Abstract
The Ewing’s family of tumors (EFT) are malignant neoplasms affecting children and young adults. Most cases arise in the long bones or the pelvis. Primary EFT of head and neck is uncommon and primary sinonasal EFT is even rarer. Previous studies have not focused on the sinonasal region specifically, and the published literature on sinonasal EFT consists of sporadic case reports. Fourteen cases of sinonasal EFT were available and had H&Es for review and immunohistochemical stains for CD99, S100, keratins, synaptophysin and desmin. FISH or RT-PCR was performed for EWSR1 abnormalities on 8 cases. The 14 identified patients included 5 males and 9 females, ranging from 7–70 years of age (mean 32.4 years). Tumors involved nasal cavity (5), sinuses (5) or both (4). Five patients had dural, orbital or brain involvement. The majority involved bone radiologically and/or microscopically. All cases were composed of small cells with variable cytoplasmic clearing. Focal or prominent nesting was noted in most cases. All cases were positive for CD99. Keratins (AE1/3 and/or CAM5.2), S100 and synaptophysin were positive in 4, 3 and 5 cases, respectively. All cases were negative for desmin. The 8 cases tested by FISH or RT-PCR were positive for EWSR1 abnormalities. Follow-up in 8 patients ranged from 1–168 months (average 11.3 m) showing 1 death due to metastatic disease, 1 death due to local disease, 1 patient alive with metastases and 5 patients disease-free at last follow-up. Interestingly, however, an analysis of the literature suggests a better prognosis for sinonasal EFT than EFT overall.
Keywords: Ewing’s family of tumors, Sinonasal, Maxillary bone, Olfactory neuroblastoma
Introduction
The Ewing’s sarcoma family of tumors (EFT) is a group of malignant neoplasms mostly affecting children and young adults [1]. It includes a spectrum of small round blue cell tumors such as osseous and extraosseous Ewing’s sarcoma, peripheral neuroectodermal tumor (PNET) and Askin’s tumor of the chest wall [1]. Most cases arise in the long bones or the pelvis [1]. Primary EFT of the head and neck is uncommon. Amongst bone EFTs, the head and neck (skull) accounts for 3.8% of cases in one large study [2]. Bone and soft tissue EFTs in the head and neck have been reported to represent anywhere from 1–7% of EFTs in some studies [3, 4] and up to 18% of EFTs in childhood in others (5). Primary sinonasal EFT is even rarer as a subgroup and represents only a small subset of these head and neck cancers [6]. The exact percentage is unknown as most studies of EFT are general studies that classify head and neck as a unified location [5, 7, 8] and have not focused specifically on the sinonasal region. Most sinonasal EFTs previously described in the literature consist of sporadic case reports that predominantly lack molecular confirmation or long-term follow-up.
The sinonasal tract is unique amongst EFTs in that the differential diagnosis is broader than at other soft tissue and bone sites, with numerous primitive tumors arising in this location, including: epithelial tumors, such as sinonasal undifferentiated carcinoma (SNUC), small cell carcinoma and the recently reported NUT midline carcinomas (NMC); mesenchymal tumors, such as rhabdomyosarcoma; and neural tumors, such as olfactory neuroblastoma [9, 10]. The histologic and immunophenotypic diversity of EFTs, including occasional keratin and neuroendocrine marker positivity [11], makes the diagnosis of this entity quite challenging particularly in small biopsies. Compounding this difficulty is the fact that other non-epithelial or neuroendocrine tumors, such as alveolar rhabdomyosarcoma, have recently been reported to occasionally stain with keratins and/or synaptophysin [12]. Herein, we describe the clinicopathologic features, immunohistochemical profile and molecular characteristics of a series of 14 cases of sinonasal tract EFT and review the literature to determine the prognostic implications of the diagnosis in this location.
Methods
Fourteen cases of “EFT” were retrieved from the archives of the authors’ institutions and re-reviewed. All had been diagnosed as “Ewing’s sarcoma” or “Peripheral neuroectodermal tumor/PNET”. All surgical specimens were fixed in 10% neutral buffered formalin. H&Es were available on all cases. PAS and PAS-D histochemical slides were available on most cases and these were also reviewed. Immunohistochemical stains had been performed on 3–5 μ thick sections from representative formalin-fixed, paraffin-embedded tissue with antibodies to keratins (CAM5.2 or pankeratin/AE1/AE3), CD99, synaptophysin, S100 and desmin. Occasional cases had one or more of the following available for review: HMB-45, Melan-A, NSE, chromogranin, CD56, myogenin and p63. Reverse transcriptase-polymerase chain reaction (RT-PCR) results for the EWS-FLI1 fusion transcript were available in one case. FISH for the rearrangement of the EWSR1 gene had been performed on 7 additional cases using a dual-color break-apart probe (Vysis®, Downer’s Grove, IL) for the 5′ region of the EWSR1 gene (spectrum green) and the corresponding 3′ region of the gene (spectrum orange).
Results
Clinical Findings
The clinical findings are summarized in Table 1. The 14 identified patients included 5 males and 9 females, ranging from 7 to 70 years of age (mean 32.4 years). All patients presented with nasal obstruction and/or epistaxis. Tumor involvement included, the nasal cavity (5/14 cases), one or more sinuses (5/14 cases) and both nasal cavity and at least one sinus (4/14 cases). Four patients presented with left-sided tumors and 5 patients with right-sided tumors, 1 patient had bilateral disease and the laterality was not specified in 4 cases. The involved sinuses were maxillary (5 cases), ethmoid (5 cases), sphenoid (2 cases) and frontal (2 cases).
