Abstract
Seven expansile jaw lesions in patients ranging from 7 to 63 years are presented to illustrate diagnostic and management issues pertaining to cases ultimately proven to be gnathic osteosarcoma (GO). Six of the cases in our series were low-grade osteoblastic and one high-grade chondroblastic. None of our cases exhibited the characteristic “sunburst” radiopaque appearance described for GO. All of our cases displayed cortical expansion and one showed development of diastema. Two occurred in the maxilla and five in the mandible. Two of the patients with mandibular lesions presented initially with pain; all other patients were asymptomatic. Lack of pain resulted in a delay in diagnosis due to postponement of consultation or biopsy. Two cases underwent initial shallow non-representative biopsies, requiring a second biopsy for definitive diagnosis, further delaying treatment. Those biopsies were initially interpreted as pyogenic granuloma and peripheral ossifying fibroma, respectively. GO should always be considered in the differential diagnosis of expansile jaw lesions. Bone biopsies of lesions exhibiting pain and expansion of cortical plates should include medullary bone in order to minimize sampling error. In addition, all rapidly growing or painful exophytic bone lesions, and presumed soft tissue lesions that may involve underlying bone, should be examined histopathologically, and receive clinical and radiographic follow-up until complete resolution or healing is evident, regardless of the diagnosis. Based on the positive outcomes of the patients in our series, the prognosis of GO appears to be relatively favorable when compared to other sarcomas and osteosarcomas of long bones.
Keywords: Osteosarcoma, Gnathic, Bone tumor, Jaws, Maxillofacial, Bone biopsy, Oral and maxillofacial pathology, Head and neck pathology
Introduction
Gnathic osteosarcoma (GO) is a malignant mesenchymal neoplasm in which the cellular stroma of the tumor directly produces osteoid or bone and may also produce variable amounts of cartilaginous matrix. The ultimate etiology of osteosarcoma is still unknown, however, most osteosarcomas contain clonal chromosomal aberrations [1]. Even though it is an infrequent tumor overall, it is the most common non-hematopoietic primary malignant tumor of long bones [2]. It has an estimated incidence of 4–5 cases per million population, or 40–60% of primary malignant bone neoplasms [3].
GO is estimated to occur at a rate of 0.7 cases per million, and has a slight male predilection [2]. Patients with jaw tumors tend to be in the 4th to 5th decades, differing from patients with long bone lesions who tend to be in the 2nd to 3rd decades. The incidence of tumors in the maxilla favors the alveolar ridge and sinus, whereas mandibular lesions are most common in the body of the mandible. Previous radiation therapy and/or Paget’s disease of bone are known predisposing factors, especially in patients older than 40 [4].
Symptoms of GO may be non-existent or vague at first, progressing to pain and swelling. Paresthesia and pathologic fracture may occur later in the disease process. A sudden and dramatic increase in size of the lesion may be secondary to intra-tumor hemorrhage, infection, or dedifferentiation. Laboratory findings are non-specific at first, but measurements of alkaline phosphatase and lactic acid dehydrogenase in advanced or treated lesions can correlate with the degree of disease activity [5].
Histologically, the neoplasm exhibits mesenchymal cells with spindle to ovoid shaped nuclei and indistinct cytoplasmic membranes. The cells exhibit a variable degree of atypical features, including hyperchromatism, pleomorphism, abnormal mitoses, and increased nuclear/cytoplasmic ratio. The tumor cells invariably produce bone matrix, even though sometimes in minimal amounts that are discovered only after studying numerous histological sections/levels of the same tumor. If the predominant matrix material is osteoid or bone, the neoplasm is referred to as an “osteoblastic osteosarcoma”. When the matrix within the tumors is mostly cartilaginous material, the tumor is referred to as a “chondroblastic osteosarcoma”. Some tumors exhibit only minimal matrix production and are then termed “fibroblastic osteosarcoma”. No prognostic significance is attributed at this time to any type or amount of matrix produced by the tumor cells [1].
The management of osteosarcoma can include preoperative chemotherapy; complete surgical excision of the tumor; postoperative chemotherapy; radiation, and combinations of these treatment modalities [6]. Recent data suggest that in those patients with osteosarcoma of the head and neck (OHN), treatment with surgery and radiation therapy improves the prognosis of a subset of patients whose surgical specimens exhibited tumor at the resection margins [7]. There is no absolute consensus at to the classification of osteosarcoma with respect to projected outcomes and treatment recommendations. However, there is a small group of early stage tumors that are cured by surgery alone. The majority of tumors though, presents in more advanced stages and require combination treatment [8]. Prognostic information can be attributed to some genetic and molecular aberrations, and to the proportion of tumor necrosis secondary to neo-adjuvant chemotherapy [9].
