Structure
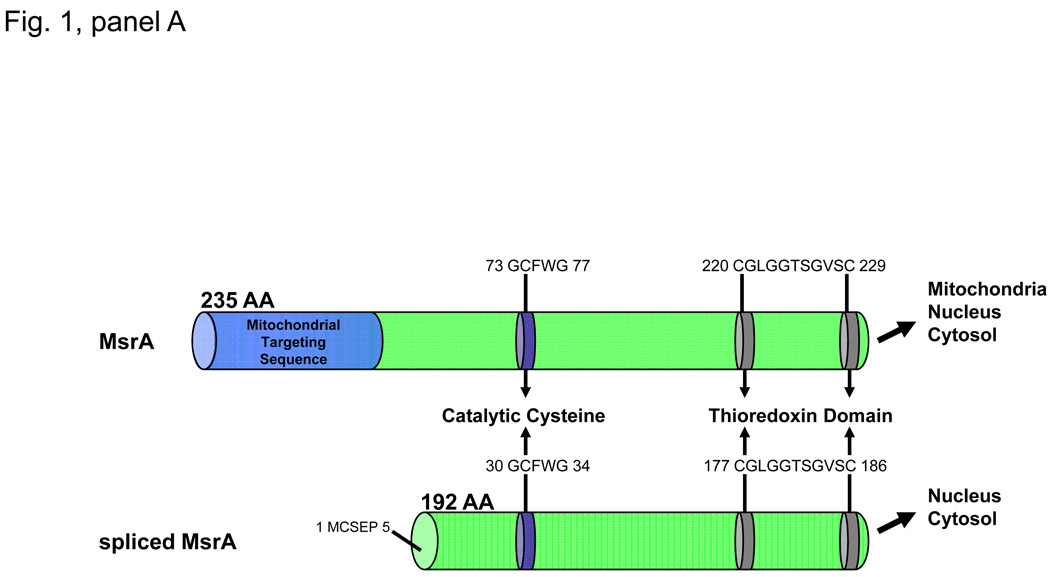
The human MSRA gene (GeneID: 4482) is located on Chromosome 8, locus 8p23.1 and encodes a 235 amino acid protein with a molecular mass of 26kDa called Methionine Sulfoxide Reductase A (MsrA) or peptide-methionine-(S)-S-oxide reductase (1.8.4.11.). There are two major transcript variants of MsrA called the long and short forms of MsrA (Kim and Gladeshev, 2007). The long form of MsrA encodes a 235 amino acid peptide containing an N-terminal mitochondrial targeting sequence, a catalytic cysteine containing Gly-Cys-Phe-Trp-Gly sequence required for its methionine sulfoxide reductase activity and a C-terminal thioredoxin binding domain (Fig. 1, panel A). The short form of MsrA lacks the mitochondrial targeting sequence while retaining the catalytic cysteine containing sequence and the thioredoxin binding domain (Fig. 1, panel A). The long form of MsrA has been localized to the mitochondria, nucleus and cytosol while the short form of MsrA has been localized to the nucleus and the cytosol (Fig. 1, panel A). To date, no functional differences between the long and short forms of MsrA have been reported.
Figure 1. Structure of MsrA splice variants and role of MsrA in lens function.