Table 1.
Clinical findings of ewing’s family tumors of the sinonasal tract
| Case | Age and gender | Site | Treatment | Follow up (months) and outcome |
|---|---|---|---|---|
| 1 | 69F | Right maxilla, maxillary sinus, nasal cavity and palate | Chemoradiation only | 6 m, NED |
| 2 | 45F | Right ethmoid and maxillary sinuses, orbit and frontal lobe | Chemoradiation only | 14 m, DOD with breast mets |
| 3 | 54 M | Right nasal cavity, maxillary bone and orbit | Chemotherapy only | 17 m, DOD |
| 4 | 13 M | Left sphenoid sinus | NA | 1 m, lung mets, AWD (lost to follow up) |
| 5 | 34 M | Bilateral ethmoid and frontal sinuses | NA | NA |
| 6 | 35F | Ethmoid sinus | NA | NA |
| 7 | 7F | Right nasal cavity | NA | NA |
| 8 | 25 M | Left maxillary sinus, nasal cavity, maxillary bone and orbit | Surgery | 26 m, NED |
| 9 | 33 M | Spheno-ethmoid recess | NA | NA |
| 10 | 13F | Left maxillary sinus | Surgery | 4 m, NED |
| 11 | 22F | Nasal cavity, cribriform plate and dura | NA | NA |
| 12 | 18F | Left nasal cavity | NA | NA |
| 13 | 70F | Right nasal cavity and infratemporal fossa | Surgery and postoperative radiation | 21 m, NED |
| 14 | 15F | Nasal cavity, maxillary, ethmoid and frontal sinuses, and orbit | Chemoradiation only | 168 m, NED |
NA not available, NED no evidence of disease, DOD dead of disease (local), AWD alive with disease, mets metastasis
In 5 patients (5/14–36%), dural, brain or orbital involvement was noted radiologically (Fig. 1). All patients with available radiology had bulky disease that was suspicious for, or definitively involving, maxillary bone. Patients were treated with surgery alone (2 patients), chemotherapy alone (1 patient), combined chemoradiation therapy (3 patients) and surgery with postoperative radiation (1 patient). Post treatment follow-up available in 8 patients ranged from 1–168 months (average 11.3 m) and showed that 1 patient died of local and metastatic disease (breast), 1 patient died of local disease, 1 patient was alive with lung metastases and the remaining 5 patients were disease free at last follow-up.
Fig. 1.
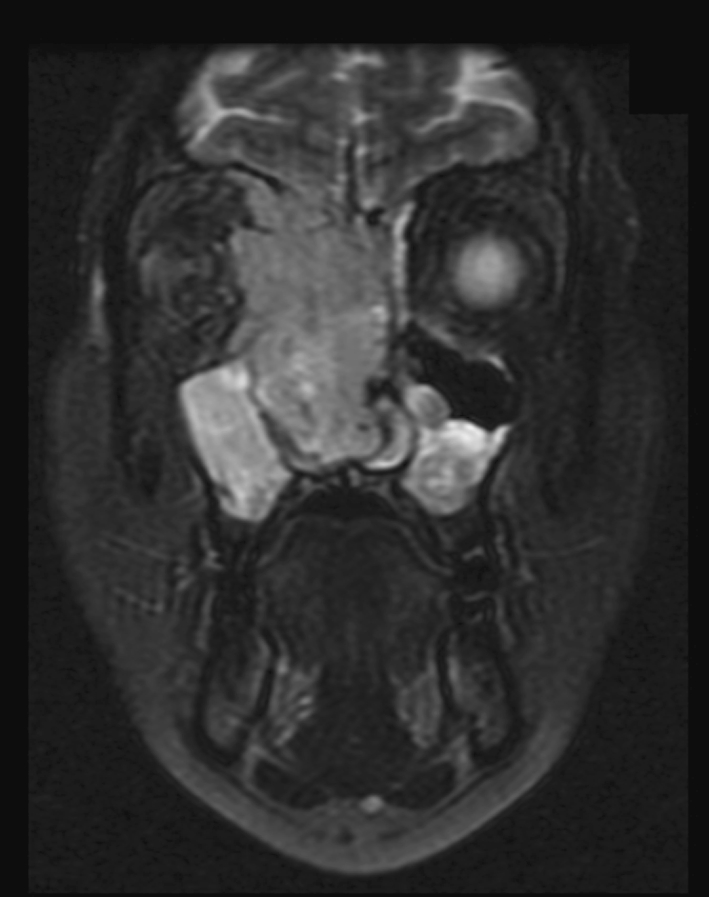
Coronal MRI demonstrating a large sinonasal mass with bone, dural and intracranial involvement
Histopathological Findings
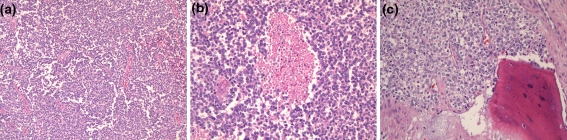
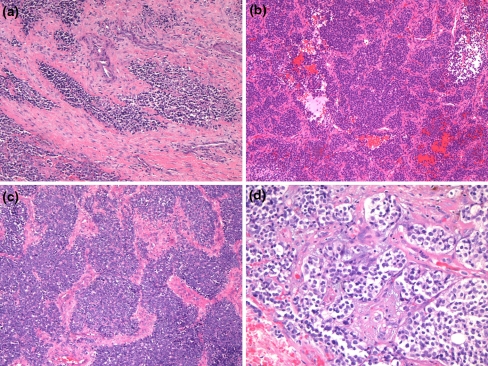
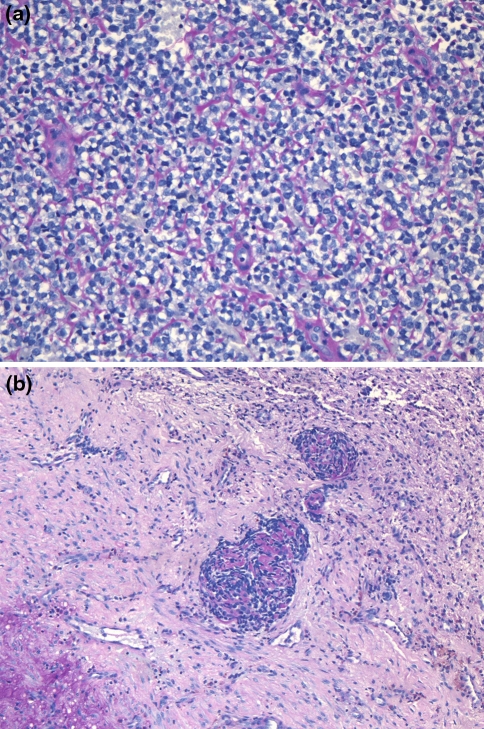
The histologic, immunohistochemical and molecular findings are summarized in Table 2. Review of the original histology sections showed that in all cases the tumors were composed of nests, cords and sheets of small round blue cells with bland nuclei and indistinct to small nucleoli (Fig. 2a). Minimal mitotic activity was present. Five cases had focal necrosis (Fig. 2b). Extensive necrosis was occasionally seen but was interpreted as surface ulceration rather than definitive tumour necrosis. Occasional Homer-Wright type rosettes were seen but were not prominent. There were no