We report seven cases that exemplify the early signs of GO and its diagnostic pitfalls.
Case Series
Case # 1
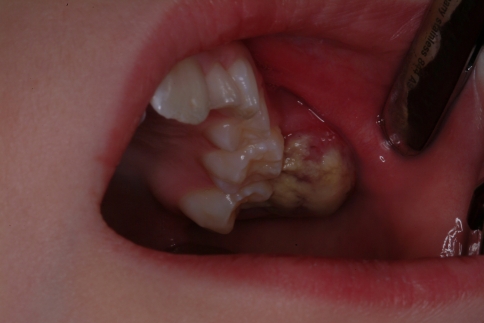
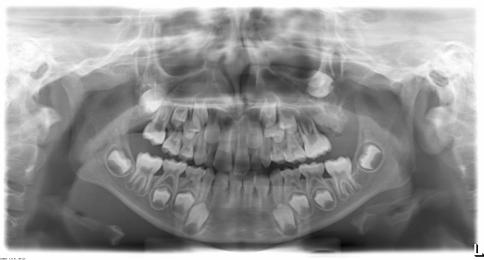
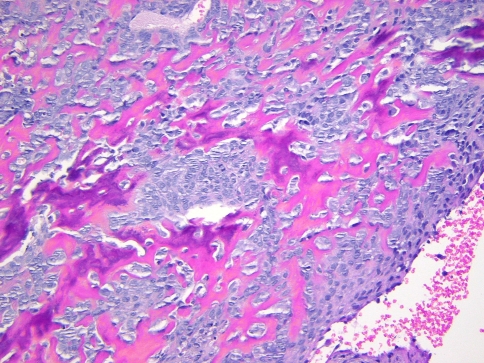
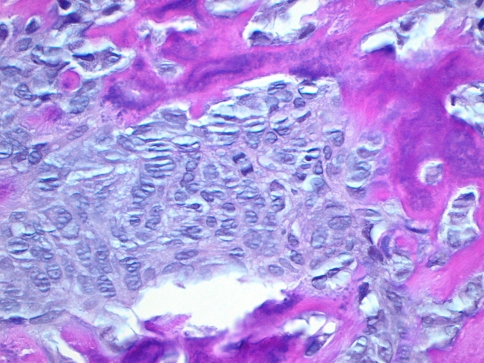
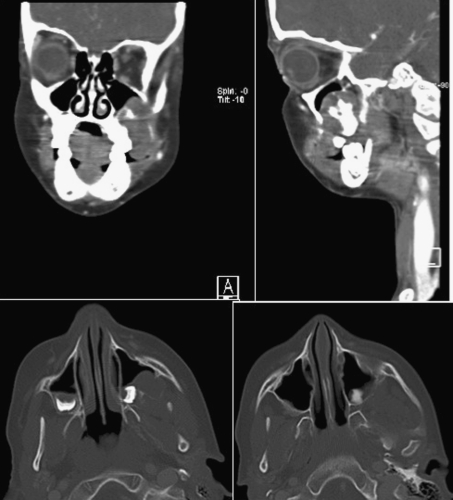
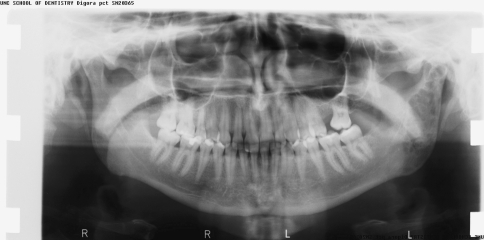
A 7-year-old boy presented to our institution referred by a pediatric dentist for evaluation of facial asymmetry and an expansile mass of the upper left maxilla associated with tooth # 14 (Fig. 1). The patient was asymptomatic and first noted the lesion 1 week prior, during toothbrushing. Upon examination, the lesion was 3-cm in diameter and was associated with a periodontal defect on the distal aspect of facially displaced tooth # 14, with probing depths greater than 10 mm. A panoramic radiograph showed displacement of impacted tooth #15, with bone destruction in the area (Fig. 2). An incisional biopsy of the lesion was procured and was consistent with osteogenic sarcoma, low to intermediate grade (Figs. 3, 4). Subsequent to the diagnosis of the lesion, the patient underwent three-dimensional imaging of the entire skeleton, but no other lesions were found (Fig. 5). A final diagnosis of osteosarcoma, primary to the maxilla, was made. The patient then underwent neoadjuvant chemotherapy with subsequent resection of the tumor. The patient is free of disease after 4 years.