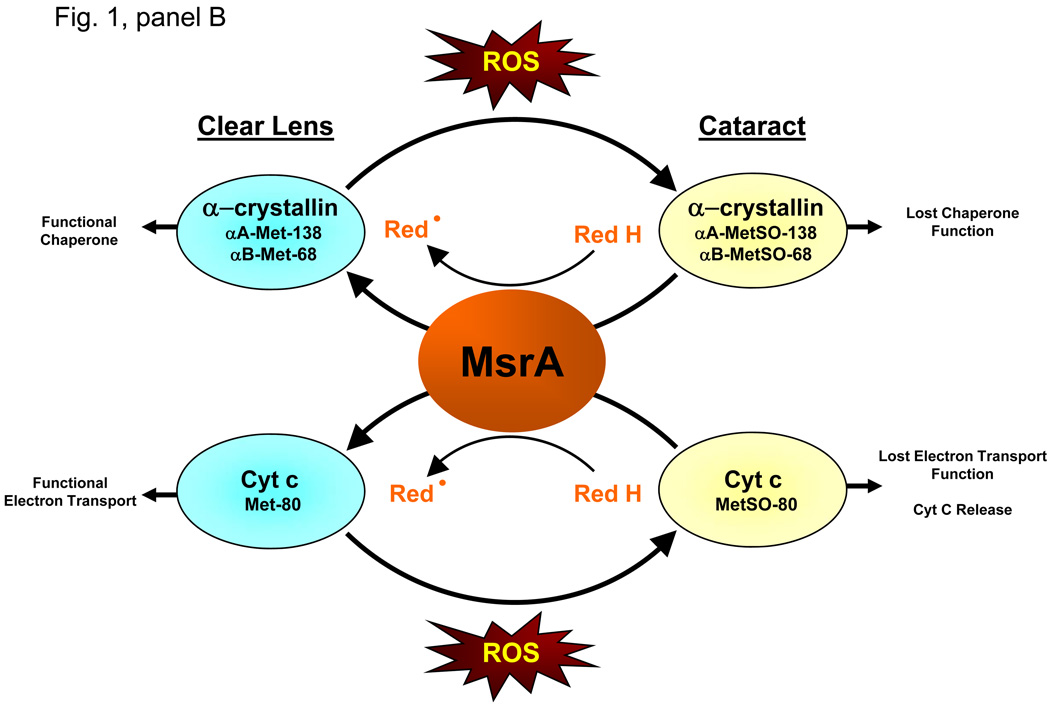
Panel A. MsrA has been shown to exist in two forms. The long form of MsrA encodes a 235 amino acid peptide containing an N-terminal mitochondrial targeting sequence, a catalytic cysteine containing Gly-Cys-Phe-Trp-Gly sequence required for its methionine sulfoxide reductase activity and a C-terminal thioredoxin binding domain. The short form of MsrA lacks the mitochondrial targeting sequence while retaining the catalytic cysteine containing sequence and the thioredoxin binding domain. The long form of MsrA has been localized to the mitochondria, nucleus and cytosol while the short form of MsrA has been localized to the nucleus and the cytosol. Panel B. Deletion of MSRA causes cataract in oxidative stress treated mice suggesting an essential role for MsrA in lens oxidative defense. Upon ROS insult, both α-crystallin and Cyt c are oxidized at methionine residues (αA-met-138, αB met-68, Cyt c met-80) to methionine sulfoxide (MetSO) in the lenses of the MSRA-knockout mice. This oxidation results in loss of chaperone and electron transport functions of these proteins, respectively. In the presence of a suitable reducing system (Red), MsrA can repair the oxidized S-methionines restoring the functions of both proteins, suggesting a possible mechanism accounting for the requirement for MsrA in lens defense against cataract formation.
All MsrAs have a globular tertiary structure which by analogy with the highly homologous structures of Bos taurus and E. Coli MsrA contains almost entirely α-helix and β-sheet secondary structures (Kim and Gladeshev, 2007). MsrA contains a conserved substrate-binding motif consisting of amino acids Gly-Cys-Phe-Trp-Gly that contains one of three cysteines required for MsrA catalytic activity. MsrA catalyzes the conversion of methionine sulfoxide, formed upon oxidation of methionine, to reduced methionine (Kim and Gladeshev, 2007). MsrA works on both free and protein-bound S-methionine sulfoxide (PMSO). Oxidation of methionine results in the equal formation of S- and R-epimers of methionine sulfoxide. MsrA is specific for the S-epimer while three other Msrs called MsrB1, MsrB2 and MsrB3 are specific for the R-epimer of methionine sulfoxide (Brennan and Kantorow, 2009 & Kim and Gladeshev, 2007). The action of MsrA depends on the presence of a suitable reducing system including DTT (in vitro) and reduced thioredoxin (Brennan and Kantorow, 2009 & Kim and Gladeshev, 2007).
The catalytic action of MsrA proceeds in three steps. The first is a nucleophilic attack by the substrate binding cysteine on the target oxidized methionine resulting in the formation of sulfenic acid intermediate. A second recycling cysteine then attacks the sulfenic acid intermediate to form an intra-monomeric disulfide bond with release of H2O. This intra-monomeric disulfide bond is then reduced using thioredoxin to catalytically reactivate the enzyme (Kim and Gladeshev, 2007).
