Abstract
Purpose
To examine the effects on erectile function of concomitant treatment with an alpha-blocker (tamsulosin) and an antimuscarinic agent (solifenacin) in patients with lower urinary tract symptoms (LUTS)/benign prostatic hyperplasia (BPH).
Materials and Methods
Fifty-seven male patients with LUTS/BPH were assessed for the degree of LUTS and erectile function. In group 1 (tamsulosin) and group 2 (tamsulosin and solifenacin), changes in the International Prostate Symptom Score [IPSS: total scores, storage symptoms (ST), voiding symptoms (VD), and quality of life (QoL)], prostate-specific antigen, trans-rectal ultrasonography, urine flowmetry, residual urine, and a 5-item version of the International Index of Erectile Function (IIEF-5) were assessed after a 3-month treatment period. In both groups, it was determined whether treatment was associated with changes in LUTS and erectile function and whether improvement in the IPSS was correlated with the IIEF-5. Comparative analysis was also done to examine the linear relationship between improved IPSS scores and IIEF-5 scores.
Results
A comparison of the degree of improvement in all the parameters indicated that both groups showed significant improvement in total IPSS, IPSS-ST, IPSS-VD, and IPSS-QoL (p<0.05). A comparison of the degree of improved sexual function associated with improved LUTS in each patient showed significant improvement in the IIEF-5 score associated with the degree of improvement in the IPSS-ST domain in group 1, but no significant associations were found in group 2. In cases in which tamsulosin was administered, the IIEF-5 score significantly improved as the IPSS-ST domain score improved. In the group in which tamsulosin and solifenacin were concomitantly administered, improvement of the IPSS-ST domain score had no significant effect on the IIEF-5 score.
Conclusions
In patients with LUTS/BPH, tamsulosin and solifenacin combination therapy was effective for LUTS, but erectile function was not significantly improved. Therefore, although effective for improving LUTS, combination therapy with an alpha-blocker and an antimuscarinic agent was not effective for improving erectile function.
Keywords: Prostatic hyperplasia; Sexual dysfunction, physiological; Solifenacin; Tamsulosin
INTRODUCTION
From a worldwide perspective, the life span of humans has been prolonged, and the proportion of elderly people in the total population has increased. Various changes occur in the human body with age. In elderly men, lower urinary tract symptoms (LUTS), erectile dysfunction (ED), and decreased libido often appear concurrently. Several community-based studies have shown a strong correlation among sexual dysfunction, increasing age, and the severity of LUTS. This coexistence of sexual problems with LUTS and benign prostatic hyperplasia (BPH) further affects quality of life (QoL) [1]. LUTS and ED share increased prevalence as age increases, and in many cases, the two are synchronously present. Thus, continuous efforts have been made to disclose the common pathophysiology between these two conditions.
No definite explanations have been given to clarify the pathophysiological mechanism of the correlation between LUTS and ED, but several hypotheses have been proposed [2-5]. One possible cause is overactivation of the autonomic nervous system and increased tension of the sympathetic nervous system. According to this hypothesis, LUTS increases sympathetic nervous system activity, which induces the occurrence of urinary storage symptoms due to the contraction of smooth muscles in the prostate gland and urinary bladder. ED occurs as the result of smooth muscle contractions in the corpus spongiosum, and the myosin light chain is phosphorylated because of increased Rho-kinase activity, which can cause smooth muscle layer contraction. It has been proposed that endothelial dysfunction in the prostate gland and corpus spongiosum and hormonal imbalance are physiological causes for the correlation between LUTS and erectile dysfunction. Psychosocial factors have been proposed in addition to physiological causes. Changes in lifestyle due to nocturia and rapid eye movement sleep disorder may affect the occurrence of erectile dysfunction. Also, the result of our preliminary study showed that age, International Prostate Symptom Score (IPSS), nocturia, and the uroflow rate correlated significantly with the 5-item version of the International Index of Erectile Function (IIEF-5) [6]. Even in cases in which age was controlled for, QoL was found to have a significant correlation with sexual function. Prostate gland volume was not significantly correlated with sexual function, implying that erectile function is not affected by subjective LUTS even with a relatively greater prostate size. Irwin et al reported that overactive bladder (OAB) was significantly associated with increased prevalence of ED and that sexual activity, sexual enjoyment, and sexual satisfaction were reduced as the result of urinary symptoms [7].