Fig. 1.
A 7-year-old boy (case # 1) with mass of the upper left maxilla
Fig. 2.
A panoramic radiograph of case #1 showing distal periodontal defect of tooth #14 with displaced impacted tooth # 15
Fig. 3.
Photomicrograph of the biopsy specimen from case # 1 exhibiting hypercellular stroma with mild cellular atypia and production of osteoid and bone. (Hematoxylin and eosin stain, 200× magnification)
Fig. 4.
Photomicrograph of case # 1 exhibiting atypical nuclear features including increased nuclear/cytoplasmic ratio, pleomorphism, and irregular chromatin distribution. Note also the production of osteoid by the malignant mesenchymal stroma. (Hematoxylin and eosin 400× magnification)
Fig. 5.
CT Scan of the maxillary region of patient in case # 1 showing the extent of the tumor. No other lesions were found elsewhere
Case # 2
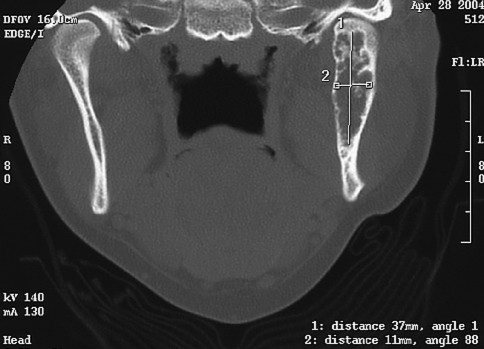
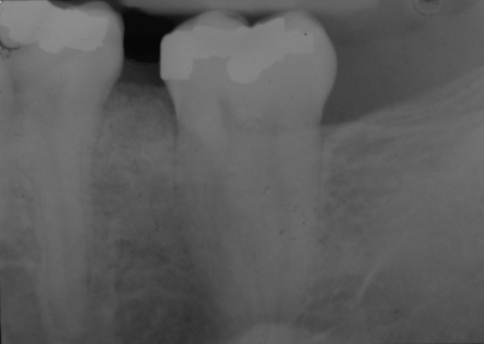
A 29-year-old White female patient presented to our institution referred by an oral and maxillofacial surgeon due to an expansile radiolucent lesion of the left posterior mandible. The patient complained of mild tenderness in the area of the left mandibular angle. A panoramic radiograph and CT scan of the area showed a multilocular radiolucency of the posterior mandible below the mandibular canal that involved the ascending ramus and the condyle (Figs. 6, 7). The lesion had been previously biopsied but the results were equivocal, based on examination of a minute, non-representative biopsy specimen, favoring a benign inflammatory lesion. A second biopsy under general anesthesia was diagnosed as GO. The patient then underwent hemimandibulectomy. The specimen margins were free of disease. Subsequent reconstruction of the mandible was achieved and the patient is free of disease 40 months after surgery.
Fig. 6.
Panoramic radiograph of lesion on case # 2. The lesion is present at mandibular angle, ramus, and condyle
Fig. 7.
Scan of the mandible of case # 2 showing expansion of the cortical plates over the radiolucent lesion
Case # 3
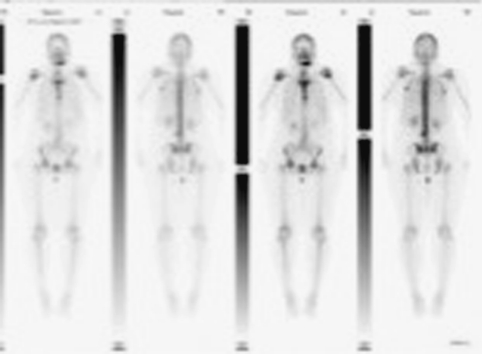
A 34-year-old African-American female presented to an oral and maxillofacial surgeon with a rapidly growing polypoid lesion of her anterior mandible (Fig. 8). Adjacent to tooth # 22, she had a history of gingival swelling and bone expansion which failed to respond to antibiotic treatment. The original lesion was a few millimeters in size and the clinical impression was “pyogenic granuloma”. Five days later, the lesion grew to approximately 5-cm in diameter. A panoramic radiograph of that area revealed significant bone destruction and small focal calcifications. An incisional biopsy was diagnosed as chondroblastic osteosarcoma. A bone scan revealed activity in the anterior mandible but nowhere else in her skeleton (Fig. 9). The patient underwent pre-operative chemotherapy and surgical removal of the tumor. She is free of disease 24 months after treatment.