Function
The major function of MsrA is to convert methionine sulfoxide to reduced methionine. Methionine oxidation occurs in cells as a consequence of exposure to reactive oxygen species (ROS) including superoxide, hydrogen peroxide, peroxynitrite and hydroxyl radical. Both exogenous sources of ROS production such as UV-light, blue light, smoke, chemical and metal exposure and endogenous sources of ROS production including free-radical formation as a consequence of inefficient mitochondrial respiration, result in direct methionine oxidation (Brennan and Kantorow, 2009). Methionine and cysteine are considered the most easily oxidized protein amino acids. Single oxidation of methionine results in the formation of methionine sulfoxide which is acted upon by MsrA while further oxidation of methionine sulfoxide results in the formation of methionine sulfonic acid which is not acted upon by MsrA.
Action of MsrA on oxidized free methionine and PMSO is believed to have many cellular and physiological consequences. These include the catalytic removal of damaging ROS through MsrA-mediated cycling of methionine sulfoxide and the repair of PMSO with consequent reactivation of proteins whose activities depend on reduced methionine. Through these actions, MsrA has been implicated in processes ranging from protection of cells against oxidative damage to the maintenance of cellular homeostasis, prevention of disease and extension of longevity (Brennan & Kantorow, 2009 & Kim and Gladeshev, 2007).
The mitochondria is believed to be a primary site of MsrA action since it is a major source of endogenous ROS and is a central mediator of several critical cellular processes including respiration and apoptotic induction. MsrA has been localized to the mitochondria in mammalian cells where it is found in the inner mitochondrial membrane. MsrA has been shown to associate with all five mitochondrial respiratory complexes (Brennan et al., 2009(A)). Importantly, MsrA has been shown to directly protect mitochondria against exogenously added oxidative stress. Deletion of MsrA from mammalian cells results in loss of mitochondrial membrane potential in combination with increased mitochondrial ROS production and ultimately death of mammalian cells (Brennan and Kantorow, 2009). Although the exact mechanism for MsrA protection of the mitochondria has not been established, it has been shown that oxidation of methionine 80 (met-80) located in the heme-pocket of the mitochondrial protein cytochrome c (Cyt c) results in loss of Cyt c oxidase and Cyt c reductase activities (Brennan et al., 2009(A)). Oxidation of Cyt c at met-80 is also believed to be a key initiator of apoptosis that promotes release of Cyt c from the mitochondrial membrane.
In the eye, in vivo studies examining the oxidized state of Cyt c in the lenses of MSRA-knockout mice revealed that MsrA is required for reduction of Cyt c met-80 sulfoxide. In vitro studies demonstrated the ability of MsrA to repair met-80 sulfoxide and restore Cyt c electron transport activities (Brennan et al., 2009(A)). Analysis of the MSRA-knockout lenses also revealed that α-crystallin, which consists of two subunits called αA- and βB-crystallin, were oxidized at met-138 and met-68, of the knockout lenses respectively (Brennan et al., 2009(B)). This PMSO form of α-crystallin exhibited significantly reduced chaperone activity then unmodified α-crystallin. Reaction of the met oxidized α-crystallin restored its chaperone activity through the direct reduction of the oxidized methionines (Brennan et al., 2009(B)). These data link the action of MsrA to both the maintenance of α-crystallin chaperone function and the electron transport function of Cyt c in the eye lens. Since the MSRA-knockout mice also get cataracts upon repeated exposure to hyperbaric oxygen, these data suggest that MsrA is required for the maintenance of lens transparency, at least in part, through its ability to maintain the reduced state of lens α-crystallin and Cyt c. This data is summarized in Fig. 1, panel B.
