Abstract
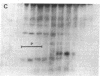
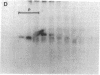
Peroxisomal function was evaluated in a male infant with clinical features of neonatal adrenoleukodystrophy. Very long chain fatty acid levels were elevated in both plasma and fibroblasts, and beta-oxidation of very long chain fatty acids in cultured fibroblasts was significantly impaired. Although the level of the bile acid intermediate trihydroxycoprostanoic acid was slightly elevated in plasma, phytanic acid and L-pipecolic acid levels were normal, as was plasmalogen synthesis in cultured fibroblasts. The latter three parameters distinguish this case from classical neonatal adrenoleukodystrophy. In addition, electron microscopy and catalase subcellular distribution studies revealed that, in contrast to neonatal adrenoleukodystrophy, peroxisomes were present in the patient's tissues. Immunoblot studies of peroxisomal beta-oxidation enzymes revealed that the bifunctional enzyme (enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase) was deficient in postmortem liver samples, whereas acyl-CoA oxidase and the mature form of beta-ketothiolase were present. Density gradient centrifugation of fibroblast homogenates confirmed that intact peroxisomes were present. Immunoblots of fibroblasts peroxisomal fractions showed that they contained acyl-CoA oxidase and beta-ketothiolase, but bifunctional enzyme was not detected. Northern analysis, however, revealed that mRNA coding for the bifunctional enzyme was present in the patient's fibroblasts. These results indicate that the primary biochemical defect in this patient is a deficiency of peroxisomal bifunctional enzyme. It is of interest that the phenotype of this patient resembled neonatal adrenoleukodystrophy and would not have been distinguished from this disorder by clinical study alone.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985 Jul;240(1):413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Beard M. E., Moser A. B., Sapirstein V., Holtzman E. Peroxisomes in infantile phytanic acid storage disease: a cytochemical study of skin fibroblasts. J Inherit Metab Dis. 1986;9(4):321–334. doi: 10.1007/BF01800481. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Falk O. Assay of the major bile acids in serum by isotope dilution-mass spectrometry. Scand J Clin Lab Invest. 1983 Apr;43(2):163–170. doi: 10.1080/00365518309168239. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen W. W., Watkins P. A., Osumi T., Hashimoto T., Moser H. W. Peroxisomal beta-oxidation enzyme proteins in adrenoleukodystrophy: distinction between X-linked adrenoleukodystrophy and neonatal adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1425–1428. doi: 10.1073/pnas.84.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks D. M., Tippett P., Adams C., Campbell P. Cerebro-hepato-renal syndrome of Zellweger. A report of eight cases with comments upon the incidence, the liver lesion, and a fault in pipecolic acid metabolism. J Pediatr. 1975 Mar;86(3):382–387. doi: 10.1016/s0022-3476(75)80967-x. [DOI] [PubMed] [Google Scholar]
- Datta N. S., Wilson G. N., Hajra A. K. Deficiency of enzymes catalyzing the biosynthesis of glycerol-ether lipids in Zellweger syndrome. A new category of metabolic disease involving the absence of peroxisomes. N Engl J Med. 1984 Oct 25;311(17):1080–1083. doi: 10.1056/NEJM198410253111704. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Coltoff-Schiller B., Chang C. H., Nigro M., Black V. H., Javitt N. B., Moser H. W., Lazarow P. B. Peroxisomal defects in neonatal-onset and X-linked adrenoleukodystrophies. Science. 1985 Jan 4;227(4682):67–70. doi: 10.1126/science.3964959. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Collins J., Rapin I., Neumann P., Neglia W., Spiro A. J., Ishii T., Roels F., Vamecq J., Van Hoof F. Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J Pediatr. 1986 Jan;108(1):25–32. doi: 10.1016/s0022-3476(86)80764-8. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. Individual peroxisomal beta-oxidation enzymes. Ann N Y Acad Sci. 1982;386:5–12. doi: 10.1111/j.1749-6632.1982.tb21403.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Fukui K., Yamasaki F. Association of the liver peroxisomal fatty acyl-CoA beta-oxidation system with the synthesis of bile acids. J Biochem. 1984 Dec;96(6):1713–1719. [PubMed] [Google Scholar]
- Hoefler G., Hoefler S., Watkins P. A., Chen W. W., Moser A., Baldwin V., McGillivary B., Charrow J., Friedman J. M., Rutledge L. Biochemical abnormalities in rhizomelic chondrodysplasia punctata. J Pediatr. 1988 May;112(5):726–733. doi: 10.1016/s0022-3476(88)80689-9. [DOI] [PubMed] [Google Scholar]
