, PA, USA) for 45 minutes. Proteins were visualized with enhanced chemiluminescence (ECL SuperSignal West Dura substrate; Pierce Biotechnologies, Rockford, IL, USA) and exposed on Fujifilm XAR film (Fuji Film Co., LTD; Tokyo, Japan). Quantification was performed using AIS 6.0 Image software (Imaging Research Inc.; St. Catharines, Ontario, Canada) to look at pixel density x total target area of each band for both acetylated histone and total histone. Data are shown as the ratio of quantified amount of acetylated histone to quantified amount of total histone.
Real-time polymerase chain reaction (RT-PCR): 1 μg of total RNA extracted from the hippocampus of each rat was processed for cDNA synthesis using random hexamers with Invitrogen First-Strand cDNA synthesis kit. cDNA was then input into Bio-Rad real-time PCR reactions in triplicate using iQ SYBR GREEN supermix (Bio-Rad Laboratories). Reactions were monitored using the BioRad iCycler machine, with SyberGreen incorporation as a tracking dye. Amplification specificity was verified with melting curve analysis and all quantification data were processed with an internal standard curve and then normalized to the housekeeping gene NADH and further analyzed via two-way ANOVA (HR/LR x defeat/non-defeat). The primer sequences were designed as follows: 5′-GACCAAGATGGGGATGACTG-3′ (Fwd) and, 5′-CCACTGATGTTTGCAACTGG-3′ (Rev) for Creb-Binding Protein (CBP); 5′-CTAGACCAGATCCGCCAGAC-3′ (Fwd) and 5′-TGGCCTGCTGTAGTTCTCCT-3′ (Rev) for HDAC3; 5′-CTGCGTTTTCCCATACCAGT-3′ (Fwd) and 5′-GAAGGGCAAAGAGAGTGCTG-3′ (Rev) for HDAC4; 5′-AGGAGGAAGAGGAGGACTGC-3′ (Fwd) and 5′-GTACACCTGGAGGGGCTGTA-3′ (Rev) for HDAC5; 5′-CTATTAATCCCCGCCTGACC-3′ (Fwd) and 5′-GGAGCTCGATTTGTTTCTGC-3′ (Rev) for NADH.
Statistical Analysis
For the sucrose preference test, data were analyzed using two-way, repeated measures ANOVAs (defeat/non-defeat x sucrose dose) for measuring sucrose dose effect and two-way ANOVAs (HR/LR x defeat/non-defeat) for analyzing individual doses for effects. Western-blots and qRT-PCR data were analyzed using two-way ANOVA with HR/LR and defeat/non-defeat as independent factors, followed by Scheffe’s post hoc test where appropriate. All the statistical analyses were performed using Statview software (version 5.0.1; SAS Institute, Inc.) with statistical significance defined as p < 0.05.
Results
Experiment 1
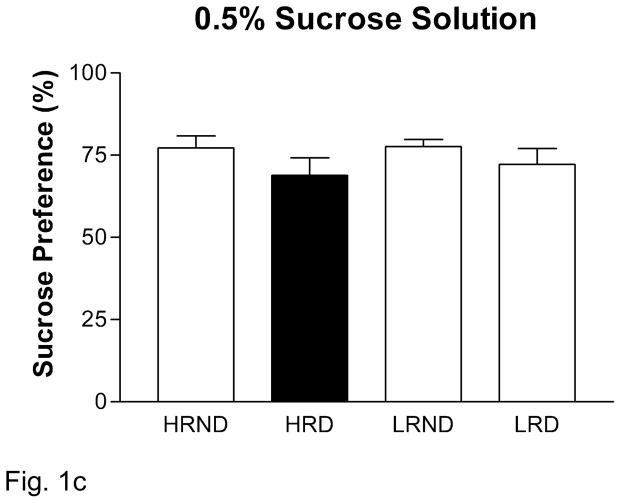
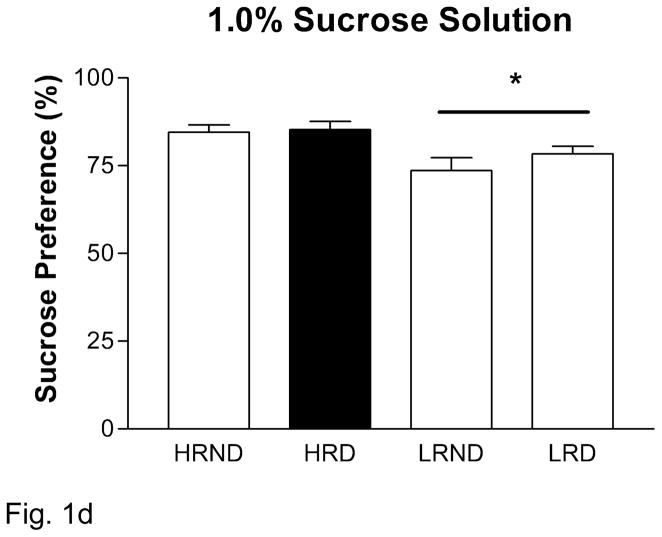
