Abstract
Histamine and prostaglandins (PGs) play a variety of physiological roles as autacoids, which function in the vicinity of their sources and maintain local homeostasis in the body. They stimulate target cells by acting on their specific receptors, which are coupled to trimeric G proteins. For the precise understanding of the physiological roles of histamine and PGs, it is necessary to clarify the molecular mechanisms involved in their synthesis as well as their receptor-mediated responses. We cloned the cDNAs for mouse l-histidine decarboxylase (HDC) and 6 mouse prostanoid receptors (4 PGE2 receptors, PGF receptor, and PGI receptor). We then characterized the expression patterns and functions of these genes. Furthermore, we established gene-targeted mouse strains for HDC and PG receptors to explore the novel pathophysiological roles of histamine and PGs. We have here summarized our research, which should contribute to progress in the molecular biology of HDC and PG receptors.
Keywords: histamine, histidine decarboxylase, prostaglandin, prostaglandin receptors, mast cell, inflammation
1. Introduction
In 1968, Tomita et al. discovered by chance that rats exhibited acute pain reactions (screaming, body writhing and biting) when they received subcutaneous injections of a solution of pyrophosphate (PPi).1) We extended this observation and further found that the injection of PPi causes immediate and temporary increases in local vascular permeability and subcutaneous histamine content. These PPi-induced responses were suppressed by a histamine H1 antagonist, diphenhydramine and closely associated with increasing number of cutaneous mast cells on the basis of histologic observation, indicating that PPi-induced inflammation was mediated by endogenous histamine from mast cells. We noticed that increasing PPi-induced vascular responses were effectively inhibited by simultaneous administration not only of epinephrine but also of theophylline, both of which mediate elevation of intracellular cAMP levels.2) In those days, Lichtenstein and Margolis also reported similar observation that allergic histamine release from human leukocytes is inhibited by catecholamines and methylxanthines, and suggested a possible regulatory role for cAMP in this process.3) We then confirmed that PPi induced histamine release from isolated rat mast cells, which was inhibited by cAMP analogues and cAMP-elevating agents such as cathecholamine.4) In addition to these suppressive effects of cAMP-elevating agents, we found that PPi-induced inflammatory responses were effectively suppressed by indomethacin and hydrocortisone, which inhibit prostaglandin (PG) generation from arachidonic acid. These observations inspired us to focus on the role of PGs in regulation of PPi-induced inflammatory responses. PGE is abundantly generated and released quickly from activated macrophages and other leukocytes accumulating in the local inflammatory environment. In a series of studies about the role of PGs in inflammatory responses, we found that PGE1 binds to its specific membrane receptors with high affinity and stimulates cAMP accumulation in P-815 cells, a murine mastocytoma cell line.5) At that time, the PGE receptor subtypes were pharmacologically classified into EP1, EP2, EP3 and EP4 based on their responses to various specific agonists and antagonists, although their molecular identities remains to be defined. This cell line with high expression levels of PGE receptors provided us with an opportunity to explore their molecular identity.
In the field of histamine research, a wide variety of histamine H1 and H2 receptor antagonists had already gained success as therapeutic agents for immediate allergy and peptic ulcer in the 1970s. The mechanism of degranulation of mast cells had also been intensively studied by many groups. However, little was known about the physiological roles of ‘nascent’ histamine, which was originally regarded as a modulator of tissue growth,6) and the molecular identity of l-histidine decarboxylase (HDC), the rate-limiting enzyme for histamine synthesis, remained to be clarified. At that time, we noticed that histamine synthesis could be induced in P-815 cells upon several stimuli and speculated that de novo synthesized histamine might have different roles from stored histamine.
In the 1980s, a vast majority of studies on histamine and PGs were performed to achieve the pharmacological understanding of the receptors using specific agonists and antagonists, and the molecular mechanisms involved in histamine synthesis and PG-mediated signal transduction remained largely unknown. During the past twenty years, however, a wide variety of biotechnological methods became available in the laboratory such as nucleotide sequencing, protein sequencing, DNA cloning, polymerase chain reaction (PCR), gene expression systems, gene targeting, immunoblotting, flow cytometry etc. Under such a biotechnology revolution, we in cooperation with our collaborators carried out many advanced studies regarding the molecular biology of HDC and PG receptors. This review summarizes our studies focusing on the molecular analyses of HDC and PG receptors and the newly-identified physiological roles of histamine and PGs.
