Abstract
Hereditary patients with a history of treated retinoblastoma (RB) have a greatly increased risk of a broad spectrum of secondary malignancies appearing many years later, with a high incidence in the head and neck region. Leiomyosarcomas (LMS) account for up to 58% of these tumors. LMS in the sinonasal region generally are uncommon and are associated with a locally aggressive course and have a poor prognosis. RB may occur in two forms. The hereditary form is generally bilateral but can present unilaterally with a positive family history and typically exhibits a germline mutation in the RB1 gene on chromosome 13. The non-hereditary form is usually unilateral but can show the same germline mutation in up to 10% of cases. Patients with hereditary RB have been shown to have a significantly higher cumulative risk of developing secondary malignancies than those with the non-hereditary form (28 vs. 1.44% respectively). Most reported cases of sinonasal LMS are in patients with a history of the bilateral hereditary form of treated RB. We report a case of LMS of the nasal sinus area in a 35-year-old African American male with a history of non-hereditary unilateral RB and radiation therapy. To the best of our knowledge, this is the first reported case of sinonasal LMS arising in a patient with a history of non-hereditary unilateral RB. The clinical history, radiology, and pathology are presented along with a brief discussion of the literature.
Keywords: Retinoblastoma, Leiomyosarcoma, Sinonasal, Secondary malignancy
Introduction
Patients with a history of treated hereditary retinoblastoma (RB) have a greatly increased risk of a broad spectrum of secondary malignancies appearing many years later [1]. A high number of these tumors arise in the head and neck region. Leiomyosarcomas (LMS) account for a high proportion of these malignancies; however, LMS in the sinonasal region generally are uncommon and are associated with a locally aggressive course and have a poor prognosis [2, 3].
Materials and Methods
A case of LMS of the nasal sinus area was identified in a patient with a history of treated non-hereditary unilateral RB and radiation therapy. The clinical history, radiology, and pathology are presented along with a brief discussion of the literature.
A search of the English-language literature was performed in PubMed using the terms “leiomyosarcoma” and “retinoblastoma” from 1970 until 2010. Cases were included if they met the following criteria: (1) history of retinoblastoma, (2) secondary malignancy of leiomyosarcoma clearly identified as sinonasal tract. Cases which described LMS primarily arising in the orbit or maxilla were excluded.
Case Report
A 35-year-old African American male presented with a 6 month history of severe right-sided nasal obstruction, sinus pressure, epistaxis, loss of sensation, and intermittent pain (at a scale of 8 of 10) in the right facial area. He reported a history of RB of the right eye at the age of 1 year which was treated with surgery and radiation therapy. The patient had no positive family history for RB, and the case was apparently sporadic.
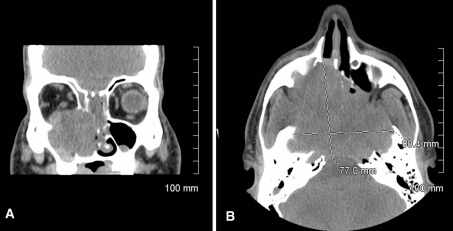
CT and MRI imaging revealed an 8.04 cm tumor mass in the central ethmoid sinuses and cribriform plate at skull base with extension into the right maxillary sinus, the right floor of the orbit, and the left sphenoid sinus. The lesion spared the frontal sinus. It also appeared to be impinging on the left orbit. The posterior aspect appeared to extend into the skull base, pterygoid bones, clivus, and sphenoid (Fig. 1a, b).
Fig. 1.
a Coronal view CT without contrast, b axial view CT without contrast showing destructive sinonasal lesion with extension to paranasal structures
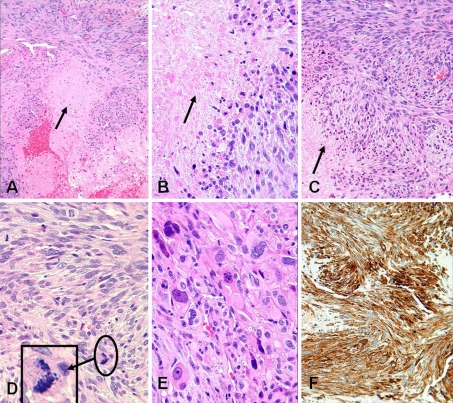
Initial biopsy diagnosis at an outside institution was a high-grade sarcoma with negative immunohistochemical (IHC) markers for keratin and CD34. The tumor was felt to be most likely consistent with a post radiation sarcoma. The patient was referred to Shands Hospital at University of Florida for treatment consultation where a review of the slides was performed. The biopsy demonstrated a high grade sarcoma composed of spindle-shaped cells arranged in fascicles featuring cigar-shaped nuclei with marked pleomorphism and a high mitotic rate with atypical mitotic figures. Significant tumor necrosis was present (Fig. 2a–e). The impression from H&E stained sections was a high grade LMS which was confirmed by the additional IHC stains performed in our institution [positive for smooth muscle actin (Fig. 2f) and negative for CD34, desmin, and myogenin markers]. The patient refused the treatment options (proton beam therapy or surgical treatment) at our institution and was lost to further follow-up.
