Abstract
This note is to report how histological preparation techniques influence the extravasation pattern of the different molecular sizes of fluorescein isothiocyanate (FITC)–dextrans, typically used as markers for blood-brain barrier leakage. By using appropriate preparation methods, false negative results can be minimized. Wistar rats underwent a 2-h middle cerebral artery occlusion and magnetic resonance imaging. After the last imaging scan, Evans blue and FITC–dextrans of 4, 40, and 70 kDa molecular weight were injected. Different histological preparation methods were used. Sites of blood-brain barrier leakage were analyzed by fluorescence microscopy. Extravasation of Evans blue and high molecular FITC–dextrans (40 and 70 kDa) in the infarcted region could be detected with all preparation methods used. If exposed directly to saline, the signal intensity of these FITC–dextrans decreased. Extravasation of the 4-kDa low molecular weight FITC–dextran could only be detected using freshly frozen tissue sections. Preparations involving paraformaldehyde and sucrose resulted in the 4-kDa FITC–dextran dissolving in these reactants and being washed out, giving the false negative result of no extravasation. FITC–dextrans represent a valuable tool to characterize altered blood-brain barrier permeability in animal models. Diffusion and washout of low molecular weight FITC–dextran can be avoided by direct immobilization through immediate freezing of the tissue. This pitfall needs to be known to avoid the false impression that there was no extravasation of low molecular weight FITC–dextrans.
Keywords: Blood-brain barrier leakage, Ischemic stroke, FITC–dextran, Permeability weighted magnetic resonance imaging
Introduction
The blood-brain barrier (BBB), formed by the cerebral microvascular endothelium, represents an essential component together with supporting structures for the health and function of the central nervous system. In several diseases, including stroke, encephalitis, and brain tumors, the permeability of the BBB is increased, and its loss of integrity may reflect the severity or progression of a disease [1]. This is the rationale for trying to quantify the blood-brain barrier permeability (BBBP), using either in vivo imaging, such as CT or MRI [2, 3] or microscopic tracers in animal models. In the latter, exogenous tracers are injected immediately before sacrificing the animal, and the fraction that extravasates is considered as a biomarker of the BBBP.
Imaging or microscopic tracers can be classified into two categories: indicators for solute and ion permeability and indicators for protein permeability [4]. Also, because disruption of the blood-brain barrier is not an “all-or-nothing” phenomenon, the molecular size of the tracers needs to be taken into consideration. Typically, several probes of increasing molecular sizes are used to make interferences about the magnitude of the BBB opening [5, 6]. If both the high and the low molecular weight tracers extravasate, the BBBP is considered to increase more than if only the low molecular weight tracers extravasate.
Gadolinium–DTPA (molecular weight, 552 Da) is typically an imaging marker for solute and ion permeability while Evans blue dye, which binds to albumin (molecular weight, ≈68 kDa), is an imaging marker for protein permeability [4]. Fluorescein isothiocyanate (FITC)–dextrans can be obtained in different molecular sizes (molecular weights ranging from 3 kDa to 2,000 kDa) and have the additional advantage that, depending on their molecular size, they can be used to test both solute and ion permeability (low molecular weight dextrans) and protein permeability (high molecular weight dextrans).
In a recent study, where we were trying to validate in vivo permeability imaging using gadolinium–DTPA by comparison with histological extravasation of Eb-Alb and FITC–dextrans in a rat model of a 2-h middle cerebral artery occlusion, we were surprised when we observed that low molecular weight FITC–dextrans seemed to extravasate less than Eb-Alb despite a smaller molecular size. A set of additional, dedicated experiments revealed that this counterintuitive observation was due to the histological preparation technique. More specifically, when the standard histological preparation technique used for Eb-Alb and high molecular weight FITC–dextrans was applied to the low molecular weight FITC–dextrans, the latter got dissolved and eliminated from the histological slides, giving the false impression that there was no extravasation of low molecular weight FITC–dextrans.
The goal of this technical note is to report this pitfall and the set of experiments that allowed to understand it so that other groups in the future can avoid it when they are using FITC–dextrans of variable molecular sizes to validate a new in vivo imaging method to assess BBBP.