2. Regulation of histamine synthesis
Histamine-mediated responses can be classified into two categories; one is mediated by stimulus-dependent release of stored histamine, and the other is accompanied by induction of histamine synthesis. HDC plays a crucial role in the latter category, and hence its identification had been anticipated for a long time. Many groups including us long faced the tough task in purification of the HDC protein, since the enzymatically active form of HDC was found to be unstable. Since fluorometric detection of histamine is sensitive and convenient, it has been used for the measurement of histamine content. Early studies demonstrated active histamine synthesis in fetal liver, pregnant mouse kidney, mastocytoma, and stomach. In 1984, Watanabe et al. succeeded in identification of histaminergic neuron with an anti-HDC antibody raised against partially purified rat HDC protein, although the molecular identity of HDC remained unknown.7)
2.1. Purification and cDNA cloning of HDC.
We determined the partial amino acid sequence of HDC for the first time through purification of the protein from the mouse mastocytoma cell line P-815.8) Since histamine synthesis was found to be drastically induced in these cells upon syngenic BDF mouse peritoneal cavity incubation, we prepared crude cell extracts from more than 2,000 mice. Mouse HDC comprises a homo-dimer, with two identical 53-kDa subunits. We then set out to clone the cDNA of mouse HDC based on its partial primary sequence. However, unexpectedly, cDNA cloning of HDC was achieved through a project which was unrelated to histamine research: Joseph et al. found a fusion transcript of putative HDC with the androgen binding protein, and identified rat HDC cDNA using the fusion transcript as a probe.9) In 1990, Joseph et al. and we reported cDNA cloning of rat and mouse HDC.9,10) Cloning of HDC cDNA revealed that HDC is translated as a 74-kDa precursor protein and might be post-translationally cleaved to a 53–55-kDa species, which forms a dimer. We further confirmed that the 53-kDa species is the dominant form in mouse stomach through purification of the HDC protein.11) Since the carboxyl-terminal (C-terminal) approximately 20-kDa region of HDC is unique in the homologous amino acid decarboxylases, such as dopa decarboxylase, we then focused on the role of this region and on the post-translational processing of HDC. Analysis of the recombinant 74-kDa species expressed in a baculovirus-insect cell expression system revealed that the mouse 74-kDa species is distributed in the insoluble fraction and exhibits lower enzyme activity in comparison with the C-terminal deleted 54-kDa species, which is localized in the cytosol.12,13) In contrast to mouse HDC, the human recombinant 74-kDa species exhibited comparable enzyme activity to its C-terminal deleted 54-kDa species.14) These results suggested that the C-terminal 20-kDa region of HDC is involved in regulation of its intracellular localization and enzymatic activity.
Cloning of HDC cDNA enabled us to determine the tissue distribution of the transcript. The expression profile of HDC mRNA was found to largely coincide with previous reports on the distribution of the enzymatic activity of HDC, with the exception of the testis. Although high levels of mRNA expression were observed in the testis, very little enzyme activity of HDC was detected. We later revealed using a specific antibody raised against HDC that HDC is expressed in the lineage of male germ cells including sperm in mice.15) Histamine was found to be released upon acrosome reaction, indicating that histamine release might be involved in fertilization. Although Joseph et al. predicted the presence of a fusion transcript of HDC and androgen binding protein, no such transcript was found in the mouse testis. It remains to be clarified as to why a large amount of the latent form of HDC is synthesized in male germ cells. HDC may have special functions in the testis, which are independent of histamine synthesis.