Fig. 8.
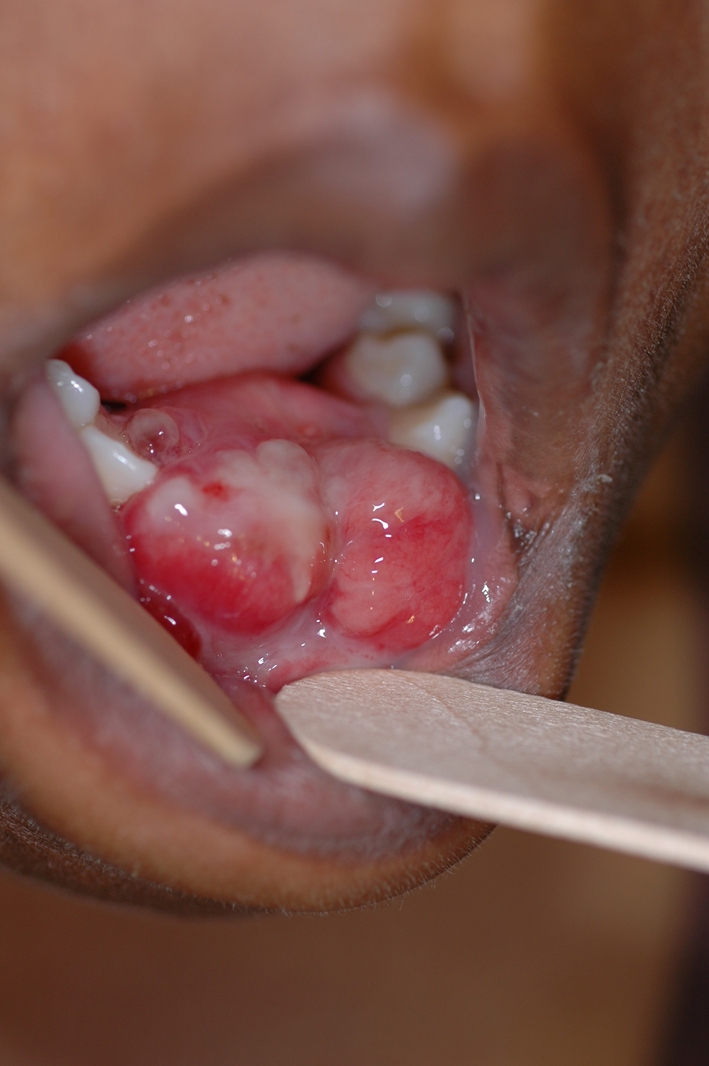
Large polypoid mass of anterior mandible (case #3) that grew to this size in 2 weeks
Fig. 9.
Bone scan (case #3) revealing a single skeletal active site located on the patient’s anterior mandible
Case # 4
A 63-year-old African-American female presented with an exophytic gingival mass facial to tooth # 9 associated with an underlying radiolucency at the apex of the tooth (Fig. 10). The lesion was clinically expansile and believed to represent an exostosis or a peripheral ossifying fibroma (POF) by the original oral and maxillofacial surgeon who excised the lesion. No histopathologic examination was requested on the specimen by the original surgeon! The lesion recurred within 12 weeks of initial removal. At this time, tooth # 9 had developed mobility and was displaced towards the palate. A second surgical removal of the lesion, along with extraction of tooth # 9 was performed. The tissue was then evaluated by an oral pathologist and a diagnosis of GO was rendered. A previous panoramic radiograph of the area revealed a mixed radiolucent-radiopaque lesion of the anterior maxilla that extended into the floor of the nose. Surgical removal of the lesion with clear margins was accomplished. The patient remained free of disease for over 4 years and was subsequently lost to follow-up.
Fig. 10.