In recent years, many studies have reported that administration of alpha-blockers improves LUTS due to BPH and sexual dysfunction [8,9]. These findings suggest that rudimentary treatments of LUTS, a risk factor for erectile dysfunction, would be mandatory to effectively improve sexual function in patients with BPH who concurrently have LUTS and ED. The primary treatment regimen for BPH is to administer alpha-blockers, for which the main mode of action is based on smooth muscle relaxation in the prostate and the resulting improvement in the urinary flow rate and voiding symptoms. However, a synchronous improvement of irritative and voiding symptoms may be the most effective treatment regimen for LUTS/BPH. It is expected that the concomitant administration of alpha-blockers and anticholinergics would improve LUTS more effectively in patients with LUTS/BPH. These effects are also expected to have a positive effect in secondarily improving sexual function. Thus, the aim of this study was to examine the effects on erectile function of a combination of the alpha-blocker tamsulosin to improve LUTS and the antimuscarinic agent solifenacin to improve irritative symptoms of the bladder.
MATERIALS AND METHODS
Male patients aged 40 years or older who had concurrent LUTS/BPH and ED were enrolled in this study. Inclusion criteria were an IPSS total score >12, an IPSS-QoL score >3, and an IIEF-5 score <20. Exclusion criteria were as follows: 1) antiandrogens administered in the 4 weeks previously for the management of BPH or LUTS; 2) sex hormone agents or PDE-5 inhibitors administered in the 4 weeks previously for the management of male sexual dysfunction; 3) surgical treatment of the prostate gland or urethra; 4) diagnosis of urethral stricture, urinary tract infection, prostatitis, prostate cancer, or bladder cancer; 5) PSA >4 mg/dl; 6) severe renal dysfunction or hepatic dysfunction; 7) residual urine >100 ml; and 8) ineligibility for the current study as judged by the investigators.
Sixty male patients between the ages of 41 and 76 years with LUTS/BPH and ED were divided into two groups by using a table of random sampling numbers: group 1 (the alpha-blocker treatment group; n=30) and group 2 (the alpha-blocker+solifenacin treatment group; n=30). In group 1, an alpha-blocker (tamsulosin 0.2 mg q.d.) was solely administered for 3 months. In group 2, an alpha-blocker and an antimuscarinic drug (tamsulosin 0.2 mg and solifenacin 5 mg q.d) were concomitantly administered during the same period. In patients who were suspected of having a significant bladder outlet obstruction (postvoiding residual urine >100 ml or maximum flow rate <5 ml/s), solifenacin was not administered.
To examine the correlation between LUTS and the degree of erectile function, IPSS and IIEF-5 were evaluated. Then, prostate-specific antigen (PSA), transrectal ultrasonography (TRUS), urine flowmetry (UFM), and residual urine (RU) were measured. IPSS was categorized into total scores, storage symptoms (ST), voiding symptoms (VD), and quality of life (QoL). In group 1, in which tamsulosin was solely administered (n=30) for 3 months, and group 2, in which tamsulosin and solifenacin were concomitantly administered (n=30) for 3 months, IPSS, IIEF-5, UFM, and RU were measured again. Comparative analyses were also done to examine the linear relationship between IPSS scores and IIEF-5 scores before and after treatment in the two groups.
1. Statistical analysis
The t-test (T) and Mann-Whitney U-test were used to compare parameters indicating the degree of LUTS and sexual function between the two groups, such as TRUS, PSA, IPSS (total, VD, and ST), IPSS-QoL, IIEF-5 total, amount of urination, amount of RU, maximal urinary flow rate (Qmax), and mean urinary flow rate (Qave). By use of the paired t-test (T) and Wilcoxon Signed Rank Test (Z), a comparative analysis was performed on each group for IPSS (VD, ST, total), IPSS-QoL, IIEF-5 total, the amount of urination, the amount of RU, Qmax, and Qave before and after treatment. Regression analysis was used to inspect the linear relationship between IPSS scores and IIEF-5 scores before and after treatment in the two groups. Statistical significance was set at p<0.05.