cases with large cell change or significant pleomorphism (“atypical Ewing’s”). Microscopic evidence of bone invasion/involvement was present in most cases (Fig. 2c). Those cases that did not exhibit bone involvement did not have bone to assess on the material available. Ten cases (10/14–71%) showed nesting with prominent intervening fibrosis or fibrovascular stroma at least focally, imparting an appearance similar to a carcinoma or olfactory neuroblastoma (Fig. 3a–d). However, the majority of each tumor was formed of sheets of bland cells. Variable cytoplasmic clearing was noted in most cases (Fig. 3d), with the remainder of the cells having minimal visible cytoplasm. One case showed focal plasmacytoid/rhabdoid cells. Three cases had pericellular eosinophilic material, which compartmentalized the tumor cells (Fig. 4a). This material showed PAS positivity. Another showed focal PAS positive basement membrane-like material with a pseudocribriform pattern (Fig. 4b). PAS also demonstrated cytoplasmic granularity due to glycogen accumulation, which was lost on PASD staining in all but one of the cases tested (7/8). An additional two tumors were reported to have glycogen on electron microscopy (not available for review). One of the cases focally involved the oral cavity and was noted to surround minor salivary glands leading to a basaloid hyperplasia of the acini.
Table 2.
Summary of pathological findings of ewing’s family tumors of the sinonasal tract
| Case | Necrosis | Bone involved | Nesting | Glycogen | CD99 | Keratin | Des | S100 | Neuroendocrine | Molecular |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | + | + PAS/D | + | − | − | − | − | EWS-FLI1 + RT-PCR |
| 2 | + | + | − | + PAS/D | + | − | − | − | + CD56 | NA |
| 3 | + | + | − | + PAS/D | + | f + CAM5.2 | − | − | − | NA |
| 4 | − | + | + | NA | + | f + AE1/3 | − | − | f + NSE | NA |
| 5 | + | − | + | NA | + | − | − | − | f + NSE | NA |
| 6 | + | + | + | + EM | + | f + AE1/3 | − | f+ | f + synaptophysin | NA |
| 7 | − | + | + | + PAS/D | + | − | − | − | f + synaptophysin | EWSR1 FISH + |
| 8 | − | + | + | − | + | − | − | − | − | EWSR1 FISH + |
| 9 | − | − | + | + PAS/D | + | − | − | − | f + CD56 and synaptophysin | EWSR1 FISH + |
| 10 | − | − | − | + PAS/D | + | − | − | f+ | f + synaptophysin | EWSR1 FISH + |
| 11 | − | + | + | +PASD | + | f + CAM5.2 and AE1/3 | − | NA | f + CD56 and synaptophysin | EWSR1 FISH + |
| 12 | − | + | + | NA | + | − | NA | NA | NA | EWSR1 FISH + |
| 13 | + | + | − | NA | + | − | − | − | − | EWSR1 FISH + |
| 14 | − | − | + | + EM | + | − | − | f+ | f + NSE | FISH equivocal |
PAS/D Periodic acid Schiff positive, diastase sensitive, EM electron microscopy, Des desmin, NSE neuron specific enolase
Fig. 2.
All tumors were composed of sheets of bland small round blue cells (a). Focal necrosis was seen in a minority of cases (b). Bone invasion, suspected clinically in all cases, was commonly seen microscopically (c)
Fig. 3.