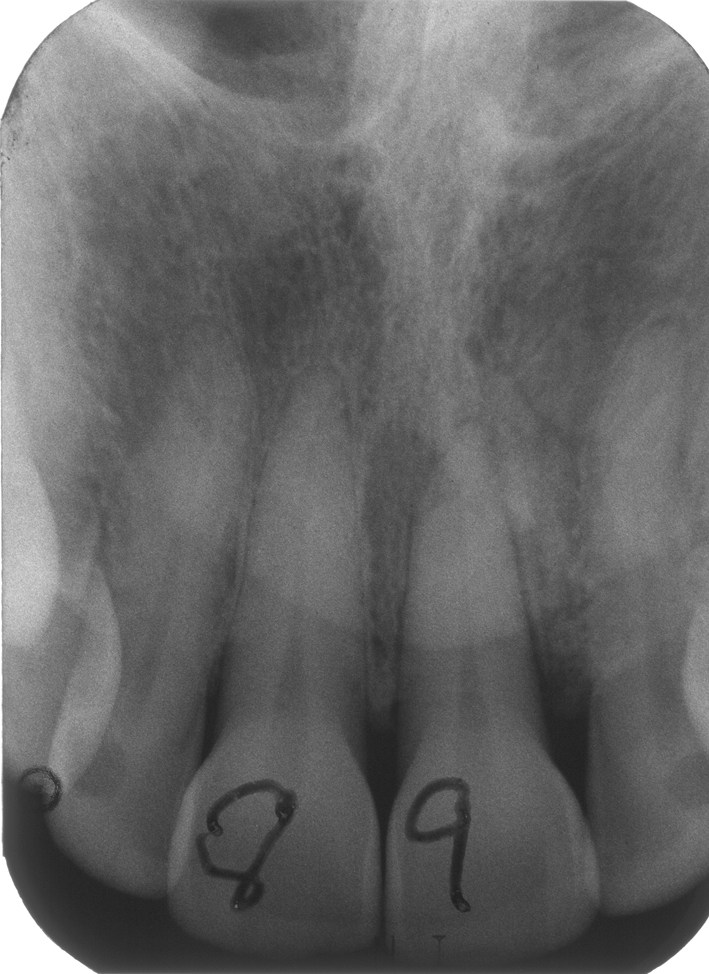
Periapical radiograph of the area before development of the lesion from case # 4
Case # 5
A 65-year-old African-American female patient presented with a chronic expansile lesion of the left mandible. She presented with mild dull pain that she could not exactly localize to a specific area. Radiographic studies were non-specific, with an ill-defined mixed density lesion and evidence of expansion of the cortical plates. A trephine bone biopsy of the body of the mandible revealed low-grade GO. A resection of the mandible was performed. The patient underwent additional surgery for resection of the distal surgical margin after tumor was discovered at that margin within the original surgical specimen. The patient remains free of disease after 12 months follow-up (Fig. 11).
Fig. 11.
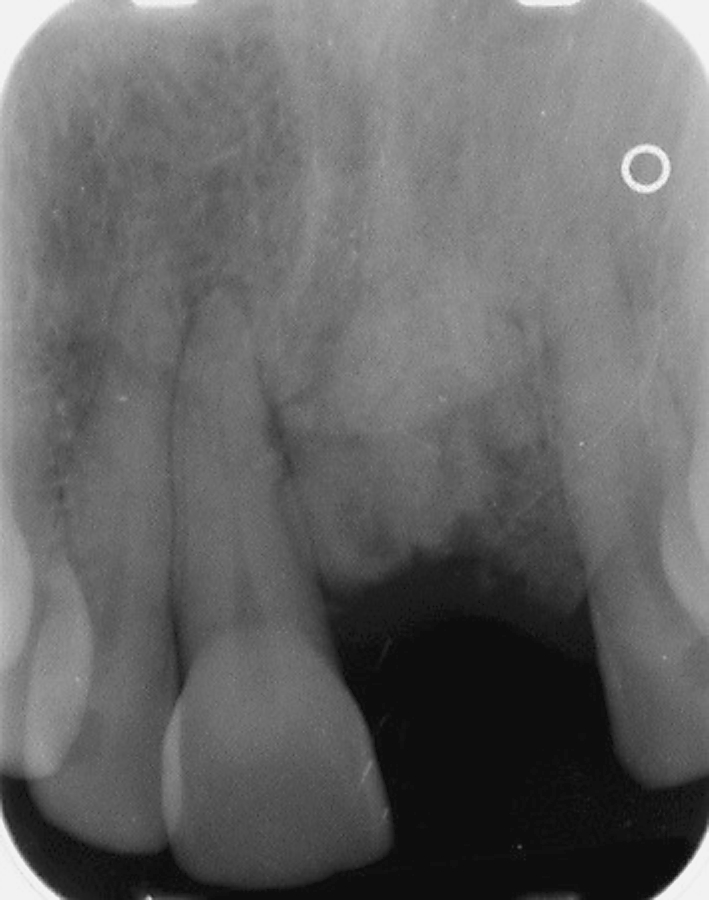
Periapical radiograph of case # 6 showing a destructive lesion of the anterior maxilla at the time of second biopsy and diagnosis of GO
Case # 6
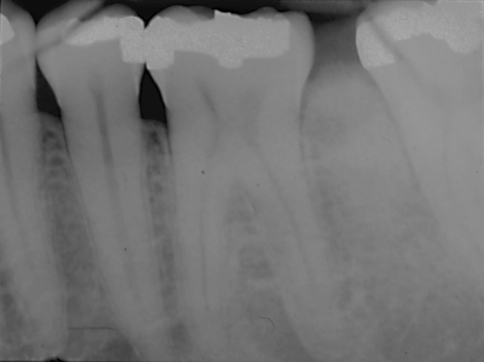
A 62-year-old male presented to a periodontist with a non-painful exophytic/expansile lesion of the mandibular gingiva between teeth 30 and 31. The clinical impression was “peripheral ossifying fibroma”. Excision of the lesion was performed. A radiograph of the area was not submitted with the biopsy specimen, which was originally read out as “peripheral ossifying fibroma”. Eighteen months later, the patient returned to the periodontist with a chief complaint that his teeth “were moving apart”. A periapical radiograph, which was submitted with the second biopsy, illustrated a diastema between 30 and 31 and increased alveolar bone production above the height of the adjacent alveolar crestal bone (Fig. 12). An additional biopsy was taken which was signed out as juxtacortical GO. Previous radiographs of the patient were then requested for comparison (Fig. 13). They illustrated that both diastema and extracortical bone formation were present, to a lesser extent, at the time of the first biopsy. After complete surgical removal of the lesion, the patient remained free of disease for 12 years, after which he was lost to follow-up.