RESULTS
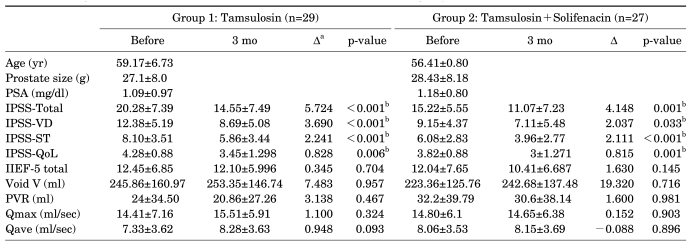
Of a total of 60 study patients, 1 patient in group 1 and 3 patients in group 2 dropped out of the 3-month follow-up study. Therefore, analyses were performed on 29 patients in group 1 and 27 patients in group 2. The incidences of hypertension and diabetes were 11 and 3 in group 1 and 9 and 5 in group 2, respectively. Before treatment, no significant differences (p<0.05) were found between groups 1 and 2, respectively, in age (59.17±6.73 yr vs. 56.41±0.80 yr), prostate gland size (27.1±8.0 g vs. 28.43±8.18 g), PSA levels (1.09±0.97 mg/dl vs. 1.18±0.80 mg/dl), amount of urination (245.86±160.97 ml vs. 223.36±125.76 ml), amount of RU (24±34.50 ml vs. 32.2±39.79 ml), Qmax (14.41±7.16 ml/s vs. 14.80±6.1 ml/s), or Qave (7.33±3.62 ml/s vs. 8.06±3.53 ml/s). No significant differences were found in IIEF-5 (12.45±6.85 vs. 12.04±7.65) or IPSS-QoL (4.28±0.88 vs. 3.82±0.88) scores. However, the IPSS total score was significantly higher (p<0.05) in group 1 than in group 2 (20.28±7.39 vs. 15.22±5.55). This was due to the exclusion of patients in group 2 who were suspected of having significant bladder outlet obstruction (postvoiding residual urine >100 ml or maximum flow rate <5 ml/s).
In both groups 1 and 2, the IPSS total, IPSS-VD, IPSS-ST, and IPSS-QoL scores were significantly improved after the 3-month treatment (p<0.05). However, no significant differences in the IIEF-5 total score, amount of urination, amount of RU, Qmax, or Qave were found before or after treatment (Table 1).
TABLE 1.
Changes in LUTS and erectile function before and after treatment in groups 1 and 2
LUTS: lower urinary tract symptoms, PSA: prostate-specific antigen, IPSS-Total: International Prostate Symptom Score total score, IPSS-VD: IPSS voiding domain, IPSS-ST: IPSS storage domain, IPSS-QoL: IPSS quality of life, IIEF-5: International Index of Erectile Function-5, Void V: voiding volume, PVR: postvoiding residual urine, Qmax: maximal urine flow rate, Qave: average flow rate, a: difference before and after treatment in group 1 and 2, b: p<0.05
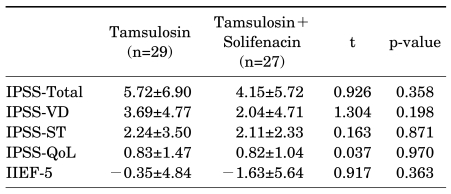
A comparison of the change (Δ) in LUTS and sexual function after treatment between group 1 and group 2 revealed no significant differences in ΔIPSS total (5.72±6.90 vs. 4.15±5.72), ΔIPSS-VD (3.69±4.77 vs. 2.04±4.71), ΔIPSS-ST (2.24±3.50 vs. 2.11±2.33), ΔIPSS-QoL (0.83±1.47 vs. 0.82±1.04), or ΔIIEF-5 (-0.35±4.84 vs. -1.63±5.64) (Table 2).
TABLE 2.