Nesting in the tumors was a common finding and ranged from discrete nests to broad anastomosing nests with an intervening fibrovascular stroma (a–c). Clear cell change was also a common finding (d)
Fig. 4.
Pericellular eosinophilic material was noted compartmentalizing tumor cells in a few cases and this material was positive for PASD (a). One case showed focal basement membrane-like material on PAS imparting a pseudocribriform pattern (b)
Immunohistochemical Findings
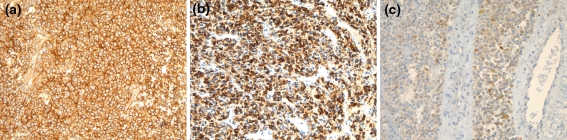
Immunohistochemical studies revealed that the neoplastic cells were diffusely positive for CD99 (Fig. 5a) in a membranous and cytoplasmic pattern (14/14–100%) and diffusely positive for vimentin (6/7–86%). Focal positivity for one or more keratins (4/14–29%), one or more neural/neuroendocrine markers (9/14–64%) and S100 (3/12–25%) was also identified (Fig. 5b-C). The S100 staining was weak and did not demonstrate a sustentacular pattern of staining. All cases tested were negative for desmin (0/13), myogenin (0/4), HMB-45 (0/4), Melan-A (0/3) and p63 (0/5).
Fig. 5.
All cases showed a strong membranous CD99 staining pattern (a). Focal keratin staining was noted in several cases. A CAM5.2 stain is shown here (b). Neuroendocrine markers were occasionally focally positive but were generally patchy or weak (c)
Molecular and FISH
In case 1, a hybrid transcript corresponding to the t(11, 22) reciprocal translocation involving the EWSR1 and FLI-1 genes was detected by RT-PCR. An additional 7 cases were tested by fluorescence in situ hybridization (FISH) and were positive for EWSR1 rearrangement. One additional case was tested by FISH for EWSR1, but showed equivocal results. No material for molecular/cytogenetic studies was available in the remaining 5 cases.
Discussion
A variety of poorly-differentiated neoplastic entities consisting of small round blue cells can arise from the sinonasal tract [10]. These include rhabdomyosarcoma, lymphoma, poorly-differentiated carcinomas, melanoma, olfactory neuroblastoma (ONB) and EFT. The differential diagnosis of these entities based on histology alone is complicated by the fact that many of them show similar histologic features and immunophenotypic overlap [10]. Among these tumors, EFTs are rare in this location and have not been extensively reported in the literature. Many of the previously published cases of EFT of the sinonasal tract have had limited or absent histologic data. In this report, we describe the histopathologic findings in 14 EFT cases of the sinonasal region/maxillary bone. This represents the largest single series of EFT in this location and the most with molecular confirmation (8 cases). While the diagnosis of EFT can be challenging, the differential diagnosis can be excluded in most cases. In general, melanoma, lymphoma and rhabdomyosarcoma can be routinely ruled out with immunohistochemistry for S100, CD45 and desmin, respectively. However, occasional cases of EFT are focally positive for S100 or desmin. In these cases, additional immunohistochemical stains for melanoma markers (HMB45, Melan-A) and specific skeletal muscle markers (myogenin, myoD1) will help exclude these entities. EFTs have never been reported to be positive for these markers, to our knowledge.
Carcinomas and ONB may present greater difficulty in the differential diagnosis. One finding noted in this study that may contribute to this confusion is the presence of a nesting pattern in many cases of EFT. This has not been noted in the literature previously for sinonasal EFT. Nesting was seen at least focally in a significant proportion of cases and may increase the likelihood of a carcinoma or ONB misdiagnosis, particularly in those cases focally positive for keratin or synaptophysin, respectively. Carcinomas, however, are generally diffusely positive for multiple keratins and do not generally occur in young patients. In contrast, EFTs, which occur in younger patients, typically stain focally for keratins and usually for only one marker. Therefore, a carcinoma can be relatively easily excluded. One of the current cases showed a pseudocribriform pattern focally that could potentially mimic a salivary tumor. However, the lack of staining for keratins ruled out a carcinoma, as well as the negativity for p63, a marker positive in most salivary tumors showing a cribriform pattern.
The nesting pattern in EFT seems to be more common in sinonasal EFTs than in their extremity counterparts, in our experience. This pattern has been referred to previously in one large study as “lobulation” in the category of “atypical EFT” [11]. None of the current cases showed nuclear pleomorphism worthy of this designation, however. Images from previous cases reported in the literature also show this nesting pattern in sinonasal EFT [13]. One possible explanation for this finding could be the rich fibrovascular nature of the native sinonasal tissue, particularly in the turbinates. Growth of tumor cells around pre-existing fibrovascular structures without destroying them may account for this nesting, to some extent, rather than to any inherent nesting quality of the EFTs. This preservation of pre-existing structures was also seen around minor salivary tissues in the palate in case 1.