Anhedonia is one of the key symptoms in human depression (DSM-IV-TR, 2000). We sought to detect the rodent-correlate of anhedonia in socially defeated rats using the sucrose preference test. For all rats, there was a significant dose-dependent increase in sucrose preference across the three doses used (Figure 1A). We found a basal difference in sucrose consumption between HR and LR rats, with LR rats consuming less sucrose at the 0.25% and 1% sucrose doses (Figure 1). Indeed we found a significant positive correlation between locomotor activity in a novel environment and overall sucrose preference (0.25%: p = 0.001, r2 = 0.36; 1.0%: p = 0.003, r2 = 0.31). Upon exposure to social defeat, we found that HR rats significantly reduce their consumption of 0.25% sucrose solution compared to HR non-defeated rats, while LR rats remain unaffected (Figure 1B). We did not see any significant individual differences or effects of defeat at the 0.5% or 1% sucrose solutions (Figure 1C, D).
Figure 1.

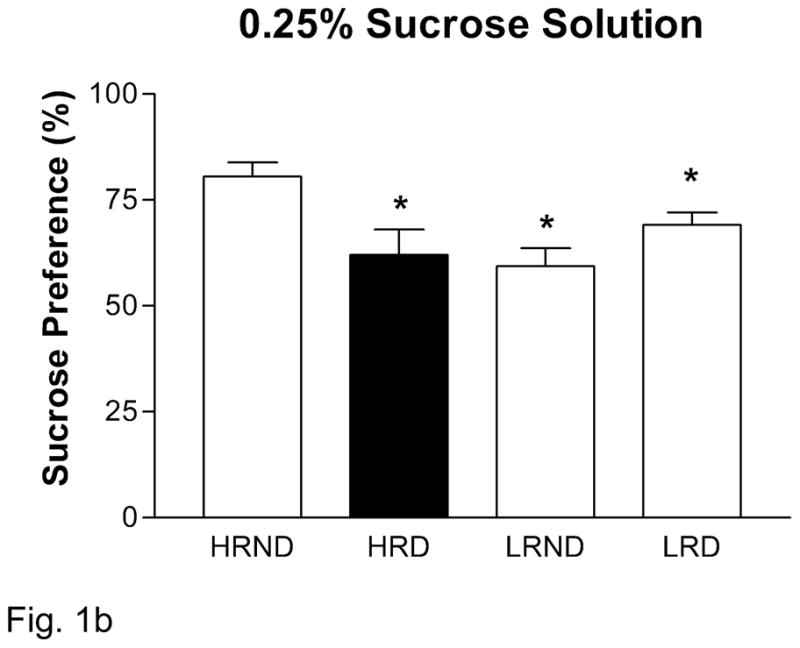
Experiment 2
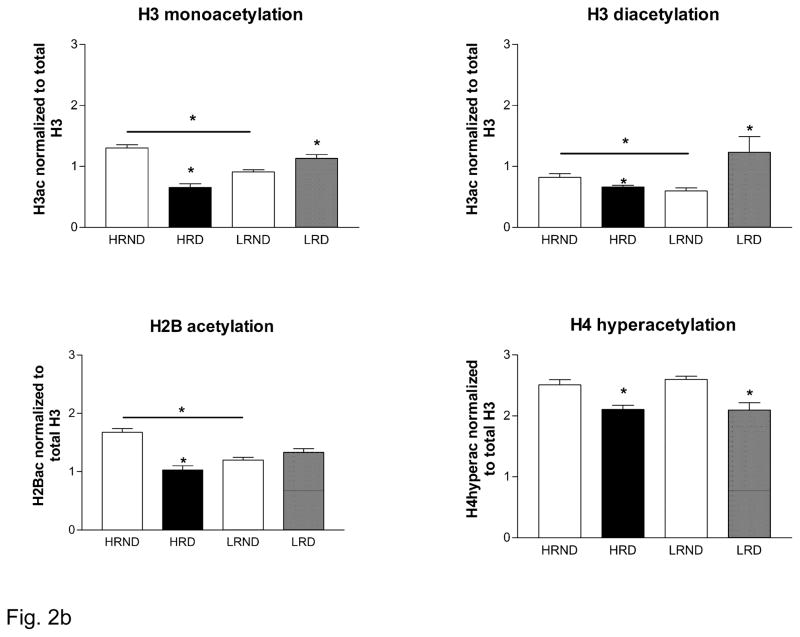
Marked histone acetylation state alterations have previously been associated with depressive-like symptoms and antidepressant treatments in rodents in several key brain areas including hippocampus (Covington et al. 2009; Tsankova et al. 2004). We therefore investigated H3, H4 and H2B acetylation states in hippocampus following a chronic social defeat exposure in the context of individual differences. Our data demonstrate that under basal conditions HR rats have higher levels of H3 acetylation (H3ac, in its mono and diacetylated forms, as indicated by an antibody specific to H3K14ac) and H2B acetylation (H2B-Ac) compared to the LR rats, suggesting that HR rats may exhibit higher rates of transcription of specific genes (Figure 2B). Repeated social defeat however induced differential acetylation in HR and LR rats. Social defeat resulted in a significant decrease in the H3ac in HR rats and H2B-Ac levels, but an increase in H3ac in LR rats (Figure 2B). There were no individual differences in the hyperacetylation of histone H4 under basal conditions, nor following social defeat as all rats exhibited a decrease in the levels of hyperacetylated H4 irrespective of their HR or LR status (Figure 2B).
Figure 2.
Experiment 3
In order to explain the differential pattern of histone acetylation between LR and HR animals hippocampi, we examined the expression levels of several relevant genes at the basal state and following exposure to the social defeat paradigm. Although no significant effect of defeat was found for any of the genes investigated, we observed a trend towards decreased HDAC3 mRNA levels following social defeat exposure (Table 1, p = 0.068). Interestingly, HR animals exhibited higher levels of CBP mRNA and lower levels of HDAC3 mRNA as compared to LR animals under basal conditions (Table 1). There were no differences between HR and LR rats in HDAC4 or HDAC5 mRNA levels.
Table 1.
| CBP | HDAC3 | HDAC4 | HDAC5 | |