- Jaffe R., Crumrine P., Hashida Y., Moser H. W. Neonatal adrenoleukodystrophy: clinical, pathologic, and biochemical delineation of a syndrome affecting both males and females. Am J Pathol. 1982 Jul;108(1):100–111. [PMC free article] [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- Kelley R. I., Datta N. S., Dobyns W. B., Hajra A. K., Moser A. B., Noetzel M. J., Zackai E. H., Moser H. W. Neonatal adrenoleukodystrophy: new cases, biochemical studies, and differentiation from Zellweger and related peroxisomal polydystrophy syndromes. Am J Med Genet. 1986 Apr;23(4):869–901. doi: 10.1002/ajmg.1320230404. [DOI] [PubMed] [Google Scholar]
- Kelley R. I. Review: the cerebrohepatorenal syndrome of Zellweger, morphologic and metabolic aspects. Am J Med Genet. 1983 Dec;16(4):503–517. doi: 10.1002/ajmg.1320160409. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y., Small G. M., Watkins P., Moser H. Presence of the peroxisomal 22-kDa integral membrane protein in the liver of a person lacking recognizable peroxisomes (Zellweger syndrome). Proc Natl Acad Sci U S A. 1986 Dec;83(23):9193–9196. doi: 10.1073/pnas.83.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Monnens L., Bakkeren J., Parmentier G., Janssen G., van Haelst U., Trijbels F., Eyssen H. Disturbances in bile acid metabolism of infants with the Zellweger (cerebro-hepato-renal) syndrome. Eur J Pediatr. 1980;133(1):31–35. doi: 10.1007/BF00444751. [DOI] [PubMed] [Google Scholar]
- Moser A. E., Singh I., Brown F. R., 3rd, Solish G. I., Kelley R. I., Benke P. J., Moser H. W. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984 May 3;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- Moser H. W., Moser A. B., Frayer K. K., Chen W., Schulman J. D., O'Neill B. P., Kishimoto Y. Adrenoleukodystrophy: increased plasma content of saturated very long chain fatty acids. Neurology. 1981 Oct;31(10):1241–1249. doi: 10.1212/wnl.31.10.1241. [DOI] [PubMed] [Google Scholar]
- Moser H. W., Moser A. B., Kawamura N., Murphy J., Suzuki K., Schaumburg H., Kishimoto Y. Adrenoleukodystrophy: elevated C26 fatty acid in cultured skin fibroblasts. Ann Neurol. 1980 Jun;7(6):542–549. doi: 10.1002/ana.410070607. [DOI] [PubMed] [Google Scholar]
- Moser H. W. New approaches in peroxisomal disorders. Dev Neurosci. 1987;9(1):1–18. doi: 10.1159/000111604. [DOI] [PubMed] [Google Scholar]
- Naidu S., Hoefler G., Watkins P. A., Chen W. W., Moser A. B., Hoefler S., Rance N. E., Powers J. M., Beard M., Green W. R. Neonatal seizures and retardation in a girl with biochemical features of X-linked adrenoleukodystrophy: a possible new peroxisomal disease entity. Neurology. 1988 Jul;38(7):1100–1107. doi: 10.1212/wnl.38.7.1100. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., Schutgens R. B., Wanders R. J., van Roermund C. W., van Wijland M. J., Schram A. W. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet. 1988 Mar;42(3):422–434. [PMC free article] [PubMed] [Google Scholar]
- Poll-The B. T., Saudubray J. M., Ogier H., Schutgens R. B., Wanders R. J., Schrakamp G., van den Bosch H., Trijbels J. M., Poulos A., Moser H. W. Infantile Refsum's disease: biochemical findings suggesting multiple peroxisomal dysfunction. J Inherit Metab Dis. 1986;9(2):169–174. doi: 10.1007/BF01799455. [DOI] [PubMed] [Google Scholar]
- Poulos A., Sharp P., Fellenberg A. J., Danks D. M. Cerebro-hepato-renal (Zellweger) syndrome, adrenoleukodystrophy, and Refsum's disease: plasma changes and skin fibroblast phytanic acid oxidase. Hum Genet. 1985;70(2):172–177. doi: 10.1007/BF00273077. [DOI] [PubMed] [Google Scholar]
- Roscher A., Molzer B., Bernheimer H., Stöckler S., Mutz I., Paltauf F. The cerebrohepatorenal (Zellweger) syndrome: an improved method for the biochemical diagnosis and its potential value for prenatal detection. Pediatr Res. 1985 Sep;19(9):930–933. doi: 10.1203/00006450-198509000-00013. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto J. M., Hadchouel M., Odievre M., Laudat M. H., Saudubray J. M., Dulac O., Beucler I., Beaune P. Infantile phytanic acid storage disease, a possible variant of Refsum's disease: three cases, including ultrastructural studies of the liver. J Inherit Metab Dis. 1982;5(2):83–90. doi: 10.1007/BF01799998. [DOI] [PubMed] [Google Scholar]
- Singh H., Derwas N., Poulos A. Very long chain fatty acid beta-oxidation by rat liver mitochondria and peroxisomes. Arch Biochem Biophys. 1987 Dec;259(2):382–390. doi: 10.1016/0003-9861(87)90504-2. [DOI] [PubMed] [Google Scholar]
- Singh I., Moser A. E., Goldfischer S., Moser H. W. Lignoceric acid is oxidized in the peroxisome: implications for the Zellweger cerebro-hepato-renal syndrome and adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4203–4207. doi: 10.1073/pnas.81.13.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg G. A., Breukelman H., Elzinga H., Trijbels J. M., Monnens L. A., Muskiet F. A. Determination of pipecolic acid in urine and plasma by isotope dilution mass fragmentography. Clin Chim Acta. 1986 Sep 30;159(3):229–237. doi: 10.1016/0009-8981(86)90056-2. [DOI] [PubMed] [Google Scholar]