Cases of EFT which have a great degree of nesting and lack keratin staining will raise the possibility of ONB, as these latter tumors are also known for a prominent nesting pattern and often occur in young patients [6]. ONB can be separated from EFT by the presence of an S100 positive sustentacular network in most cases as well as diffuse positivity for neuroendocrine markers and negativity for CD99 [6, 14]. While EFTs can be positive for synaptophysin and other neuroendocrine markers, they usually only stain focally. Moreover, S100 staining in EFTs was generally weak in this study and only stained tumor cells, unlike the sustentacular pattern of ONB. In addition, EFTs stain with CD99 in almost 100% of cases, in contrast to ONB [11]. However, ONB cases without sustentacular cells will present an additional diagnostic challenge, although these cases tend to be higher in Hyams’ morphologic grade [6] while EFTs are most often monotonous in appearance. Another useful clue includes superior nasal cavity/cribriform plate involvement in ONB, while EFTs can occur anywhere in the sinonasal tract and most often involve at least one sinus. If necessary, molecular techniques can be used in equivocal cases, as the vast majority of EFTs harbor the EWS-FLI1 fusion transcript, while ONBs do not [14]. Initially, there were reports published in the literature of ONB with the t(11;22) [15] as well as studies suggesting ONB is a primitive neuroectodermal tumor [16]. However, larger studies have shown the complete lack of the t(11;22) in classical ONB [14] and those cases harboring this molecular signature should always be regarded as being in the EFT family, particularly given the great histologic diversity known for EFTs in other locations [11].
Cytogenetics and other molecular techniques, such as FISH and RT-PCR, have provided new tools for diagnosis of this group of small round blue cell neoplasms, and may be particularly useful in the more difficult cases. Molecular studies are increasingly being requested by clinicians who are reluctant to treat patients without them and insist on the molecular diagnostics for therapeutic decision-making [11]. Molecular diagnostics should not be considered a prerequisite for the diagnosis, however. The cases in this study were all considered diagnostic without molecular confirmation, although FISH or RT-PCR, was performed in 8 cases and shown to be positive. A recent study by Cordes et al. demonstrated a EWSR1-FLI1 fusion transcript by RT-PCR on frozen tissue in only 4 of 10 cases of typical sinonasal EFT [17]. A negative RT-PCR study does not rule out alternative fusion gene partners for EWSR1, present in as much as 15% of EFT cases in other locations [1]. In addition, FISH for EWSR1, which will catch all potential gene partners, will still miss those cases where FUS replaces EWSR1 [1].
Due to advances in the treatment of EFT in recent years, survival rates (for all sites) are reported to have improved and have reached up to 86% in patients with non-metastatic disease at initial presentation [7]. Those with metastases have poorer outcomes [7]. Approximately 12–38% had metastases at first presentation [5, 7]. Approximately 32% of patients overall died of disease in one study [7] and those with metastases had an overall survival of only 21% in another study [5]. The majority of cases included in these series consisted of extremity osseus and soft tissue tumors. There was a slight male predominance [7] and the majority of patients were less than 30 years of age [6] with a median age of 16 years [7]. Although in some series the prognosis has been suggested to be more favorable for the head and neck region [4, 8, 18], there have been no large studies of EFT in the sinonasal area specifically and most of the previously reported cases have lacked molecular confirmation. Soft tissue EFT has been reported to have a greater chance of survival for those cases that achieve complete tumor removal prior to chemotherapy [5]. This latter point, coupled with the difficulty in achieving margin negative resections in the sinonasal tract, would suggest a worse prognosis for this location, despite the proposed better outcomes for head and neck EFTs as a whole [5].
A total of 49 cases of proposed sinonasal EFT have been reported in the English language literature thus far [3, 8, 13, 15, 17–41], 11 of which were confirmed with molecular studies [15, 17, 19, 26, 37–39] and 21 of which have reported follow-up or outcomes [8, 13, 15, 19, 20, 22–26, 33, 35–37, 39, 40]. The pertinent information on previous cases of EFT with follow-up available is presented in Table 3. Many of these cases, however, provided limited information regarding clinical parameters, histology or immunohistochemistry. Combining previous cases and those in this report, there have been a total of 63 cases of EFT of the sinonasal region, with follow-up available on 29 cases and molecular confirmation on 19 cases. The average age for this group was 24 years with a range of 7–70 and there was a slight female predominance (1.13:1). Follow-up on these 29 cases ranged from 1–276 months (average 50.6 m). There were 3 local recurrences and 5 metastases in this group. Of these 29 cases, 23 (79%) had no evidence of disease (NED) at last follow-up. Of these, 2 patients were retreated after a late local failure at 192 months, or bone metastases after 30 months and are alive with NED at 207 and 122 months, respectively [37, 40]. Four patients (14%) were dead of disease (DOD) at last follow-up with 2 having local failure, 1 distant failure (bone) and 1 showing local and distant failure (breast) [36, 40]. Two (7%) additional patients had metastases (lung and chest wall) and were subsequently lost to follow-up [15].
Table 3.