Fig. 2.
a, b Lower and high power views of tumor with central necrosis (arrow), c, d fascicles of spindle tumor cells with necrosis and brisk abnormal mitoses (arrow and insert), e prominent nuclear pleomorphism and f strong diffuse positivity for smooth muscle actin (SMA). (H&E stain: a ×10, b ×40, c ×20, d ×40, e ×40 and f, peroxidase-antiperoxidase immunostain with anti-SMA ×20)
Discussion
RB may occur in hereditary and non-hereditary forms. The hereditary form is generally bilateral but can present unilaterally with a positive family history and typically exhibits a germline mutation in the RB1 gene on chromosome 13. The non-hereditary form is usually unilateral but can show the same germline mutation in up to 10% of cases [4]. It has been suggested that the germline mutation predisposes to a lifelong increased risk of many forms of cancer [5]. Patients with hereditary RB have a higher cumulative risk of developing secondary malignancies than those with the non-hereditary form [2, 6, 7]. In one study, hereditary RB patients had an increased risk of secondary malignancy 40 years after RB treatment of approximately 28% as compared to 1.44% in patients with the non-hereditary form [6]. It has also been demonstrated that hereditary RB survivors had a higher mortality from secondary malignancies of bone and soft tissue whereas patients with non-hereditary RB showed a higher mortality from myeloid leukemia, ovary, and kidney cancers [5]. In addition, radiation therapy for RB treatment is believed to further increase the risk for secondary malignancies in hereditary cases [8].
LMS is uncommonly found in the head and neck region and has been estimated to compose only approximately 2% of sinonasal mesenchymal tumors [3]. However, there is an increased risk of LMS occurring as a secondary tumor in patients with a history of treated RB regardless of the history of radiation therapy. MacCarthy et al. [2] found in a cohort study that LMS is the most often reported secondary malignancy accounting for 58% of cases followed by other soft tissue sarcomas, osteosarcoma, carcinoma, CNS tumors, melanoma, leukemia, and other types in order of decreasing prevalence. Similarly, Kleinerman et al. [1] found that LMS was the most represented of soft tissue sarcomas in patients with a history of RB, accounting for 33% of secondary tumors in a cohort study of 963 patients with RB. Interestingly, both in this study and supported by the study of Marees et al. [6] it was found that LMS arose more commonly outside the field of prior radiation therapy, while the other subtypes more often arose inside the field. LMS tend to arise later after RB than other soft tissue sarcomas with 78% of LMS arose 30 years or more after initial diagnosis as compared to the majority of fibrosarcoma and rhabdomyosarcoma that arose within the first 20 years of diagnosis [1].
When determining the incidence of sinonasal LMS after treated RB several difficulties arise. First, there is some degree of anatomic overlap in the primary tumor origin in the craniofacial area, which makes classifying tumors that are purely sinonasal in origin difficult. Table 1 lists 14 reported cases of LMS clearly identified as primarily arising from the sinonasal tract of patients with a history of RB selected in addition to the present case [3, 4, 9–14]. In the large cohort studies of Kleinerman et al. [1] and MacCarthy et al. [2] of patients with a history of RB developing secondary malignancy the studies did not specify the location in conjunction with the tumor type and thus patients specifically matching the criteria of sinonasal LMS could not be identified. In the previously mentioned cohort study of Kleinerman et al. [1], 963 patients with a history of RB developed 69 secondary malignancy tumors. Of these, 23 were identified as LMS. In addition, out of the 69 cases it was identified that the sinonasal tract was the location of 16 of the tumors. However, the location of the tumors was not matched to the tumor type. The other large cohort study of MacCarthy et al. [2] included patients with secondary tumors, but the locations were also not matched to secondary tumor type. In addition, ten cases in the literature of LMS arising in patients with a history of RB originated in areas adjacent to the sinonasal cavity (orbit, maxilla) for which it is possible the sinonasal tract was involved by extension but did not appear to be the original or significantly involved site [10, 15–20].
Table 1.