|---|---|---|---|---|
| HRND | 1.147 ± 0.074** | 1.064 ± 0.055 | 1.301 ± 0113 | 0.974 ± 0.045 |
| HRD | 1.183 ± 0.134 | 0.934 ± 0.023 | 1.232 ± 0.068 | 1.085 ± 0.096 |
| LRND | 0.848 ± 0.039 | 1.129 ± 0.059 | 1.249 ± 0.086 | 1.098 + 0.023 |
| LRD | 1.054 ± 0 075* | 1.075 ± 0.047 | 1.383 ± 0.141 | 1.188 ± 0.014 |
Discussion
The sucrose preference test provides a measure of anhedonia in rodents. While all groups increased consumption upon presentation of increased sucrose content, we found individual differences in depressive-like behavior in response to repeated social defeat. HR rats exposed to repeated defeat exhibited a decreased sucrose preference from HR non-defeated rats, while LR rats’ preference for sucrose solution remained unaffected. This suggests that HR rats are more susceptible to the behavioral effects of social defeat. Interestingly, while LR rats showed no change in sucrose consumption upon exposure to defeat, they did drink significantly less sucrose solution than HR rats in basal conditions. This basal difference is in line with previous studies investigating HR/LR responses to the rewarding stimulation of drugs of abuse. While HR animals self administer cocaine at higher levels and rates than LR animals in basal conditions, their intake becomes indistinguishable from that of LR animals following exposure to social defeat (Kabbaj et al., 2001), suggesting that HR and LR animals respond similarly to both natural rewards and drugs of abuse. Taghzouti et al. (1999) reported a similar pattern of depressive-like behavior in HR/LR rats using the forced swim test. They found that under basal conditions, LR rats spent a significantly longer time immobile in the ‘test’ phase than HR rats but were unaffected by repeated swimming stress exposure (Taghzouti et al., 1999). Under basal conditions, HR rats spent significantly more time swimming than LR’s, however; HR’s nearly tripled their immobility duration upon exposure to stress (Taghzouti et al., 1999). Additionally, subchronic administration of the antidepressant fluoxetine prior to the forced swim test greatly reduced immobility and increased swimming behaviors in LR animals while producing the opposite response in HR animals (Taghzouti et al., 1999). Administration of a different class of antidepressant, desipramine, produced similar behavioral results with LR animals decreasing their immobility and HR animals decreasing their active escape behavior (Jama et al., 2008). These findings suggest a basal difference in affect level between HR and LR rats, with HR rats exhibiting higher levels of positive affect, but also greater vulnerabilities to the effects of stress and resistance to traditional antidepressant treatment.