Previously reported Ewing’s family tumors of the sinonasal tract with follow up
| Study | # of Cases | Age and gender | Site | Molecular confirmation? | Treatment | Follow up |
|---|---|---|---|---|---|---|
| Whaley [40] | 2 | 9F | Ethmoid Sinus | No | Chemoradiation | Bone mets-30 m, NED-122 m |
| 17 M | Sphenoid Sinus | No | Chemoradiation | Bone mets-11 m, DOD-29 m | ||
| Whang-Peng [15] | 1 | 22 M | Nasal cavity, maxillary sinuses | Yes | Surgery + postop radiation | Chest wall met-29 m, AWD-29 m |
| Csokonai [24] | 1 | 19 M | Right middle concha polyp | No | Surgery + postop radiation | NED-72 m |
| Aferzon [19] | 1 | 14F | Left nasal cavity | Yes | Surgery and chemoradiation | NED-6 m |
| Gray [26] | 2 | 15F | Left ethmoid and orbital bone erosion | Yes | Surgery and chemoradiation | NED-23 m |
| 17 M | Left ethmoid and orbit extension | Yes | Chemoradiation | NED-20 m | ||
| Wexler [39] | 1 | 9F | Right nasal and maxillary sinus | Yes | Chemotherapy and surgery | NED-36 + m |
| Infante-Cossio [13] | 1 | 17 M | Left maxillary sinus and zygoma | No | Chemotherapy and surgery | NED-96 m |
| Thariat [37] | 1 | 10 M | Left maxillary sinus | Yes | Chemotherapy and surgery | Local relapse-192 m, NED 15 m after further treatment-207 m |
| Boor [22] | 1 | 20F | Nasal cavity | No | Surgery and chemoradiation | NED-12 m |
| Kawabata [33] | 1 | 12 M | Left maxillary sinus and bone | No | Chemoradiation | NED-20 m |
| Coskun [23] | 1 | 16F | Right maxillary sinus and orbit | No | Chemoradiation | NED-12 m |
| Alobid [20] | 1 | 23F | Left maxillary sinus and orbital bone | No | NA | NED-59 m |
| Pontius [35] | 1 | 39F | Nasal cavity and ethmoid | No | Chemoradiation | NED-24 m |
| Windfuhr [8] | 1 | 7 M | Right maxillary sinus and orbit | No | Surgery and chemoradiation | NED-17 m |
| Fernandez [25] | 2 | NA | Maxillary sinus | No | Chemoradiation | NED |
| Strong [36] | 3 | NA | Maxillary sinus | No | Chemoradiation | DOD, 1 NED48 m, 1 NED 276 m |
NA not available, NED no evidence of disease, DOD dead of disease (local), AWD alive with disease at last follow up, mets metastasis
The subgroup of sinonasal EFTs (in the literature and this report) having both molecular confirmation and follow-up data includes 10 cases [15, 19, 26, 37, 39]. The follow-up ranged from 4–207 months (average 37.8 m). Of these, 9 (90%) showed NED at last follow-up, with 1 having had retreatment for a local relapse at 192 months (total 207 months follow-up). One case was alive with a chest wall metastasis but was lost to follow-up. There were no cases of confirmed mortality in a patient with a molecular proven EFT of the sinonasal tract. These findings seem to suggest a better outcome for sinonasal EFTs, similar to previous reports for the head and neck as a whole [5].
Although these cases, and those reported in the literature tend to be labeled as sinonasal or nasal cavity tumors, the majority of cases in this series demonstrated unequivocal involvement of bone either by radiology or by histology. This bone involvement was centered on a portion of the maxillary bone in most cases. This is not surprising as maxillary bone is intimately associated with sinonasal mucosa owing to the membranous ossification that it arises from. EFT is currently listed in the WHO blue book for head and neck tumors as a sinonasal neuroectodermal tumor [6] with a presumed neuroectodermal cell origin. It is quoted as having rare bone erosion only and is listed separately from sinonasal bone tumors, implying a mucosal or extra-osseus origin [6]. Previous cases in the literature have also been specifically designated as “extraskeletal” Ewing sarcoma of the nose, nasal fossa or sinus [22, 23, 30, 34, 35]. There is the possibility, however, that most or all sinonasal EFTs arise in maxillary bone primarily (osseus EFT) and secondarily expand to involve the sinonasal region. This is suggested by the frequency of bone involvement in this series. It would also be in keeping with the significantly rarer presentation of EFT in other mucosal sites, such as the lower respiratory, urologic, gynecologic and gastrointestinal tracts, none of which are closely apposed to bone. It does not, however, entirely explain why mandibular EFTs are less common than sinonasal EFTs and at least some of these tumors may truly be extra-osseus tumors. The difficulty in knowing the tissue of origin warrants grouping these neoplasms together as “sinonasal/maxillary EFT”.
In summary, we have presented the largest series of sinonasal EFT to date, most of which have molecular confirmation. EFT appears to be less aggressive in this location than more typical extremity bone and soft tissue tumors; however, the limited follow-up in the literature precludes a definitive statement on behavior of EFT in this site. A less aggressive course is surprising given the complexity of the anatomy of the primary site, difficulty in achieving a negative-margin resection, late presentation and early access by the tumor to vital structures. Sinonasal EFTs show a great degree of histologic and immunophenotypic diversity, similar to what has been seen in EFT at other sites. However, the sinonasal location is particularly problematic owing to the large number of potential mimics, including epithelial, mesenchymal and neural tumors, most of which are more common than EFT. This diagnostic challenge can be resolved, nonetheless, by the judicious use of immunohistochemistry combined with radiographic and clinical correlation.
References
- 1.Ushigome S, Machinami R, Sorensen PH. Ewing sarcoma/primitive neuroectodermal tumor (PNET). In: Fletcher CD, Unni KK, Mertens F, editors. World Health Organization classification of tumours. Pathology and genetics of bone and soft tissue tumours Lyon. France: IARC Press; 2002. p. 298–300.