Fig. 12.
Periapical radiograph of case # 6 taken 18 months before the diagnosis of GO, which was submitted with the second biopsy, illustrated a diastema between 30 and 31 and increased alveolar bone production above the height of the adjacent alveolar crestal bone
Fig. 13.
Previous periapical radiograph of patient in case # 6 before the appearance of any lesion
Case # 7
A 9-year-old male was referred to an oral and maxillofacial surgeon for biopsy of a spherical non-painful exophytic mass of the right posterior mandible, buccal to tooth # 30. It had been detected 1 year previously but the patient had not been referred for a biopsy. The lesion had no significant increase in size according to the measurements noted previously. Incisional biopsy was ultimately performed and, following multiple consultations, the lesion was diagnosed as a low-grade GO. A segmental resection of the mandible was performed and the patient was immediately reconstructed and grafted. The patient was alive and free of disease 5 years post-surgery and was subsequently lost to follow-up.
Results
Seven new cases of GO were analyzed in order to determine issues in diagnosis and patient management. The results are summarized in Table 1. The ages of the patients at initial presentation ranged from seven to sixty-three years. All of our cases presented with evidence of cortical bone expansion. The distribution of the lesions favored the mandible 5:2. The two patients with maxillary lesions were asymptomatic at initial presentation. Two of our five patients with mandibular lesions presented initially with pain as the chief complaint. One case presented initially with recent onset of a diastema in the posterior mandible. Three cases were given the clinical diagnosis of peripheral ossifying fibroma and one the diagnosis of pyogenic granuloma by their respective contributors.
Table 1.