The cause of such differences in behavior between HR and LR rats is relatively unknown. We investigated histone modifications as a potential mechanism for such differences using TAU gels that separate post-translationally modified histones. We found basal differences in histone acetylation between HR and LR rats, with higher mono- and diacetylated H3 and H2B acetylation in HR hippocampi. Such increased acetylation may account for the observed increases in hippocampal gene expression in HR rats in previous studies (Kabbaj et al., 2004). Exposure to social defeat resulted in differential changes in acetylation. Acetylation was decreased in HR defeated rats on histones H3 and H2B, but increased in LR defeated rats on H3 only. Increased H3K14 acetylation in the hippocampus has been linked to antidepressive-like behavior following chronic electroconvulsive shock therapy (ECS; Tsankova et al., 2004), which may explain the lack of behavioral effects seen in LR rats following social defeat. The consequences of histone H2B acetylation are not as widely studied as those of histones H3 and H4, however a very recent study indicates a similar role for this histone’s modifications in synaptic plasticity (Maharana et al., 2010). To our knowledge, this is the first demonstration of H2B modification in the context of social stress and depression. We found no differences in H4 acetylation between HR and LR rats under basal conditions. Following exposure to social defeat, both HR and LR rats show a significant decrease in H4 acetylation. Decreases in H4 acetylation were observed following chronic ECS treatment at specific promoters of Brain Derived Neurotrophic Factor (BDNF) while H3 acetylation was increased at other promoter sites (Tsankova et al., 2004). It was hypothesized that H4 acetylation was decreased to suppress specific BDNF promoters so that H3 regulation could occur (Tsankova et al., 2004). It will be interesting to investigate H4 acetylation further at such specific promoter regions, such as BDNF, to see if a similar pattern emerges. Alternatively, we can not rule out the possibility that antibody specificity for histone H4 acetylation may not be as precise as that which was used for H3 acetylation. The H3 hyperacetylated antibody specifically recognizes acetylation at lysine residues 9 and 14. The H4 antibody used recognizes global acetylation on all four lysine residues, and therefore may be less sensitive to acute changes at specific lysine residues on the H4 N-terminal tail.
We sought to uncover the mechanism behind this differential change in acetylation by investigating levels of expression of one histone acetyltransferases (HATs) and several histone deacetylases (HDACs). We chose these two classes of enzymes as they are both directly responsible for changes in acetylation on histones (Wade 2001). HAT enzymes use an acetyltransferase catalytic domain to add acetyl groups to lysine residues on histone N-terminal tails (Roth et al. 2001), thereby weakening their interaction with the DNA and facilitating transcriptional activation (Bannister and Kouzarides 1996; Ogryzko et al. 1996; for review see Roth et al., 2001). One such HAT is the CREB-binding protein (CBP), a transcriptional coactivator that interacts with numerous transcriptional regulators and facilitates the assembly of the transcriptional machinery (Chrivia et al. 1993; Janknecht 2002). Importantly, CBP preferentially acetylates H3K14 (Cheung et al. 2000a; Lo et al. 2000; McManus and Hendzel 2001). HDAC enzymes exert their effect by removing the negatively charged acetyl groups from acetylated histones, thereby increasing the net positive charge and the affinity of histones for the negatively charged DNA. This strengthening of histone-DNA contacts upon histone deacetylation interferes with transcriptional activation. Thus HDACs are considered active transcriptional repressors. Accordingly, we investigated three HDACs recently implicated in depression and CBP. We observed a significant increase in CBP and decrease in HDAC3 in HR animals, as compared to LR rats. Although this regulation remains to be confirmed at the protein level, HR animals appear to exhibit an increase in CBP and a decrease in HDAC3, both acting in a coherent manner to explain their higher acetylation level of histones H3 and H2B (cf. Figure 2). In contrast, we didn’t find any regulation of the other investigated targets following social defeat. While this result may appear surprising in light of the observed modifications in histone acetylation, it is in line with similar analyses of several class I and II HDAC mRNA levels, including HDACs 4 and 5, in hippocampus after chronic social defeat in mice (Tsankova et al., 2006). Indeed, the authors reported no significant influence of social defeat alone on the levels of HDAC expression (Tsankova et al., 2006). It may be the case that post-translational modification of HDACs may underlie the observed changes in histone acetylation as class II HDACs have the ability to shuttle between the nucleus and cytoplasm depending on the received signal (de Ruijter et al., 2003). Also, we can not rule out the possibility that other HDACs, HATs, or other brain structures are not implicated. Recent work has implicated HDAC2 in the regulation of acetylation in the nucleus accumbens following repeated exposure to social defeat (Covington et al., 2009). Also, GCN5 is a HAT that preferentially acetylates lysine 14 on H3 (Lo et al., 2000). Both of these are potential targets for future investigation.