Reported cases of sinonasal LMS and history of RB including present case
| Author | Age/gender | Type RB | Time | Site of LMS | Side |
|---|---|---|---|---|---|
| Martin-Hirsch et al. [9] | 45 M | Bilateral | 43 | Nasal fossa | I |
| Rubin et al. [10] | 12 U | Bilateral | 10.5 | Ethmoid sinus | NA |
| Rubin et al. [10] | 23 U | Bilateral | 21 | Sphenoid sinus | NA |
| Rubin et al. [10] | 32 U | Bilateral | 31 | Ethmoid sinus | NA |
| Dunkel et al. [4] | 12 U | Bilateral | NA | Ethmoid sinus | I |
| Dunkel et al. [4] | 23 U | Bilateral | NA | Sphenoid sinus | C |
| Dunkel et al. [4] | 32 U | Bilateral | NA | Ethmoid sinus | I |
| Dunkel et al. [4] | 10 U | Bilateral | NA | Max sinus | I |
| Dunkel et al. [4] | 18 U | Bilateral | NA | Max sinus | I |
| Klippenstein et al. [11] | 29 M | Bilateral | 28 | Max sinus | NA |
| Newman et al. [12] | 40 M | Bilateral | 39 | Ethmoid and max sinus | NA |
| Sedghizadeh et al. [13] | 30 M | Unilateral with FH | 28 | Max sinus | I |
| Ulrich et al. [3] | 23 M | Bilateral | 20 | Nasal cavity and bilateral sinus | NA |
| Qureshi et al. [14] | 15 M | Bilateral | 13.5 | Nasal cavity infratemporal fossa and ethmoid sinus | NA |
| Present case | 35 M | Unilateral without FH | 34 | Ethmoid, maxillary, sphenoid sinus | I |
LMS leiomyosarcoma, RB retinoblastoma, U gender unknown, M male, FH family history positive for RB, Time elapsed time between treatment of RB and diagnosis of LMS (in years), Side LMS arose in relationship to radiation field: (I ipsilateral, C contralateral, NA not available)
Of the 15 cases identified as sinonasal LMS after treated RB (including the current case), the median age at onset of secondary LMS was 25 years old, with a range from age 10–45. Gender predilection could not be accurately quantified because many cases did not list the gender, but of the seven cases where gender was known, all patients were male. Previous studies showed that the majority of LMS arising after RB in males occurred in the head and neck, while LMS after RB in females mainly arose in the pelvic region [1]. Of the 10 cases that reported the age of RB treatment, the median time between treatment of RB and diagnosis of LMS was 26.8 years. The reported cases of sinonasal LMS complicated the bilateral hereditary form of treated RB in 13 of the 15 cases. In the only other previously reported case with history of unilateral RB, the patient involved had a positive family history for RB as his three children each subsequently developed unilateral RB in infancy [13]. Thus to the best of our knowledge, the present case appears to be the first case of sinonasal LMS arising in a patient with a history of non-hereditary unilateral RB. By site, 4 out of 15 cases involved the ethmoid sinus, 2 involved the sphenoid sinus, 4 involved the maxillary sinus, 1 involved the nasal fossa, and 4 involved a combination of sites including nasal cavity, ethmoid, maxillary, and sphenoid sinuses. All patients received radiation therapy for treatment of RB. The majority of cases that listed the relationship of the tumor to the field of radiation reported that the secondary tumor arose on the ipsilateral side including the current case [4, 9, 13]. One case reported a secondary tumor arising on the contralateral side of radiation field [4].
The majority of head and neck LMS are of moderate or high histologic grade, behave aggressively, and show little response to chemotherapy or radiation [19, 21].
Prognosis of LMS of the sinonasal tract is dependent on location and extent of tumor. Treatment generally consists of radical resection, and chemotherapy and radiation therapy have been used with limited success [3]. Tumors confined to the nasal cavity in which complete excision can be achieved have a high rate of survival [19]. However, in many cases of sinonasal LMS complete resection is not possible with negative margins, and in these cases mortality is much higher [19].