Material and Methods
Animal Preparation, Surgery, and Magnetic Resonance Imaging
The experimental animals were cared for in accordance with the Animal Welfare Act. The experimental protocol was performed in compliance with our institutional guidelines for animal research and was approved by the University of California San Francisco Committee on Animal Research. All experiments—surgery, MR imaging, and tracer injection—were conducted while the animals were anesthetized. Anesthesia was induced by isoflorane 3.5% and then maintained at 2% using a face mask. Body temperature was maintained at 37°C using a water-heated circulating pad and monitored via an intrarectal thermocouple. Wistar rats (n = 8) between 220 and 280 g were subjected to a 2-h filament occlusion of the right middle cerebral artery following published procedures [7]. Magnetic resonance imaging was performed with a 2T Bruker Omega CSI system (Bruker, Fremont, California) equipped with Acustar S-150 self-shielded gradients (±20 G/cm, 15 cm inner diameter). Diffusion and perfusion-weighted magnetic resonance imaging using gadolinium–DTPA (molecular weight, 552 Da) were obtained during occlusion, as well as 4 and 24 h after reperfusion. Immediately after the 24-h MRI scan, fluorescent tracers were administered (times indicated in the next section).
Injection and Circulation Time of the Fluorescent Tracers
Three FITC–dextrans of different molecular sizes (molecular weights; 4, 40, and 70 kDa) and Evans blue albumin (molecular weight, ≈68 kDa) were used as gold standard for the evaluation of BBBP. Immediately after the last MRI scan, the animal was intravenously injected with Evans blue (Sigma Chemical Co., St. Louis, MI, USA; 0.5 ml of a 2% solution in saline), which circulated for 30 min. After 28 min of Evans blue circulation, one of three FITC–dextrans (Sigma Chemical Co., St. Louis, MI, USA; 0.5 ml of a 100-mg/ml solution in saline of 40 and 70 kDa; 0.5 ml of a 300-mg/ml solution in saline of 4 kDa) was administered intravenously to circulate for 2 min. At the end of the circulation period, the rats were killed by decapitation, their brains were quickly removed.
A shorter circulation time of 2 min has been chosen for the FITC–dextran tracer, as the tracer clears more rapidly from the system than the Evans blue dye, which binds to plasma albumin immediately after injection. Plasma proteins have been shown not to bind to FITC–dextran. Dextrans are mainly eliminated from the body by renal excretion [8, 9] while Evans blue is mainly cleared from the intravascular compartment by phagocytosis into the reticuloendothelial system. As a result, approximately 30–50% of the initial plasma concentration of Evans blue-tagged albumin is still found 24 h after injection [10].
In addition, a higher concentration for the low molecular FITC–dextran was used. Indeed, the renal excretion rate of dextrans is an inverse function of their molecular weight. It follows biphasic clearance kinetics [9] with a significantly lower glomerular filtration rate of the dextran moiety fraction with a molecular weight >50 kDa, which represents the renal threshold.
Histological Tissue Preparation
The following four different fixation methods were tested (Fig. 1):
Brains, which had been quickly removed after decapitation, were fixed by immersion in 4% paraformaldehyde (PFA) for 24 h, then kept 48 h in 30% sucrose for dehydration. Afterwards, brains were embedded in optimum cutting temperature (OCT) cryostat-embedding compound (Tissue-Tek, Torrance, CA), frozen on dry ice, cut into 50-μm thick sections on a cryostat (Leica Microsystems, Wetzlar, Germany) at −19°C, and mounted on gelatinized slides. The slides were not allowed to thaw. Sections were washed by covering them with saline for 30 s in order to dissolve the OTC compound before being coverslipped. Sections were subsequently directly coverslipped with Dapi mounting media (Vector, Burlingame, CA) and analyzed on the microscope right afterwards.
Same as #1, but without saline washing. In order to compare preparations #1 and #2, the same sections were first coverslipped without washing. After being analyzed on the microscope, the coverslip was removed, the slide was washed for 30 s by covering the slides with saline, re-coverslipped and analyzed on the microscope.
The brains were fixed by immersion in 4% paraformaldehyde for 24 h and afterwards snap frozen with liquid nitrogen. Frozen brains were embedded in OCT cryostat-embedding compound (Tissue-Tek, Torrance, CA), cut into 50-μm thick sections on a cryostat (Leica Microsystems, Germany) at −19°C, and mounted on gelatinized slides. The slides were not allowed to thaw. Brain sections were directly coverslipped with Dapi mounting media (Vector, Burlingame, CA) and analyzed on the microscope right afterwards.
