Abstract
Nut midline carcinomas are uncommon carcinomas characterized by chromosomal rearrangements that involve the gene encoding the nuclear protein of the testis (NUT). This article reviews the clinicopathologic features of these malignancies. Ancillary testing is discussed as well as the pathologic differential diagnosis.
Keywords: Nut midline carcinoma, t(15;19), Immunohistochemistry, Fluorescent in situ hybridization
Introduction
NUT midline carcinomas (NMCs) are malignant epithelial tumors that have chromosomal rearrangements of the gene encoding nuclear protein of the testis (NUT) at 15q14. The malignancies range from entirely undifferentiated carcinomas to carcinomas with prominent squamous differentiation. As of now, these carcinomas are defined by having the disrupted NUT gene and have significant histologic and clinical overlap with other carcinomas found at the sites they occur. The translocations appear to be unique, however, are associated with a relatively consistent phenotype, and are functional.
In 1991, two cases of carcinoma were described involving the thorax that were shown to have t(15;19) [1, 2]. Both involved the mediastinum and were considered thymic in origin. To date, at least 28 cases have been reported (Table 1) [1–21]. Of the 14 tumors believed to originate in the head and neck, 6 involved primarily the nasal cavity and/or sinuses, 2 involved the nasopharynx, 2 involved the orbit, 2 involved the major salivary glands, 1 involved the supraglottic larynx and 1 involved the epiglottis. Fourteen of the 28 NMCs so far reported have developed in girls or women. The tumors have been noted to occur over a vast age range (Newborn-78 years) and about half of the cases have been reported in patients over the age of 20 years.
Table 1.
Clinical features of Nut midline carcinomas
| Age | Range = 0–78 yr |
| Sex | 14 Female; 14 Male |
| Location | Head and neck = 14 |
| Sinonasal = 6 | |
| Nasopharynx = 2 | |
| Larynx = 1 | |
| Epiglottis = 1 | |
| Orbit = 2 | |
| Major salivary gland = 2 | |
| Thorax = 11 | |
| Mediastinum/Thymus = 7 | |
| Lung = 1 | |
| Trachea = 1 | |
| Other = 3 |
Originally, the tumors had been believed to primarily affect children and young adults, perhaps reflecting the increased likelihood that tumors from these patients are analyzed for karyotype. In a recent series of primarily undifferentiated carcinomas from the upper aerodigestive tract, 4 of the 5 patients found to have translocations by fluorescent in situ hybridization were older than any previously reported patients, including a 78-year-old woman with an undifferentiated carcinoma of the larynx [12]. This is particularly interesting given the fact that many of the cases tested came from younger patients (age range 15–88 years). Another tumor has recently been described involving the nasal cavity of a 54-year-old woman [18].
Patients with NMCs frequently present with mass-related symptoms, generally due to the assumed primary tumor, although many present with apparent metastases at the time of their diagnosis [2, 4, 10, 11, 13, 14, 18]. Non-specific symptoms such as fever and weight loss have also been noted.
Pathologic Findings
As most tumors have presented at advanced stage (lymph node metastases, bone metastases, pleural carcinomatosis), tumors have only infrequently been resected [5, 6, 14, 18]. As a result, gross descriptions are lacking. Of the 3 gross specimens seen at the University of Virginia, two were sinonasal and received piecemeal and showed grossly obvious hemorrhage and necrosis. One lesion was present in a laryngectomy specimen and was described as a 3 cm variegated, somewhat circumscribed mass. Nodal metastases were characterized by enlarged nodes with necrotic, non-specific changes.