Summary of information on GO patients
| Case # | Age/gender | Location | Duration | Symptoms | Bone expansion | TX | Outcome | Follow up |
|---|---|---|---|---|---|---|---|---|
| 1 | 07-M | Maxilla | 1 week | None | Yes | C & S | DF | 48 M |
| 2 | 29-F | Mandible | 8 weeks | Pain | Yes | S | DF | 40 M |
| 3 | 34-F | Mandible | 2 weeks | None | Yes | C & S | DF | 24 M |
| 4 | 63-F | Maxilla | 12 weeks | None | Yes | S | DF | 50 M |
| 5 | 61-F | Mandible | 24 months | Pain | Yes | S | DF | 12 M |
| 6 | 62-M | Mandible | Unknown | None | Yes, with new diastema | S | DF | 144 M |
| 7 | 09-M | Mandible | 12 months | None | Yes | S | DF | 60 M |
DF free of disease, TX treatment, C & S preoperative chemotherapy and surgery, S surgery alone, GO gnathic osteosarcoma, Mo months
Discussion
The differential diagnosis for an expansile and/or exophytic lesion of the jaws may include a large number of entities, for example: peripheral ossifying fibroma, peripheral giant cell granuloma, peripheral odontogenic tumors, fibrous dysplasia, ossifying fibroma, cysts, and benign and malignant neoplasms. The presence of local irritants within the periodontal apparatus will suggest or favor reactive conditions. Radiographic changes detected will suggest a central lesion causing expansion and possible perforation of the cortex. Lack of signs and symptoms of inflammation will suggest neoplastic conditions unless the neoplasm has become secondarily infected. Correlation of all clinical, radiographic, and pathologic features of each lesion ultimately leads to a definitive diagnosis.
Patients with GO typically present 10–20 years later than patients with osteosarcoma of long bones; however, the age range at presentation for GO is wide, as illustrated by the age distribution in our series (7–63 years). Symptoms in GO may be absent or minimally apparent during early disease, but without treatment, are expected to eventually progress to a painful expansile mass of the jaws, with neurosensory changes such as paresthesia. The elapsed time between our patients being aware of having a lesion of the jaws and their presentation to an oral health care provider varied between 1 week and 2 years. In our series, only two patients, both with mandibular lesions, were already symptomatic at initial presentation, and all of our patients exhibited cortical bone expansion. The reason for this difference may rest on the density of the bone being greater in the mandible than in the maxilla, as well as the greater vascularity of the maxillary bone, therefore allowing for better resolution of inflammation-driven changes in early expansile lesions. Our case with a newly developed diastema between the teeth showed histological evidence of GO infiltrating the interdental medullary and alveolar bone. Such an infiltrative pattern induced expansion of the cortical plate, buccal/distal repositioning of teeth, radiopaque expansion of the alveolar crest, and formation of the diastema.
It is vital for the oral health care provider to be alert to the potential variability in patient’s age, symptoms, location of the lesion, and presence of cortical bone expansion of early GO. Patients with GO may also present to the oral health care provider on an emergency basis with pain that may be interpreted as odontogenic in origin.
The radiographic presentation of GO has been described historically as a progression from radiolucent to mixed radiolucent-radiopaque lesions with cortical plate expansion and sometimes a “sunburst” radiopaque pattern arising from of the cortical bone [10, 11]. However, none of our cases exhibited the classical “sunburst” pattern. In addition, case 4 in this series had an initial radiographic presentation that did not show overt signs of a malignant matrix-producing tumor but rather suggested an inflammatory lesion. As the disease advanced and matured, the mixed radiolucent-radiopaque nature of GO became evident after enough bone matrix was produced within the lesion to be evident in the radiograph. The availability of previous radiographic records for comparison was critical for this observation.
When obtaining biopsy specimens of lesions suspected to be GO, it is important to procure the best and most abundant specimen that is practical in order to increase the accuracy of the pathologic interpretation. Superficial biopsies of such lesions may not be representative of the underlying GO, as was evidenced in two of our cases. The superficial component of the lesion may only reveal mucosal ulceration, and/or granulation tissue that can be confused with a pyogenic granuloma (PG). In addition, if the GO is well differentiated, it can be misinterpreted as a peripheral ossifying fibroma (POF) or other benign oral reactive osseous lesion. If a superficial biopsy of GO lacks osteoid or bone matrix, it can be interpreted as some other non-matrix-producing lesion like fibrosarcoma. The histopathologic presentation in six of our seven cases displayed equivocal histologic features, resulting in the need for inter-pathologist consultation. Multiple consults were required on two cases due to lack of significant numbers of mitotic figures, amounts of osteoid and cytologic atypia.
An appropriate biopsy specimen is also desired in case ancillary diagnostic or prognostic studies need to be carried out on the sample. Since the histopathologic features of GO will be obfuscated when pre-operative chemotherapy is given to the patient, and the final surgical resection specimen may show minimal areas consistent with GO due to tumor necrosis, the information gathered from the biopsy specimen can be of paramount importance in guiding the management and prognosis of GO.