The brains were freshly frozen with 2-methylbutane on dry ice. Frozen brains were embedded in OCT cryostat-embedding compound (Tissue-Tek, Torrance, CA), cut into 50-μm thick sections on a cryostat (Leica Microsystems, Germany) at −19°C, and mounted on gelatinized slides. The slides were not allowed to thaw. Brain sections were directly coverslipped with Dapi mounting media (Vector, Burlingame, CA) and analyzed on the microscope right afterwards.
Fig. 1.
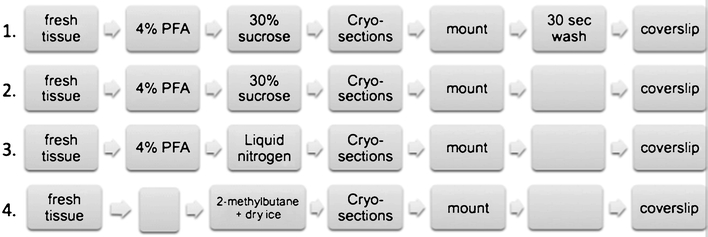
Four different fixation methods that were tested
Microscopy
The brain sections were visualized using a fluorescent microscope and standard filter set. Adjacent sections were stained with cresyl violet in order to delineate the infarcted area. On the cresyl violet sections, the infarct core was identified on light microscopy as a region of pallor that contained shrunken cell bodies characteristic of neuronal cell death.
Extravasation of the FITC–dextran was determined as clear specific fluorescence occurring outside the vessel lumina. Vascular leakage of the Evans blue-tagged albumin represented two different patterns, extravasated tracer outside the vessel lumina and cellular uptake. Extravasation was classified as weak (+), medium (++), strong (+++), and diffuse (++++) extravasation, and the extravasation profile of free tracers was characterized. Weak extravasation was defined as a faint cloud, clearly associated with one vessel site of the tracer leakage. Medium extravasation was defined as a bright cloud, clearly associated with one vessel site of the tracer leakage. Strong extravasation was defined as a bright cloud, not clearly associated with one-vessel leakage, but a group of vessels. Diffuse extravasation was defined as extensive leakage of the tracer without clear association of specific vessels.
Results
All animals showed a complete middle cerebral artery occlusion on the diffusion-weighted images during occlusion and no blood flow in the middle cerebral artery territory on the perfusion-weighted images.
Using preparation methods #1 and #2, tracer leakage was detected in the infract core in all animals which had been injected with the 40 and the 70-kDa dextran. Including a 30-s washing step (preparation #2) resulted in a decrease of the intensity of the FITC–dextran extravasation. (Table 1; Fig. 2)
Table 1.
Apparent degree of extravasation of the different tracers used for preparation methods #1 and #2
| EB-Alb | 70 kDa dextran | 40 kDa dextran | 4 kDa dextran | |
|---|---|---|---|---|
| Preparation #1 (PFA + sucrose + wash) | Extravasation + | Extravasation + | Extravasation + | No extravasation seen |
| Preparation #2 (PFA + sucrose) | Extravasation + | Extravasation ++ | Extravasation ++ | No extravasation seen |
EB-Alb Evans blue dye, which binds to albumin
Fig. 2.
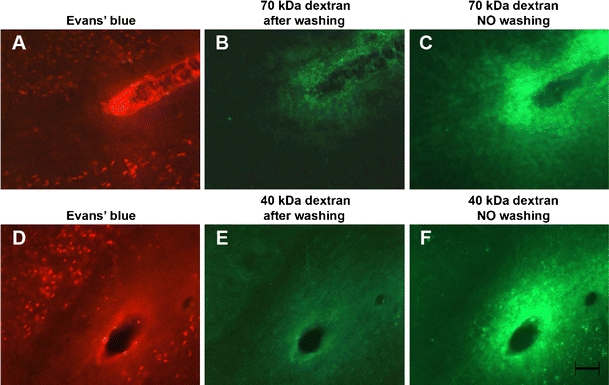
A set of images showing the distribution of Evans blue-tagged albumin and 70 kDa and 40 kDa FITC dextrans in the infarction core after washing and without washing, in a region surrounding a vessel where BBB opening occurred. The extravasated Evans blue albumin is visible as a faint cloud around the vessel and also internalized in cells around the vessel. a, d For both the 70 kDa and 40 kDa FITC dextrans, a faint green cloud surrounds the lumen of the vessel after the 30-s washing step. b, e If the section does not undergo washing and is not exposed to saline—and therefore the FITC–dextran is not dissolved—the cloud around the vessel lumen at the leakage site appears much brighter. c, f A 20-μm space bar appears in f and holds for all images
Animals, which had been injected with the 4-kDa dextran did not show any apparent leakage of FITC–dextran if fixed by immersion in paraformaldehyde and sucrose (preparations #1 and #2) or just in paraformaldehyde (preparation #3). Interestingly, for all preparations, 4 kDa dextran could be identified within the lumen of the vessels on the contralateral side to the ischemic lesions. When freshly frozen sections were used (preparation #4), leakage of the 4-kDa dextran could be visualized on the infarcted ipsilateral side. Compared to the larger molecular weight dextrans, the extravasated 4-kDa dextran tends to spread out further away from the vessels into the extravascular space (Table 2; Fig. 3).