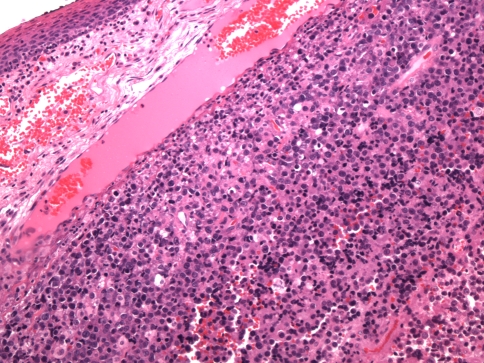
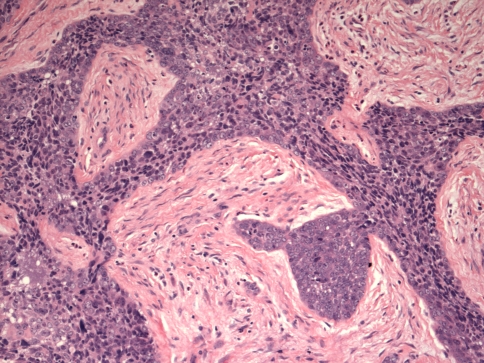
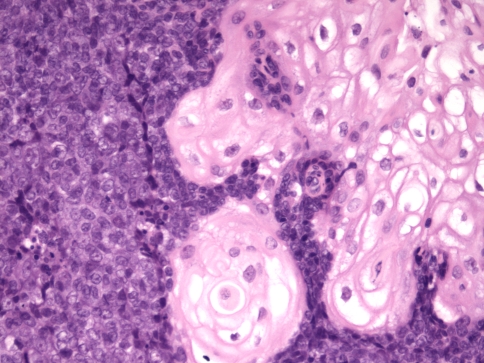
Histologically, tumors are usually composed of sheets of undifferentiated cells (Fig. 1) [5, 6, 14, 22]. Occasional cases may have more nested malignant cells within a desmoplastic stroma (Fig. 2). Large areas of coagulative necrosis may be present. The cells have scant amphiphilic or eosinophilic cytoplasm. Nuclei have irregular contours although they tend to be somewhat uniform in size. They have fine to vesicular chromatin and prominent nucleoli. Mitotic figures and apoptotic bodies are common. Squamous differentiation is frequently seen and does not necessarily predict the partner gene, as once believed [5]. The differentiation may be subtle with slight streaming of the malignant cells or it can be very distinct, with maturing squamous cells and extracellular keratin formation. Some authors have noted that the keratinization may appear abrupt, with sheets of immature cells juxtaposed to well-differentiated, mature, often benign appearing squamous nests (Fig. 3) [12]. The well-differentiated areas may even become cystic. A single case of the parotid gland has been described that additionally showed chondroid differentiation similar to a carcinosarcoma [16].
Fig. 1.
A NUT midline carcinoma composed of sheets of undifferentiated cells immediately beneath metaplastic squamous epithelium of the larynx
Fig. 2.
A sinonasal NUT midline carcinoma with infiltrating trabeculae of undifferentiated cells separated by desmoplastic stroma
Fig. 3.
A NUT midline carcinoma with abrupt squamous differentiation
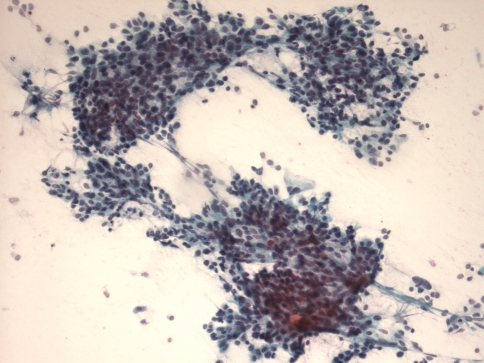
The cytologic features of NUT-translocation associated carcinoma have only recently been described [23]. The three cases in this report demonstrated highly cellular aspirate specimens with cohesive and discohesive epithelial cells akin to nasopharyngeal Epstein Barr virus (EBV)-associated squamous cell carcinoma (Fig. 4). Scant, delicate cytoplasm was present in two cases, while the third case had denser cytoplasm. Cells had irregular nuclear contours, discrete nucleoli, and fine to vesicular chromatin. Abundant mitotic figures, naked nuclei, karyorrhectic debris, and nuclear crush artifact were noted. Overt squamous differentiation was not identified in these cases.