The histopathologic differential diagnosis for LMS in this area includes other high grade spindle cell sarcomas: fibrosarcoma (FS), malignant peripheral nerve sheath tumor (MPNST), rhabdomyosarcoma (RMS), myofibroblastic sarcoma (or myofibrosarcoma), and the fibrous monophasic variant of synovial sarcoma. FS is an important differential diagnosis especially in the setting of a patient with a history of previous radiation therapy and the sinonasal tract is not an uncommon location within the head and neck for occurrence [22]. FS generally presents with a more tapered cell nuclei and “herring bone” fascicular pattern than LMS and may show focal areas of staining for smooth muscle markers reflecting myofibroblastic differentiation but does not show the extent of diffuse positivity to smooth muscle markers as LMS [23]. MPNST has been reported rarely in the sinonasal tract and differs from LMS in showing a characteristic appearance of wavy or buckled cells and alternating zones of cellular and less cellular areas which may appear in a vaguely nodular pattern [22, 24]. Spindled MPNST will be negative for smooth muscle actin and show focal S-100 positivity, whereas LMS is the opposite [22]. The pleomorphic form of RMS may exhibit areas of spindle cell fascicular pattern [25]. The spindle cell variant of embryonal RMS may also be seen in the sinonasal tract and may resemble LMS but generally occurs in young patients [26]. Pleomorphic and embryonal RMS like LMS are positive for desmin and SMA, but unlike LMS they are positive for myoglobin or myoD1 indicating a skeletal muscle origin [24]. Myofibroblastic sarcoma (or myofibrosarcoma) may be included in the differential diagnosis, but these lesions are generally of a low grade, exhibit fusiform rather than the cigar-shaped nuclei seen in LMS, have a paler eosinophilic cytoplasm than LMS, and are only focally positive for SMA [24]. In addition, staining for h-caldesmon is generally negative in myofibroblastic sarcoma but positive in LMS [24]. A final entity to consider is the monophasic fibrous variant of synovial sarcoma (SS). Rare cases of both monophasic and biphasic SS have been reported in the sinonasal tract [27]. Monophasic SS generally exhibits abundant wiry collagen, branching vascular pattern, and stromal calcification [22]. This lesion could be differentiated from LMS by the presence of positivity for cytokeratin staining (AE 1/3) at least in focal areas, CD99 positivity, and bcl-2 positivity which LMS will not exhibit [22, 28]. In addition, a specific chromosomal translocation (X; 18) is associated with SS that is not noted in LMS [27].
Conclusion
Patients with a history of RB have been shown to have a dramatically increased risk of developing secondary malignancies later in life likely due to both a genetic predisposition and complications of radiation therapy to the original tumor. These patients may develop malignancies in the head and neck and at an earlier age than most patients developing a primary malignancy in these areas. Early identification of these tumors is critical due to their locally aggressive nature and poor prognosis. Given the excellent survival of treated RB patients, the awareness of the phenomenon of increased risk of secondary malignancy in these patients has important implications. Among these implications is counseling of survivors and the clinical follow-up with thorough investigation of any symptom or sign in adult patients for the early detection of secondary malignancy to prevent a fatal outcome.
Conflict of interest
There is no financial conflict of interest related to this manuscript.
References
- 1.Kleinerman RA, Tucker MA, Abramson DH, et al. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst. 2007;99:24–31. doi: 10.1093/jnci/djk002. [DOI] [PubMed] [Google Scholar]
- 2.MacCarthy A, Bayne AM, Draper G, et al. Non-ocular tumours following retinoblastoma in Great Britain 1951 to 2004. Br J Opthamol. 2009;93:1159–1162. doi: 10.1136/bjo.2008.146035. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich CT, Feiz-Erfan I, Spetzler RF, et al. Sinonasal leiomyosarcoma: review of literature and case report. Laryngoscope. 2005;115:2242–2248. doi: 10.1097/01.mlg.0000183767.97518.09. [DOI] [PubMed] [Google Scholar]
- 4.Dunkel IJ, Gerald WL, Rosenfield NS, et al. Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy: the memorial sloan-kettering experience. Med Pediatr Oncol. 1998;31:59–62. doi: 10.1002/(SICI)1096-911X(199801)30:1<59::AID-MPO14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Acquaviva A, Ciccolallo L, Rondelli R, et al. Mortality from second tumour among long-term survivors of retinoblastoma: a retrospective analysis of the Italian retinoblastoma registry. Oncogene. 2006;25:5350–5357. doi: 10.1038/sj.onc.1209786. [DOI] [PubMed] [Google Scholar]