Changes in LUTS and erectile function following treatment in both groups
LUTS: lower urinary tract symptoms, IPSS-Total: International Prostate Symptom Score total score, IPSS-VD: IPSS voiding domain, IPSS-ST: IPSS storage domain, IPSS-QoL: IPSS quality of life, IIEF-5: International Index of Erectile Function-5
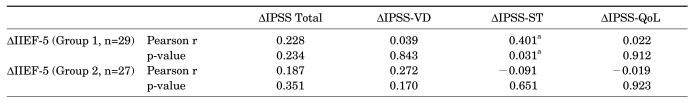
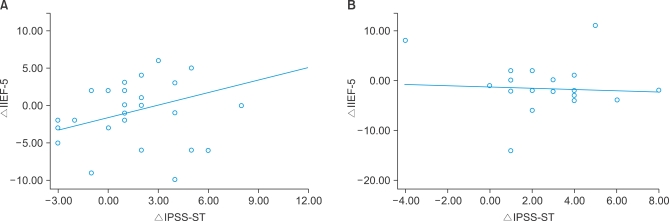
A comparison of the degree of improved sexual function (ΔIIEF-5) associated with improved LUTS in each patient (ΔIPSS total, ΔIPSS-VD, ΔIPSS-ST, and ΔIPSS-QoL) showed significant improvement in the IIEF-5 score associated with the degree of improvement in the IPSS-ST domain (ΔIPSS-ST) in group 1, but no significant associations were found in group 2 (Table 3, Fig. 1). In cases in which tamsulosin was administered, the IIEF-5 score significantly improved as the IPSS-ST domain score improved. In the group in which tamsulosin and solifenacin were concomitantly administered, improvement of the IPSS-ST domain score had no significant effect on the IIEF-5 score.
TABLE 3.
Effects of improvement in LUTS on erectile function
LUTS: lower urinary tract symptoms, IPSS-Total: International Prostate Symptom Score total score, IPSS-VD: IPSS voiding domain, IPSS-ST: IPSS storage domain, IPSS-QoL: IPSS quality of life, IIEF-5: International Index of Erectile Function-5, Δ: change rate (difference), r: coefficient of correlation, a: p<0.05
FIG. 1.
Changes in IIEF-5 scores (IIEF-5) associated with improved IPSS-ST domain (IPSS-ST) in groups 1 and 2. In group 1 (tamsulosin solely administered), the storage (or irritative) symptom score (IPSS-ST) was significantly associated with erectile function (IIEF-5) depending on the degree of improvement (F=5.176, p=0.031; R2=0.16) (A). In group 2 (tamsulosin and solifenacin concomitantly administered), storage symptoms (IPSS-ST) showed no significant association with erectile function (IIEF-5) in relation to the degree of improvement (B). IIEF-5: International Index of Erectile Function-5, IPSS-ST: IPSS storage domain.
DISCUSSION
LUTS/BPH is the most common disease of lower urinary tract obstruction that occurs in men aged 60 years or older. In most cases, it develops slowly, but in some patients, it progresses rapidly. LUTS aggravated by BPH causes decreased quality of life and sexual dysfunction. The incidence of both BPH and ED is increased in elderly people, and the synchronous occurrence of LUTS and ED is considered to happen as the result of aging. Even in cases in which age and underlying disease such as cardiovascular disease, diabetes mellitus, and hypertension were statistically controlled, epidemiologic studies have shown a definite correlation between BPH and ED [10]. These two disease entities are not due to aging, but they share a common pathophysiology. Thus, a synergistic effect whereby the treatment of one disease would lead to improvement in the other disease is expected. Lowe reported that treatment of BPH led to improved sexual function [11]. Conversely, Köhler and McVary reported that the improved sexual function resulting from treatment with PDE-5 inhibitors led to improved LUTS [12]. A questionnaire study of 12,815 men aged 50 years or older found a significant correlation between BPH and ED. Furthermore, age and LUTS were found to be greater risk factors for ED than were organic factors such as diabetes mellitus, hypertension, and hyperlipidemia [13]. In our series, age had the highest degree of correlation with sexual function [6]. Voiding symptoms of the IPSS, storage symptoms, QoL, nocturia symptoms, residual urine, maximal urinary flow rate, and mean urinary flow rate were significantly correlated with the degree of sexual satisfaction as well as with erectile function.