Table 2.
Apparent degree of extravasation of the 4-kDa FITC–dextran for all preparation methods used
| EB-Alb | 4 kDa dextran | |
|---|---|---|
| Preparation #1 (PFA + sucrose + wash) | Extravasation + | No extravasation seen |
| Preparation #2 (PFA + sucrose) | Extravasation + | No extravasation seen |
| Preparation #3 (PFA + freezing) | Extravasation + | No extravasation seen |
| Preparation #4 (direct freezing) | Extravasation ++ | Extravasation ++++ |
Fig. 3.
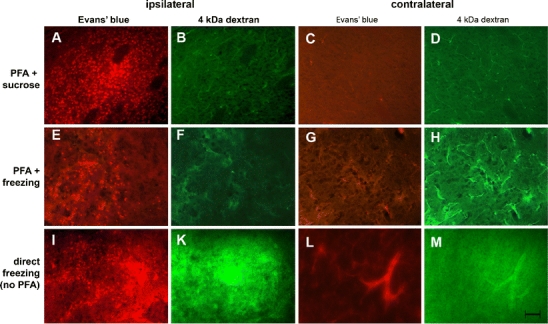
Representing images showing the leakage pattern of Evans blue-tagged albumin and of the 4-kDa FITC–dextran in the infarction core after different histologic processing procedures on the sides ipsilateral and contralateral to the infarct. On the ischemic side, the extravasation of Evans blue-tagged albumin resulted in diffuse and a punctuated distribution (cellular uptake) of the red fluorescence. a, e If the brain was frozen directly, free Evans blue-tagged albumin was more important. i On the nonischemic side, Evans blue-tagged albumin remains in the vessel lumen. c, g, l The appearance of the 4-kDa FITC–dextran for the same fields depends upon the processing method. On the ipsilateral side, using preparation #2 (PFA + sucrose) and #3 (PFA + freezing), no leakage of 4 kDa FITC–dextran can be detected. b, f If frozen directly, a bright green fluorescent cloud diffuses out at the site of the BBB opening. k On the contralateral side, 4 kDa FITC–dextran can be detected intravascularly when PFA + sucrose (preparation #1) or only PFA are used (preparation #2). d, h A 20-μm space bar appears in m and holds for all the images
Discussion
Our interpretation of our results was as follows: The extravasation of FITC–dextrans in colliquative necrosis is an inverse function of their molecular weight, and low molecular weight FITC–dextrans always extravasate more than high molecular weight FITC–dextrans. In addition, dextrans are water soluble molecules, and their solubility also depends on the molecular weight [11]. Smaller dextrans are more soluble in PFA and in sucrose and more readily washed out the slides when immersed in these reactants. As a result, low molecular weight FITC–dextrans are not detected on the slides when such histological preparations are used. On the other hand, if brains are freshly frozen with 2-methylbutane on dry ice while all other steps of the experiment are kept the same (type of occlusion, duration of occlusion, and slide preparation), the extravasation of low molecular weight FITC–dextrans can be visualized and is seen to be more important than the extravasation of high molecular weight FITC–dextrans.