- 6.Marees T, Molle AC, Imhof SM, et al. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100:1771–1779. doi: 10.1093/jnci/djn394. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher O, Easton D, Anderson K, et al. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96:357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 8.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Hirsch DP, Habashi S, Benbow EW, et al. Post-irradiation leiomyosarcoma of the maxilla. J Laryngol Otol. 1991;105(12):1068–1071. doi: 10.1017/S0022215100118213. [DOI] [PubMed] [Google Scholar]
- 10.Rubin CZ, Rosenfield NS, Abramson SJ, et al. The location and appearance of second malignancies in patients with bilateral retinoblastoma. Sarcoma. 1997;1:89–93. doi: 10.1080/13577149778353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klippenstein KA, Welsey RE, Glick AD. Orbital leiomyosarcoma after retinoblastoma. Opthalmic Surg Lasers. 1999;30(7):579–583. [PubMed] [Google Scholar]
- 12.Newman SA, Yoo J, Jones N, et al. Radiation-induced leiomyosarcoma of the ethmoid sinus. Skull Base. 2003;13(3):179–182. doi: 10.1055/s-2003-43329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedghizadeh PP, Angiero F, Allen CM, et al. Post-irradiation leiomyosarcoma of the maxilla: report of a case in a patient with prior radiation treatment for retinoblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:726–731. doi: 10.1016/j.tripleo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi SS, Mistry RC, Natrajan G, et al. Leiomyosarcoma of the maxilla as second malignancy in retinoblastoma. Indian J Cancer. 2008;45:123–125. doi: 10.4103/0019-509X.44069. [DOI] [PubMed] [Google Scholar]
- 15.Font RL, Jurco S, Brechner RJ. Posradiation leiomyosarcoma of the orbit complicating bilateral retinoblastoma. Arch Opthalmol. 1983;101:1557–1561. doi: 10.1001/archopht.1983.01040020559011. [DOI] [PubMed] [Google Scholar]
- 16.Folberg R, Cleasby G, Flanagan JA, et al. Orbital leiomyosarcoma after radiation therapy for bilateral retinoblastoma. Arch Opthalmol. 1983;101:1562–1565. doi: 10.1001/archopht.1983.01040020564012. [DOI] [PubMed] [Google Scholar]
- 17.Mihara F, Gupta KL, Kartchner ZA, et al. Leiomyosarcoma after retinoblastoma radiotherapy. Radiat Med. 1991;9(5):183–184. [PubMed] [Google Scholar]
- 18.Marta U, Zsuzsanna S, Jozsef B, et al. Rare incidence of three consecutive primary tumors in the maxillofacial region: retinoblastoma, leiomyosarcoma, and choriocarcinoma: case report. J Craniofac Surg. 2001;12:464–484. doi: 10.1097/00001665-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery E, Goldblum JR, Fisher C. Leiomyosarcoma of the head and neck: a clinicopathological study. Histopathology. 2002;40:518–525. doi: 10.1046/j.1365-2559.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 20.Demirci H, Marentette LJ, Nelson CC. The transglabellar/subcranial approach for surgical excision of periocular second tumors in retinoblastoma. Orbit. 2008;27:285–291. doi: 10.1080/01676830802222951. [DOI] [PubMed] [Google Scholar]
- 21.Kuruvilla A, Wenig BM, Humphrey DM, et al. Leiomyosarcoma of the sinonasal tract. A clinicopathologic study of nine cases. Arch Otoloryngol Head Neck Surg. 1990;116:1278–1286. doi: 10.1001/archotol.1990.01870110050005. [DOI] [PubMed] [Google Scholar]
- 22.Folpe AL. Soft-tissue tumors of the head and neck. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: Saunders Elsevier; 2009. pp. 647–727. [Google Scholar]
- 23.Weiss SWW, Goldblum JR. Fibrosarcoma. In: Weiss SWW, Goldblum JR, editors. Enzinger and Weiss’s soft tissue tumors. 5. Edinburgh, Scotland: Mosby Elsevier; 2008. pp. 303–330. [Google Scholar]
- 24.Minovi A, Basten O, Hunter B, et al. Malignant peripheral nerve sheath tumors of the head and neck: management of 10 cases and literature review. Head Neck. 2007;29:439–445. doi: 10.1002/hed.20537. [DOI] [PubMed] [Google Scholar]
- 25.Weiss SWW, Goldblum JR. Rhabdomyosarcoma. In: Weiss SWW, Goldblum JR, editors. Enzinger and Weiss’s soft tissue tumors. 5. Edinburgh, Scotland: Mosby Elsevier; 2008. pp. 595–631. [Google Scholar]
- 26.Wenig BM. Undifferentiated malignant neoplasms of the sinonasal tract. Arch Pathol Lab Med. 2009;133:699–712. doi: 10.5858/133.5.699. [DOI] [PubMed] [Google Scholar]
- 27.Gallia GL, Sciubba DM, Hann CL, et al. Synovial sarcoma of the frontal sinus. J Neurosurg. 2005;103:1077–1080. doi: 10.3171/jns.2005.103.6.1077. [DOI] [PubMed] [Google Scholar]
- 28.Weiss SWW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SWW, Goldblum JR, editors. Enzinger and Weiss’s soft tissue tumors. 5. Edinburgh, Scotland: Mosby Elsevier; 2008. pp. 1161–1220. [Google Scholar]