LUTS accompanied by BPH can be divided mainly into voiding (obstructive) symptoms and storage (irritative) symptoms. It has been well established that LUTS/BPH is closely linked to ED. However, little is known about which of the various symptoms are more closely associated with ED. It remains unclear which LUTS might effectively improve the concurrent presence of ED, and a substantial number of studies in recent years have aimed to resolve this issue. This study was also performed to investigate which symptoms of BPH, obstructive or irritative symptoms, are closely related to erectile function. Morant et al reported that both irritative and voiding symptoms are significantly correlated with sexual function [14]. Tsai et al reported that irritative symptoms are more highly correlated with ED than are obstructive symptoms [15]. A preliminary study that we conducted in patients with BPH found a significant correlation between sexual function and various irritative and voiding symptoms, QoL, nocturia, residual urine, and urine flow rates. Following a 3-month treatment of LUTS with alpha-blockers, the improvement in voiding symptoms, QoL, and average flow rate was significantly correlated with improved sexual function [6]. To date, however, few studies have reported similar results, and controversy remains. This matter should be explored further.
In 50% to 75% of patients with LUTS/BPH, OAB is also present [16]. Even after the treatment of BPH, approximately 38% of total cases have been reported to have persistent OAB [17]. Storage symptoms due to OAB may cause a higher degree of pain than do voiding symptoms [18]. In cases of BPH accompanied by OAB, a single use of alpha-blockers is insufficient for obtaining treatment effects. For this reason, the use of anticholinergic agents (or antimuscarinic agents) has been attempted in patients with OAB accompanied by BPH. Several previous studies have confirmed the efficacy and safety of concomitant administration of alpha-blockers and anticholinergic agents [19]. Even so, in some cases, residual urine is increased or voiding symptoms are aggravated following the use of anticholinergic agents, and in patients with LUTS/BPH, anticholinergic agents are used selectively. In our series, we excluded patients who likely had a significant bladder outlet obstruction (PVR >100 ml or Qmax <5 ml/s) and who had LUTS/BPH; we therefore found significantly improved LUTS with no complications such as acute urinary retention.
Common side effects following the use of anticholinergic (or antimuscarinic) agents are dry mouth, constipation, blurred vision, headache, and dry eye, but almost no reports have described the effects on sexual function. Some concern exists that the use of anticholinergic (or antimuscarinic) agents in patients with BPH may lead to aggravation of voiding difficulty or the development of urinary retention. To date, urologists have not actively used anticholinergic (or antimuscarinic) agents, but many types of antimuscarinic agents have been used in recent years to treat OAB in a clinical setting. As related experiences have accumulated, these drugs have been selectively used for patients with LUTS/BPH [20]. Almost no reports have addressed the effects of antimuscarinic agents on sexual function in LUTS/BPH patients. In a study of 39 men with BPH and LUTS in whom previous alpha-blocker therapy had failed, Kaplan et al found normal sexual function in 27 (63%) and 29 (67%) patients before and after administration, respectively [21]. Furthermore, mean IIEF-EF domain scores improved by 6.9 points. No reports have indicated that the administration of anticholinergic (or antimuscarinic) agents significantly impairs sexual function. In our series, a comparison of IIEF-5 scores before and after concomitant drug treatment with solifenacin and tamsulosin revealed no statistically significant changes. Thus, it can be inferred that administration of anticholinergic (or antimuscarinic) agents had no significant effect on sexual function. In patients with LUTS/BPH, concomitant treatment with alpha-blockers and antimuscarinics may lead to improvement in LUTS, but this treatment cannot be expected to improve sexual function.
CONCLUSIONS
In patients with LUTS/BPH and ED, concomitant use of tamsulosin and solifenacin was effective for improving LUTS. However, sexual function was not significantly improved by the concomitant administration of alpha-blockers and antimuscarinics.
Footnotes
The authors have nothing to disclose.