Compared to the ischemic side the nonischemic side, where the BBBP is not increased and the tissue structure is intact, less extravasation of low molecular weight FITC–dextrans from the intravascular to the extravascular compartment occurs. These dextrans can be visualized within the vessels even when PFA and sucrose are used for the histological preparation because most of the dextran molecules never made it into the extravascular space where they would have been washed by the PFA or sucrose. This is in accordance with other studies, which examined intact brain structure and detected 4 kDa dextran or NaFl using standard histologic processing including PFA [12, 13].
Exposing the sliced brains with dextrans of any size to fluid decreases the intensity of the visualized extravasation of these dextrans, especially in colliquative necrosis which occurs after ischemic infarction. Therefore, steps as washing, processing methods like floating sections, further examination of the tissue using antibody solutions, or any kind of exposure to fluid should be used with caution.
FITC–dextrans represent a valuable tool to characterize the BBB and quantify how much its permeability is altered/increased in animal models. The use of low molecular weight FITC–dextran for histological experiments, however, does require special attention and modification of the standard tissue preparation. Diffusion and washout of the tracer can be avoided by direct immobilization through immediate freezing of the tissue. This pitfall needs to be known to avoid the false impression that there was no extravsation of low molecular weight FITC–dextrans.
Acknowledgements
This study was supported by a seed grant from the UCSF Department of Radiology and Biomedical Imaging. Joerg Bredno is an employee of Philips Healthcare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Dankbaar JW, Hom J, Schneider T, Cheng SC, Lau BC, van der Schaaf I, et al. Age- and anatomy-related values of blood-brain barrier permeability measured by perfusion-CT in non-stroke patients. J Neuroradiol. 2009;36(4):219–227. doi: 10.1016/j.neurad.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Knight RA, Karki K, Ewing JR, Divine GW, Fenstermacher JD, Patlak CS, et al. Estimating blood and brain concentrations and blood-to-brain influx by magnetic resonance imaging with step-down infusion of Gd-DTPA in focal transient cerebral ischemia and confirmation by quantitative autoradiography with Gd-[(14)C]DTPA. J Cereb Blood Flow Metab. 2009;29(5):1048–1058. doi: 10.1038/jcbfm.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nag S. Blood-brain barrier permeability using tracers and immunohistochemistry. Meth Mol Med. 2003;89:133–144. doi: 10.1385/1-59259-419-0:133. [DOI] [PubMed] [Google Scholar]
- 5.Juhler M, Barry DI, Offner H, Konat G, Klinken L, Paulson OB. Blood-brain and blood-spinal cord barrier permeability during the course of experimental allergic encephalomyelitis in the rat. Brain Res. 1984;302(2):347–355. doi: 10.1016/0006-8993(84)90249-X. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraja TN, Keenan KA, Fenstermacher JD, Knight RA. Acute leakage patterns of fluorescent plasma flow markers after transient focal cerebral ischemia suggest large openings in blood-brain barrier. Microcirculation. 2008;15(1):1–14. doi: 10.1080/10739680701409811. [DOI] [PubMed] [Google Scholar]
- 7.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Björn Ingelman AG, Gelin L-E, Eliasson R. Properties and applications of dextrans. Stockholm: Almquist and Wiksell; 1969. [Google Scholar]
- 9.Emmrich P, Baumann W, Stechele U. Studies on the kinetics and renal excretion of low and high molecular weight dextrans in preterm babies, newborns and young infants. Eur J Pediatr. 1977;125(3):181–190. doi: 10.1007/BF00480595. [DOI] [PubMed] [Google Scholar]
- 10.Wolman M, Klatzo I, Chui E, Wilmes F, Nishimoto K, Fujiwara K, et al. Evaluation of the dye–protein tracers in pathophysiology of the blood-brain barrier. Acta Neuropathol. 1981;54(1):55–61. doi: 10.1007/BF00691332. [DOI] [PubMed] [Google Scholar]
- 11.Thorball N. FITC–dextran tracers in microcirculatory and permeability studies using combined fluorescence stereo microscopy, fluorescence light microscopy and electron microscopy. Histochemistry. 1981;71(2):209–233. doi: 10.1007/BF00507826. [DOI] [PubMed] [Google Scholar]
- 12.Budinger E, Laszcz A, Lison H, Scheich H, Ohl FW. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res. 2008;1220:2–32. doi: 10.1016/j.brainres.2007.07.084. [DOI] [PubMed] [Google Scholar]
- 13.Kozler P, Pokorny J. Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52(5):607–614. [PubMed] [Google Scholar]