Fig. 4.
This aspirate of a metastatic NUT midline carcinoma contains cohesive clusters of poorly differentiated epithelial cells
Tumor cells typically appear polyhedral [1, 14]. Intermediate junctions and desmosomes are seen, consistent with epithelial differentiation. Some cells have been noted to contain branching tonofilament bundles within the cytoplasm consistent with squamous differentiation. Evidence of glandular differentiation has not been noted.
NUT midline carcinomas are epithelial and react with antibodies to keratins (although staining may be focal) [5, 6, 22]. In our series, NMCs were mostly immunoreactive with antibodies to p63, consistent with squamous differentiation. The majority of tumors have been reported to be immunoreactive with antibodies to cytokeratin (CK)7 and focal immunoreactivity with antibody to CK20 has sometimes been noted [6]. CD34 immunoreactivity was seen in a little more than half the cases studied in one review, a unique finding for an epithelial malignancy [6]. Immunoreactivity with other antigens expressed in small blue cells tumors of childhood has not been noted, and tumors have reportedly been non-reactive with antibodies to desmin, myoglobin, smooth muscle actin, muscle actin, chromogranin, synaptophysin, leukocyte common antigen, placental alkaline phosphatase, S100 protein, alpha-fetoprotein, neuron specific enolase, CD57, CD99, and HMB45. Evidence of EBV and human papillomavirus (HPV) infection has not been identified [5].
Both polyclonal and monoclonal antibodies to the NUT protein have been developed [12, 24]. We reported the results for the polyclonal antibody in our series of undifferentiated carcinomas of the upper aerodigestive tract. Using 50% nuclear staining as a cutoff, the antibody showed 80% sensitivity and 96% specificity when compared to FISH as the gold standard. A newer monoclonal antibody has improved function with a sensitivity of 87% and specificity of 100% [24].
The most common translocation involving the NUT gene is the t(15;19) (q14;p13.1) [5]. This translocation fuses the NUT gene on chromosome 15 to the BRD4 gene. In about one-third of cases, however, the NUT gene is fused to a different partner gene (NUT-variant midline carcinoma). Rarely, the NUT gene is fused to the BRD3 gene at 9q34.2 [9]. The BRD proteins are known to bind transcriptionally active chromatin [5]. The function of the NUT protein is not known, although it is only constitutively expressed in early germ cells and within the brain (ciliary ganglion) [5]. It is believed that the fusion protein can be localized to the nucleus secondary to the expressed portions of the BRD4 and BRD3 proteins that are retained. Some recent data suggest that the fusion proteins block epithelial and squamous differentiation. Indeed, si-RNA-induced withdrawal of the fusion proteins leads to squamous differentiation and cell cycle arrest [9].
Because of the rarity of these tumors, FISH may currently be the diagnostic test needed for the establishment of a diagnosis. Probes have been developed for the regions flanking the typical break point of the NUT gene on chromosome 15 and for the typical breakpoints with BRD4 and BRD3, however, they are not commercially available. Primers are also developed for both known fusion proteins, although RT–PCR would be necessarily less sensitive than FISH.
Differential Diagnosis
The differential diagnosis for NMCs of the upper aerodigestive tract includes other undifferentiated malignancies including undifferentiated carcinomas and poorly differentiated squamous cell carcinomas. It includes pediatric small blue cell tumors (e.g., primitive neuroectodermal tumor, rhabdomyosarcoma, desmoplastic small round cell tumor, etc.), melanoma, olfactory neuroblastoma, high-grade hematologic malignancies, endocrine carcinomas and other undifferentiated or poorly differentiated carcinomas including sinonasal undifferentiated carcinomas and EBV-driven non-keratinizing squamous cell carcinomas which occur most frequently in the nasopharynx.