- 2.Cotterill SJ, Ahrens S, Paulussen M, Jürgens HF, Voûte PA, Gadner H, Craft AW. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma study group. J Clin Oncol. 2000;18(17):3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 3.Siegal GP, Oliver WR, Reinus WR, Gilula LA, Foulkes MA, Kissane JM, Askin FB. Primary Ewing’s sarcoma involving the bones of the head and neck. Cancer. 1987;60(11):2829–2840. doi: 10.1002/1097-0142(19871201)60:11<2829::AID-CNCR2820601139>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Vaccani JP, Forte V, Jong AL, Taylor G. Ewing’s sarcoma of the head and neck in children. Int J Pediatr Otorhinolaryngol. 1999;48(3):209–216. doi: 10.1016/S0165-5876(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 5.Raney RB, Asmar L, Newton WA, Jr, Bagwell C, Breneman JC, Crist W, Gehan EA, Webber B, Wharam M, Wiener ES, Anderson JR, Maurer HM. Ewing’s sarcoma of soft tissues in childhood: a report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J Clin Oncol. 1997;15(2):574–582. doi: 10.1200/JCO.1997.15.2.574. [DOI] [PubMed] [Google Scholar]
- 6.Wenig BM, Dulguerov P, Kapadia SP, Prasad ML, Fanburg-smith JC Thompson LD. Neuroectodermal tumors. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours Lyon. France: IARC Press; 2005. p. 65–70.
- 7.La TH, Meyers PA, Wexler LH, Alektiar KM, Healey JH, Laquaglia MP, Boland PJ, Wolden SL. Radiation therapy for Ewing’s sarcoma: results from Memorial Sloan-Kettering in the modern era. Int J Radiat Oncol Biol Phys. 2006;64(2):544–550. doi: 10.1016/j.ijrobp.2005.07.299. [DOI] [PubMed] [Google Scholar]
- 8.Windfuhr JP. Primitive neuroectodermal tumor of the head and neck: incidence, diagnosis, and management. Ann Otol Rhinol Laryngol. 2004;113(7):533–543. doi: 10.1177/000348940411300705. [DOI] [PubMed] [Google Scholar]
- 9.Stelow EB, Bellizzi AM, Taneja K, Mills SE, Legallo RD, Kutok JL, Aster JC, French CA. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32(6):828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 10.Wenig BM. Undifferentiated malignant neoplasms of the sinonasal tract. Arch Pathol Lab Med. 2009;133(5):699–712. doi: 10.5858/133.5.699. [DOI] [PubMed] [Google Scholar]
- 11.Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, Karzeladze A, Savelov N, Petrov S, Alvarado-Cabrero I, Mihaila D, Terrier P, Lopez-Guerrero JA, Picci P. Histological heterogeneity of Ewing’s sarcoma/PNET: an immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch. 2009;455(5):397–411. doi: 10.1007/s00428-009-0842-7. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21(7):795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 13.Infante-Cossio P, Gutierrez-Perez JL, Garcia-Perla A, Noguer-Mediavilla M, Gavilan-Carrasco F. Primary Ewing’s sarcoma of the maxilla and zygoma: report of a case. J Oral Maxillofac Surg. 2005;63(10):1539–1542. doi: 10.1016/j.joms.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Argani P, Perez-Ordoñez B, Xiao H, Caruana SM, Huvos AG, Ladanyi M. Olfactory neuroblastoma is not related to the Ewing family of tumors: absence of EWS/FLI1 gene fusion and MIC2 expression. Am J Surg Pathol. 1998;22(4):391–398. doi: 10.1097/00000478-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Whang-Peng J, Freter CE, Knutsen T, Nanfro JJ, Gazdar A. Translocation t(11;22) in esthesioneuroblastoma. Cancer Genet Cytogenet. 1987;29(1):155–157. doi: 10.1016/0165-4608(87)90043-4. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen PH, Wu JK, Berean KW, Lim JF, Donn W, Frierson HF, Reynolds CP, López-Terrada D, Triche TJ. Olfactory neuroblastoma is a peripheral primitive neuroectodermal tumor related to Ewing sarcoma. Proc Natl Acad Sci USA. 1996;93(3):1038–1043. doi: 10.1073/pnas.93.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordes B, Williams MD, Tirado Y, Bell D, Rosenthal DI, Al-Dhahri SF, Hanna EY, El-Naggar AK. Molecular and phenotypic analysis of poorly differentiated sinonasal neoplasms: an integrated approach for early diagnosis and classification. Hum Pathol. 2009;40(3):283–292. doi: 10.1016/j.humpath.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allam A, El-Husseiny G, Khafaga Y, Kandil A, Gray A, Ezzat A, Schultz H. Ewing’s Sarcoma of the head and neck: a retrospective analysis of 24 cases. Sarcoma. 1999;3(1):11–15. doi: 10.1080/13577149977811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aferzon M, Wood WE, Powell JR. Ewing’s sarcoma of the ethmoid sinus. Otolaryngol Head Neck Surg. 2003;128(6):897–901. doi: 10.1016/S0194-5998(03)00452-2. [DOI] [PubMed] [Google Scholar]