In addition to Cyt c and α-crystallin, at least 8 other proteins have been identified to be affected by methionine oxidation and are repaired by MsrA. These include the shaker potassium channel, HIV-2 protease, the Ffh prokaryotic signal recognition particle component, calmodulin, alpha-1-prtoteinase inhibitor, ribosomal protein L12, Hsp21 and a-synuclein (Brennan and Kantorow, 2009 & Kim and Gladeshev, 2007). MsrA has also been localized to the mammalian retina and has been shown to protect retinal pigmented epithelial cells (RPE) against exogenously added oxidative stress (Brennan and Kantorow, 2009). It has been proposed that MsrA has little or no target specificity and is therefore likely to act on surface exposed PMSO of many proteins.
Disease Involvement
To date, the exact role of MsrA in homeostasis and disease has not been established but the action of MsrA is linked to multiple disease states. In the eye lens, deletion of the MSRA gene causes cataract in oxidative stress-treated but not untreated mice (Brennan et al., 2009(A)). It is also known that in the human lens PMSO levels increase with age, and upon cataract formation as much as 60% of total lens protein is found as PMSO (Brennan & Kantorow, 2009). These data suggest that MsrA plays a key role in defense of the lens against oxidative insult, and that decreased MsrA activity in association with accumulation of PMSO upon aging and insult likely is a factor in human cataract formation. Although no direct role for MsrA has been established for other tissues of the eye, the ability of MsrA to protect RPE cells against oxidative stress damage implicates MsrA as important for retinal cell maintenance and indicates a potential role for MsrA in retinal degenerations including age-related macular degeneration. PMSO levels are also known to increase in many non-ocular age-related degenerative diseases including Alzheimer’s disease and Parkinson’s disease (Kim and Gladeshev, 2007).
Future Studies
A major goal in understanding the function of MsrA in homeostasis, oxidative stress defense and disease is to determine why PMSO levels increase upon aging and if this increase occurs as a consequence reduced MsrA function. The activity of MsrA depends on the availability of a reducing system needed to catalytically regenerate the enzyme after its action on methionine sulfoxide, and, to date, thioredoxin is the only established MsrA reducing system. Analysis of the levels of reduced thioredoxin coupled with analysis of the levels of MsrA will likely provide valuable information towards understanding the accumulation of PMSO in aging and disease. It is well accepted that the levels of multiple cellular reducing systems decrease upon aging, and it is likely that this decrease affects the activity of MsrA. In addition, it is likely that MsrA uses multiple reducing systems in addition to thioredoxin, and identifying these novel reducing systems and analyzing their levels in specific tissues and sub-cellular components will likely reveal unique activation and regulatory roles for the action of these reducing proteins on MsrA. It is also likely that multiple targets for MsrA regulate a wide array of cellular functions and identifying these targets in specific tissues and disease states will provide direct information of the function of MsrA. Finally, MsrA works on only the S-epimer of PMSO and understanding how MsrA acts in concert with the MsrB enzymes that act on the R-epimer of PMSO is likely to reveal important insight into those mechanisms by which the Msr system maintains cellular homeostasis and defends against aging, oxidative stress and disease.
Acknowledgements
We would like to acknowledge the many people who have made essential collaborative contributions towards this work including: Dr. Lisa Brennan, Dr. Maria Marchetti, Dr. J.F. Hejtmancik, Dr. Frank Giblin, Dr. Larry David, Dr. Ignacio Rodriquez, Dr. Herbert Weissbach, Dr. John Hawse and Dr. Rod Levine. We also wish to express our gratitude to the National Eye Institute, without whose support, these studies would not have been possible. This work was supported by EY13022-MK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marc Kantorow, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431 Phone: (561) 297-2910 Fax:(561)297-2221 mkantoro@fau.edu.
Wanda Lee, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431.
Daniel Chauss, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431.
References
- Brennan LA, Kantorow M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009;88:195–203. doi: 10.1016/j.exer.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, Lee W, Cowell T, Giblin F, Kantorow M. Deletion of mouse MsrA results in HBO-induced cataract: MsrA repairs mitochondrial cytochrome c. Mol Vis. 2009;15:985–999. (A) [PMC free article] [PubMed] [Google Scholar]
- Brennan LA, Lee W, Giblin F, David L, Kantorow M. Methionine sulfoxide reductase A (MsrA) restores a-crystallin chaperone activity lost upon methionine oxidation. Biochim Biophys Acta. 2009;1790:1665–1672. doi: 10.1016/j.bbagen.2009.08.011. (B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem. J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]