Treatment and diagnosis of depression varies at the individual level. This study found both behavioral and molecular individual differences at the basal level and in response to stress that may correspond to depression-prone vulnerabilities or resistances. More research is needed in this arena, particularly studies to uncover differences in histone acetylation at specific gene promoter regions using ChIP-chip in response to stress. Investigating the effects of social defeat in the context of the HR/LR model of individual differences provides both a new level of complexity but also a more personalized approach towards treatment for this devastating disease.
Acknowledgments
This work was funded by two National Institute of Mental Health (NIMH) grants (R21 MH081046-0182 and R01 MH087583-01A1). F. Hollis is supported by the Florida State University Department of Biomedical Sciences Graduate program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, Henderson RP. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders text revision (DSM-IV-TR) Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Dietz DM, Dietz KC, Moore S, Ouimet CC, Kabbaj M. Repeated social defeat stress-induced sensitization to the locomotor activating effects of d-amphetamine: role of individual differences. Psychopharmacology (Berl) 2008;198:51–62. doi: 10.1007/s00213-008-1078-y. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86:347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Forsberg EC, Bresnick EH. Histone acetylation beyond promoters: long-range acetylation patterns in the chromatin world. Bioessays. 2001;23:820–830. doi: 10.1002/bies.1117. [DOI] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Gunjan A, Sittman DB, Brown DT. Core histone acetylation is regulated by linker histone stoichiometry in vivo. J Biol Chem. 2001;276:3635–3640. doi: 10.1074/jbc.M007590200. [DOI] [PubMed] [Google Scholar]
- Gunjan A, Verreault A. A Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell. 2003;115:537–49. doi: 10.1016/s0092-8674(03)00896-1. [DOI] [PubMed] [Google Scholar]
- Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl) 211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Ann N Y Acad Sci. 1992;654:444–447. doi: 10.1111/j.1749-6632.1992.tb25993.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991a;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991b;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Ito K, Adcock IM. Histone acetylation and histone deacetylation. Mol Biotechnol. 2002;20:99–106. doi: 10.1385/MB:20:1:099. [DOI] [PubMed] [Google Scholar]
- Janknecht R. The versatile functions of the transcriptional coactivators p300 and CBP and their roles in disease. Histol Histopathol. 2002;17:657–68. doi: 10.14670/HH-17.657. [DOI] [PubMed] [Google Scholar]
- Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Inter-individual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198:333–340. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jakovcevski M, Bharadwaj R, Connor C, Schroeder FA, Lin CL, Straubhaar J, Martin G, Akbarian S. Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J Neurosci. 30:7152–7167. doi: 10.1523/JNEUROSCI.1314-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Akil H. Individual differences in novelty-seeking behavior in rats: a c-fos study. Neuroscience. 2001;106:535–545. doi: 10.1016/s0306-4522(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Evans S, Watson SJ, Akil H. The search for the neurobiological basis of vulnerability to drug abuse: using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47(Suppl 1):111–122. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Price RH, Wortman CB. Social factors in psychopathology: stress, social support, and coping processes. Annu Rev Psychol. 1985;36:531–572. doi: 10.1146/annurev.ps.36.020185.002531. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- Maharana C, Sharma KP, Sharma SK. Depolarization induces acetylation of histone H2B in the hippocampus. Neuroscience. doi: 10.1016/j.neuroscience.2010.02.023. [DOI] [PubMed] [Google Scholar]
- McManus KJ, Hendzel MJ. CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol. 2001;79:253–66. [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJ, Benning MA, Koolhaas JM, Van den Hoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Behav. 1996;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats. Psychopharmacology (Berl) 1991;104:181–6. doi: 10.1007/BF02244176. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Roth SY. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Schurman T. Hormonal correlates of agonistic behavior in adult male rats. Prog Brain Res. 1980;53:415–20. doi: 10.1016/S0079-6123(08)60079-5. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Lamarque S, Kharouby M, Simon H. Interindividual differences in active and passive behaviors in the forced-swimming test: implications for animal models of psychopathology. Biol Psychiatry. 1999;45:750–758. doi: 10.1016/s0006-3223(98)00156-5. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–93. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10:693–8. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Histone Genes: Structure, Organization, and Regulation. John Wiley & Sons; New York: 1984. pp. 339–371. [Google Scholar]