- 20.Alobid I, Bernal-Sprekelsen M, Alos L, Benitez P, Traserra J, Mullol J. Peripheral primitive neuroectodermal tumour of the left maxillary sinus. Acta Otolaryngol. 2003;123(6):776–778. doi: 10.1080/00016480310001213. [DOI] [PubMed] [Google Scholar]
- 21.Amin MN, Islam KM, Ahmed AN, Datta PG, Amin AS, Abdullah M. Ewing’s sarcoma of maxilla-a case report. Bangladesh Med Res Counc Bull. 1990;16(1):42–45. [PubMed] [Google Scholar]
- 22.Böör A, Jurkovic I, Friedmann I, Plank L, Kocan P. Extraskeletal Ewing’s sarcoma of the nose. J Laryngol Otol. 2001;115(1):74–76. doi: 10.1258/0022215011906885. [DOI] [PubMed] [Google Scholar]
- 23.Coskun BU, Cinar U, Savk H, Basak T, Dadas B. Isolated maxillary sinus Ewing’s sarcoma. Rhinology. 2005;43(3):225–228. [PubMed] [Google Scholar]
- 24.Csokonai LV, Liktor B, Arató G, Helffrich F. Ewing’s sarcoma in the nasal cavity. Otolaryngol Head Neck Surg. 2001;125(6):665–667. doi: 10.1067/mhn.2001.119486. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez CH, Lindberg RD, Sutow WW, Samuels ML. Localized Ewing’s sarcoma–treatment and results. Cancer. 1974;34(1):143–148. doi: 10.1002/1097-0142(197407)34:1<143::AID-CNCR2820340121>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Gray ST, Chen YL, Lin DT. Efficacy of proton beam therapy in the treatment of Ewing’s sarcoma of the paranasal sinuses and anterior skull base. Skull Base. 2009;19(6):409–416. doi: 10.1055/s-0029-1220207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Gupta OP, Mehrotra S, Mehrotra D. Ewing sarcoma of the maxilla: a rare presentation. Quintessence Int. 2009;40(2):135–140. [PubMed] [Google Scholar]
- 28.Harman M, Kiroglu F, Kosem M, Unal O. Primary Ewing’s sarcoma of the paranasal sinus with intracranial extension: imaging features. Dentomaxillofac Radiol. 2003;32(5):343–346. doi: 10.1259/dmfr/17098962. [DOI] [PubMed] [Google Scholar]
- 29.Hossfeld DK, Seeber S, Siemers E, Schmidt CG, Scherer E. Early results of combined modality therapy of patients with Ewing’s sarcoma. Recent Results Cancer Res. 1982;80:124–127. doi: 10.1007/978-3-642-81685-7_21. [DOI] [PubMed] [Google Scholar]
- 30.Howard DJ, Daniels HA. Ewing’s sarcoma of the nose. Ear Nose Throat J. 1993;72(4):277–279. [PubMed] [Google Scholar]
- 31.Howard DJ, Lund VJ. Primary Ewing’s sarcoma of the ethmoid bone. J Laryngol Otol. 1985;99(10):1019–1023. doi: 10.1017/S0022215100098108. [DOI] [PubMed] [Google Scholar]
- 32.Howarth KL, Khodaei I, Karkanevatos A, Clarke RW. A sinonasal primary Ewing’s sarcoma. Int J Pediatr Otorhinolaryngol. 2004;68(2):221–224. doi: 10.1016/j.ijporl.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata M, Yoshifuku K, Sagara Y, Kurono Y. Ewing’s sarcoma/primitive neuroectodermal tumour occurring in the maxillary sinus. Rhinology. 2008;46(1):75–78. [PubMed] [Google Scholar]
- 34.Lane S, Ironside JW. Extra-skeletal Ewing’s sarcoma of the nasal fossa. J Laryngol Otol. 1990;104(7):570–573. doi: 10.1017/S0022215100113192. [DOI] [PubMed] [Google Scholar]
- 35.Pontius KI, Sebek BA. Extraskeletal Ewing’s sarcoma arising in the nasal fossa. Light- and electron-microscopic observations. Am J Clin Pathol. 1981;75(3):410–5. [DOI] [PubMed]
- 36.Strong LC, Herson J, Osborne BM, Sutow WW. Risk of radiation-related subsequent malignant tumors in survivors of Ewing’s sarcoma. J Natl Cancer Inst. 1979;62(6):1401–1406. [PubMed] [Google Scholar]
- 37.Thariat J, Italiano A, Peyrade F, Birtwisle-Peyrottes I, Gastaud L, Dassonville O, Thyss A. Very late local relapse of Ewing’s sarcoma of the head and neck treated with aggressive multimodal therapy. Sarcoma. 2008;2008:854141. doi: 10.1155/2008/854141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velche-Haag B, Dehesdin D, Proust F, Marie JP, Andrieu-Guitrancourt J, Laquerriere A. Ewing’s sarcoma of the head and neck: a case report. Ann Otolaryngol Chir Cervicofac. 2002;119(6):363–368. [PubMed] [Google Scholar]
- 39.Wexler LH, Kacker A, Piro JD, Haddad J, Jr, Close LG. Combined modality treatment of Ewing’s sarcoma of the maxilla. Head Neck. 2003;25(2):168–172. doi: 10.1002/hed.10156. [DOI] [PubMed] [Google Scholar]
- 40.Whaley JT, Indelicato DJ, Morris CG, Hinerman RW, Amdur RJ, Mendenhall WM, Keole SR, Marcus RB Jr. Ewing Tumors of the Head and Neck. Am J Clin Oncol. 2009 Oct 16. [Epub ahead of print]. [DOI] [PubMed]
- 41.Woodruff G, Thorner P, Skarf B. Primary Ewing’s sarcoma of the orbit presenting with visual loss. Br J Ophthalmol. 1988;72(10):786–792. doi: 10.1136/bjo.72.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]