Identification of clear-cut epithelial differentiation rules out many of the tumors in the differential diagnosis, although one should remember that some mesenchymal tumors can rarely show immunoreactivity with antibodies to epithelial antigens, e.g., olfactory neuroblastomas and primitive neuroectodermal tumor [25, 26]. For tumors without obvious differentiation seen histologically, immunohistcochemistry can be used. Epithelial differentiation can almost always be demonstrated for NMCs with a pankeratin immunococktail. Obviously, negative results for other antigens such as S100 protein, CD45, muscle antigens, neuroendocrine antigens, germ cell antigens, are also helpful. p63 immunoreactivity is also helpful as it points toward squamous differentiation. It should be remembered, however, that p63 is also expressed by salivary gland tumors that have myoepithelial differentiation such as myoepithelial carcinomas and adenoid cystic carcinomas and that these tumors can appear undifferentiated, especially in small biopsy specimens [27, 28]. Using antibodies to other myoepithelial antigens, such as smooth muscle actin, may be helpful to exclude some salivary gland-type carcinomas. Reactivity with antibody to p63 may make some diagnoses, such as SNUC or pituitary adenoma less likely [29].
NMCs have not been shown to be associated with carcinoma-related viruses such as EBV and HPV [5]. In situ hybridization for these viruses can be used and the identification of the viruses virtually excludes the diagnosis of NMC. Surrogate markers such as p16 immunohistochemistry for the diagnosis of HPV-related malignancy have not been studied.
The definitive diagnosis of an NMC and the distinction of such a tumor based solely on histology and immunohistochemistry (excluding antibody to the NUT protein) is not considered possible (see above). It is unclear whether definitive distinction will be possible using the monoclonal antibody to the NUT protein alone although the early results, reported above, appear good. It is also unclear whether such a distinction is necessary in general practice, other than for prognostic purposes.
Treatment and Prognosis
There is no established treatment for NMCs. Most patients have received combination multi-drug chemotherapy and radiation. Only occasional cases have undergone subsequent resection. Of the patients with adequate follow-up in one series, all but one (15/16) have died of disease with an average survival time of 9 to 10 months [5]. It is interesting to note that the single patient with an NMC in that review who had survived for an extended period of time had had a tumor of the iliac bone that had originally been diagnosed as Ewing Sarcoma and had been treated as such [11]. A patient with an NMC of the mediastinum has since been reported alive and without disease at 34 months after his diagnosis.
References
- 1.Kees UR, Mulcahy MT. Willoughby ML: intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19) Am J Pediatr Hematol Oncol. 1991;13:459–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T. Miyoshi I: novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 3.Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, Roberts JR. Carbone DP: chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst. 2000;92:1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 4.Engleson J, Soller M, Panagopoulos I, Dahlen A, Dictor M, Jerkeman M. Midline carcinoma with t(15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer. 2006;6:69. doi: 10.1186/1471-2407-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2008;63:492–6. [DOI] [PubMed]
- 6.French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, Nose V, Vargas SO, Moschovi M, Tzortzatou-Stathopoulou F, Miyoshi I, Perez-Atayde AR, Aster JC. Fletcher JA: midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 7.French CA, Miyoshi I, Aster JC, Kubonishi I, Kroll TG, Dal Cin P, Vargas SO, Perez-Atayde AR. Fletcher JA: BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19) Am J Pathol. 2001;159:1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR. Fletcher JA: BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 9.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, Kutok JL, Toretsky JA, Tadavarthy AK, Kees UR, Fletcher JA. Aster JC: BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 10.Lee AC, Kwong YI, Fu KH, Chan GC, Ma L, Lau YL. Disseminated mediastinal carcinoma with chromosomal translocation (15;19). A distinctive clinicopathologic syndrome. Cancer. 1993;72:2273–2276. doi: 10.1002/1097-0142(19931001)72:7<2273::AID-CNCR2820720735>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49:1015–7. [DOI] [PubMed]