References
- 1.Rosen RC, Wei JT, Althof SE, Seftel AD, Miner M, Perelman MA. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology. 2009;73:562–566. doi: 10.1016/j.urology.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JM, Desouza R, Wang R. Common approach to managing lower urinary tract symptoms and erectile dysfunction. Asian J Androl. 2008;10:45–53. doi: 10.1111/j.1745-7262.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 3.McVary KT, McKenna KE. The relationship between erectile dysfunction and lower urinary tract symptoms: epidemiological, clinical, and basic science evidence. Curr Urol Rep. 2004;5:251–257. doi: 10.1007/s11934-004-0047-1. [DOI] [PubMed] [Google Scholar]
- 4.McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005;47:838–845. doi: 10.1016/j.eururo.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:824–837. doi: 10.1016/j.eururo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Jung JH, Jae SU, Kam SC, Hyun JS. Correlation between lower urinary tract symptoms (LUTS) and sexual function in benign prostatic hyperplasia: impact of treatment of LUTS on sexual function. J Sex Med. 2009;6:2299–2304. doi: 10.1111/j.1743-6109.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- 7.Irwin DE, Milsom I, Reilly K, Hunskaar S, Kopp Z, Herschorn S, et al. Overactive bladder is associated with erectile dysfunction and reduced sexual quality of life in men. J Sex Med. 2008;5:2904–2910. doi: 10.1111/j.1743-6109.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- 8.Chung BH, Lee JY, Lee SH, Yoo SJ, Lee SW, Oh CY. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impot Res. 2009;21:122–128. doi: 10.1038/ijir.2009.2. [DOI] [PubMed] [Google Scholar]
- 9.Nickel JC, Elhilali M, Emberton M, Vallancien G. The beneficial effect of alfuzosin 10 mg once daily in 'real-life' practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006;97:1242–1246. doi: 10.1111/j.1464-410X.2006.06171.x. [DOI] [PubMed] [Google Scholar]
- 10.Rhoden EL, Riedner CE, Fornari A, Fuchs SC, Ribeiro EP. Evaluation of the association between lower urinary tract symptoms and erectile dysfunction, considering its multiple risk factors. J Sex Med. 2008;5:2662–2668. doi: 10.1111/j.1743-6109.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowe FC. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: sexual function. BJU Int. 2005;95(Suppl 4):12–18. doi: 10.1111/j.1464-410X.2005.05486.x. [DOI] [PubMed] [Google Scholar]
- 12.Köhler TS, McVary KT. The relationship between erectile dysfunction and lower urinary tract symptoms and the role of phosphodiesterase type 5 inhibitors. Eur Urol. 2009;55:38–48. doi: 10.1016/j.eururo.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Morant S, Bloomfield G, Vats V, Chapple C. Increased sexual dysfunction in men with storage and voiding lower urinary tract symptoms. J Sex Med. 2009;6:1103–1110. doi: 10.1111/j.1743-6109.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CC, Liu CC, Huang SP, Li WM, Wu WJ, Huang CH, et al. The impact of irritative lower urinary tract symptoms on erectile dysfunction in aging Taiwanese males. Aging Male. 2010;13:179–183. doi: 10.3109/13685531003586975. [DOI] [PubMed] [Google Scholar]
- 16.Rosier PF, de la Rosette JJ, Wijkstra H, Van Kerrebroeck PE, Debruyne FM. Is detrusor instability in elderly males related to the grade of obstruction? Neurourol Urodyn. 1995;14:625–633. doi: 10.1002/nau.1930140604. [DOI] [PubMed] [Google Scholar]
- 17.Mitterberger M, Pallwein L, Gradl J, Frauscher F, Neuwirt H, Leunhartsberger N, et al. Persistent detrusor overactivity after transurethral resection of the prostate is associated with reduced perfusion of the urinary bladder. BJU Int. 2007;99:831–835. doi: 10.1111/j.1464-410X.2006.06735.x. [DOI] [PubMed] [Google Scholar]
- 18.Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6(11 Suppl):S580–S590. [PubMed] [Google Scholar]
- 19.Novara G, Galfano A, Ficarra V, Artibani W. Anticholinergic drugs in patients with bladder outlet obstruction and lower urinary tract symptoms: a systematic review. Eur Urol. 2006;50:675–683. doi: 10.1016/j.eururo.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Gallegos PJ, Frazee LA. Anticholinergic therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Pharmacotherapy. 2008;28:356–365. doi: 10.1592/phco.28.3.356. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan SA, Roehrborn CG, Chancellor M, Carlsson M, Bavendam T, Guan Z. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int. 2008;102:1133–1139. doi: 10.1111/j.1464-410X.2008.07761.x. [DOI] [PubMed] [Google Scholar]