As previously stated, early diagnosis of GO increases the chance of successful treatment outcome as it triggers treatment of the tumor either before micrometastasis or widespread metastasis occur. Accurate and prompt diagnosis is also invaluable in cases in which the disease is extensive and GO-specific preoperative chemotherapy must be given. The proportion of tumor necrosis can be subsequently measured in the entire surgical specimen. Prognostic value is related to the degree of necrosis of the lesion [9]. A greater proportion of tumor undergoing necrosis after chemotherapy indicates a better the prognosis for the patient. Therefore, the earlier an oral health care provider triggers the diagnostic process of any potential GO lesion, the better the chances of cure for the patient. We suggest that any expansile and painful lesion of the jaws in any patient in whom a clear inflammatory etiology is not evident, be promptly evaluated radiographically and pathologically, as it may represent GO.
The treatment outcome of the cases from this series suggest that the prognosis of GO appears to be relatively favorable, especially when compared to osteosarcomas in other anatomic sites and other sarcomas of the gnathic region [12].
Therefore, in summary:
For clinicians:
Osteosarcoma should always be considered in the differential diagnosis of exophytic and expansile lesions of the jaws.
Pain is not always associated with GO.
Pain associated with GO can be interpreted as odontogenic in origin, resulting in needless endodontic therapy or extraction, and delay in obtaining the final diagnosis.
Follow-up of all patients with excised pyogenic granulomas and POFs is critical to make sure there is not an underlying GO or another lesion.
Movement of teeth in the area of a lesion suggests a neoplastic process.
Radiographic findings are not always pathognomonic for GO; e.g., the “sunburst” pattern is frequently not present.
If a biopsy is too superficial it will rarely yield the right diagnosis and additional surgical sampling will be required.
For pathologists:
Beware of the shallow biopsy. Sampling error may result in the pathologist rendering an erroneous diagnosis.
Superficial biopsies may be interpreted as non-matrix producing lesions, such as fibrosarcoma, or reactive entities, such as POF or PG. Consider requesting radiographs on POFs and PGs.
Small biopsies may not capture the osteoid component of GO.
The initial radiographic presentation of GO may suggest an inflammatory lesion rather than a matrix-producing tumor.
The GO frequently presents equivocal histopathologic features. Examination of the specimen by multiple consultants or by an experienced oral pathologist with specialty training may be desirable.
References
- 1.Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002. [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 3.Fernandez R, Nikitakis NG, Pazoli A, Ord RA. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg. 2007;65(7):1286–1291. doi: 10.1016/j.joms.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YS, Wright JM, Walstad WR, Finn MD. Osteosarcoma arising in Paget’s disease of the mandible. Oral Oncol. 2002;38(8):785–792. doi: 10.1016/S1368-8375(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Bramer JA, Abdulu AA, Tillman RM, Carter SR, Sumathi VP, Grimer RJ. Pre- and post-chemotherapy alkaline phosphatase levels as prognostic indicators in adults with localised osteosarcoma. Eur J Cancer. 2005;41(18):2846–2852. doi: 10.1016/j.ejca.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Nissanka EH, Amaratunge EA, Tilakaratne WM. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Dis. 2007;13(1):82–87. doi: 10.1111/j.1601-0825.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 7.Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer. 2009;115(14):3262–3270. doi: 10.1002/cncr.24297. [DOI] [PubMed] [Google Scholar]
- 8.Niswander LM, Kim SY. Stratifying osteosarcoma: minimizing and maximizing therapy. Curr Oncol Rep. 2010;12(4):266–270. doi: 10.1007/s11912-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eftekhari F. Imaging assessment of osteosarcoma in childhood and adolescence: diagnosis, staging, and evaluating response to chemotherapy. Cancer Treat Res. 2010;152:33–62. doi: 10.1007/978-1-4419-0284-9_3. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: a 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(3):323–332. doi: 10.1067/moe.2000.108274. [DOI] [PubMed] [Google Scholar]
- 11.Givol N, Buchner A, Taicher S, Kaffle I. Radiological features of osteogenic sarcoma of the jaws. A comparative study of different radiographic modalities. Dentomaxillofac Radiol. 1998;27(6):313–320. doi: 10.1038/sj.dmfr.4600383. [DOI] [PubMed] [Google Scholar]
- 12.Vencio EF, Reeve CM, Unni KK, Nascimento AG. Mesenchymal chondrosarcoma of the jaw bones: clinicopathologic study of 19 cases. Cancer. 1998;82(12):2350–2355. doi: 10.1002/(SICI)1097-0142(19980615)82:12<2350::AID-CNCR8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]