- 12.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, Aster JC. French CA: NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 13.Toretsky JA, Jenson J, Sun CC, Eskenazi AE, Campbell A, Hunger SP, Caires A, Frantz C, Hill JL. Stamberg J: translocation (11;15;19): a highly specific chromosome rearrangement associated with poorly differentiated thymic carcinoma in young patients. Am J Clin Oncol. 2003;26:300–306. doi: 10.1097/00000421-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Vargas SO, French CA, Faul PN, Fletcher JA, Davis IJ, Dal Cin P. Perez-Atayde AR: upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer. 2001;92:1195–1203. doi: 10.1002/1097-0142(20010901)92:5<1195::AID-CNCR1438>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Stelow EB. French CA: carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas) Adv Anat Pathol. 2009;16:92–96. doi: 10.1097/PAP.0b013e31819923e4. [DOI] [PubMed] [Google Scholar]
- 16.Bakker MA, Beverloo BH, Heuvel-Eibrink MM, Meeuwis CA, Tan LM, Johnson LA, French CA. van Leenders GJ: NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka N, French CA, Cameron MJ. Kratzke RA: long-term survival of a patient with squamous cell carcinoma harboring NUT gene rearrangement. J Thorac Oncol. 2010;5:1704–1705. doi: 10.1097/JTO.0b013e3181ebaa20. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MS, French CA, Liang CW, Hsiao CH: NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol. 2009. doi:10.1177/1066896909353600. [DOI] [PubMed]
- 19.Nelson BA, Lee EY, French CA, Bauer DE. Vargas SO: BRD4-NUT carcinoma of the mediastinum in a pediatric patient: multidetector computed tomography imaging findings. J Thorac Imaging. 2010;25:W93–W96. doi: 10.1097/RTI.0b013e3181b5d84d. [DOI] [PubMed] [Google Scholar]
- 20.Shehata B, Steelman CK, Abramowsky CR, Olson T, French C, Saxe D, Ricketts R, Katzenstein H: NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a two-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2009. doi:10.2350/09-10-0727-CR.1. [DOI] [PubMed]
- 21.Ziai J, French CA. Zambrano E: NUT gene rearrangement in a poorly-differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelow EB, Mills SE. Squamous cell carcinoma variants of the upper aerodigestive tract. Am J Clin Pathol. 2005;124(Suppl):S96–S109. doi: 10.1309/CR5JXUY3J2YGTC1D. [DOI] [PubMed] [Google Scholar]
- 23.Bellizzi AM, Bruzzi C, French CA. Stelow EB: the cytologic features of NUT midline carcinoma. Cancer Cytopathol. 2009;117:508–515. doi: 10.1002/cncy.20044. [DOI] [PubMed] [Google Scholar]
- 24.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, Hong SM, Schwartz BE, Cameron MJ, Rubin MA, Chang MC, Aster JC. French CA: diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frierson HF, Jr, Ross GW, Mills SE, Frankfurter A. Olfactory neuroblastoma. Additional immunohistochemical characterization. Am J Clin Pathol. 1990;94:547–553. doi: 10.1093/ajcp/94.5.547. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb I, Goldstein D. Perez-Ordonez B: primary extraskeletal Ewing family tumor with complex epithelial differentiation: a unique case arising in the lateral neck presenting with Horner syndrome. Am J Surg Pathol. 2008;32:1742–1748. doi: 10.1097/PAS.0b013e3181706252. [DOI] [PubMed] [Google Scholar]
- 27.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007;31:44–57. doi: 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 28.Seethala RR, Hunt JL, Baloch ZW, Livolsi VA, Leon Barnes E. Adenoid cystic carcinoma with high-grade transformation: a report of 11 cases and a review of the literature. Am J Surg Pathol. 2007;31:1683–1694. doi: 10.1097/PAS.0b013e3180dc928c. [DOI] [PubMed] [Google Scholar]
- 29.Bourne TD, Bellizzi AM, Stelow EB, Loy AH, Levine PA, Wick MR. Mills SE: p63 expression in olfactory neuroblastoma and other small cell tumors of the sinonasal tract. Am J Clin Pathol. 2008;130:213–218. doi: 10.1309/TEDD2FCWH8W0H4HA. [DOI] [PubMed] [Google Scholar]