2.2. Transcriptional regulation of HDC.
Accumulating evidence has indicated that histamine synthesis is induced in various kinds of cells including gastric enterochromaffin-like cells. Prolonged psychological stress often results in peptic ulcer, for which H2 receptor antagonists are used as potent therapeutic drugs, indicating that stress responses lead to histamine-mediated acid hypersecretion. We hypothesized that the stress-induced glucocorticoid–ACTH pathway is involved in this increase in histamine synthesis. Indeed, administration of hydrocortisone significantly augmented the enzymatical activity of HDC.16) Dexamethasone, a synthetic analog of glucocorticoid, potently induced histamine synthesis in P-815 cells.17) Our finding that an anti-inflammatory drug, dexamethasone, induced the synthesis of histamine, which is a potent pro-inflammatory mediator, was interesting and raised the possibility that histamine is involved not only in inflammatory responses but also in other physiological responses. We further demonstrated the positive effects of dexamethasone on the induction of histamine synthesis in mouse fetal liver cells,18) suggesting that the activation of glucocorticoid–ACTH pathway immediately prior to parturition might be involved in the regulation of fetal hematopoiesis through histamine synthesis. We then focused on the regulation of gene expression of HDC in P-815 cells. A drastic induction of HDC was found in the cells treated with a combination of dexamethasone and phorbol ester, as well as with a combination of a cAMP analogue and Ca2+ ionophore.19,20) Both studies demonstrated the stimulus-dependent synergistic induction of HDC, indicating that histamine synthesis could be induced upon various combination of stimuli.
Cloning of HDC cDNA enabled us to perform subsequent genomic cloning and promoter analysis of the HDC gene. The mouse HDC 5′-flanking region was found to contain a TATA-like box and a GC box.21) Ohtsu et al. suggested that down-regulation of NF-E2 leads to transcriptional activation of the mouse HDC gene upon peritoneal cavity incubation in BDF1 mice.22) Wang et al. have continuously performed promoter analyses of the rat and human HDC gene.23) They found a series of suppressive elements inversely involved in gastrin-mediated induction of HDC. Since few elements that positively regulate the gene expression of HDC have been identified, further analyses are required to reveal the whole mechanism of gene expression regulation of HDC.
We determined the structure of the human HDC gene, which spans approximately 24 kb and contains 12 exons, and confirmed that HDC is encoded by a single copy gene.24) Investigation of the 5′-flanking region of the human HDC gene suggested that the c-Myb binding motif is involved in the specific expression of HDC in human basophilic leukemia cells, KU-812-F.25) Kuramasu et al. found that mast cell- or basophil-specific expression of human HDC is regulated by CpG methylation in the promoter region.26) DNA methylation-mediated regulation was also found in the cell type-specific expression of mouse HDC.27) However, it remains largely unknown as to how tissue specific expression of HDC is regulated. Since transcriptional control of the local induction of the HDC gene might contribute to appropriate modulation of histamine-mediated responses, particularly, in inflammatory and immune responses, the mechanism of HDC gene expression should be clarified.
2.3. Post-translational regulation of HDC.
Purification, cDNA cloning, and comparison with the other homologous amino acid decarboxylases indicated that the C-terminal 20-kDa region of HDC is deleted by post-translational cleavage of the 74-kDa form. However, it remained unknown how the post-translational processing of HDC is regulated and what the physiological roles of the cleavage are. We succeeded in raising a specific antibody against the amino-terminal (N-terminal) region of mouse HDC. This antibody was found to be useful for immunohistochemistry and immunoprecipitation, and has made a great contribution in the field of histamine research. We investigated the turnover rate of the nascent HDC protein and clarified its post-translational processing by immunoprecipitation with this antibody. In a rat basophilic/mast cell line, RBL-2H3, we found that the 74-kDa form of de novo synthesized HDC was very unstable. Further analysis revealed that the 74-kDa form of HDC was degraded through the ubiquitin–proteasome pathway.28) In this cell line, the 74-kDa form was found to be localized in the cytosol, whereas the 53-kDa form was in the granule fraction upon sucrose density gradient fractionation.29) Both fractions contained enzymatic activity of HDC, indicating that the post-translational cleavage determines the intracellular localization of HDC, rather than its enzymatic activation, in this cell line. Granular localization of the 53-kDa form was also observed in elicited mouse peritoneal neutrophils.30) Transient expression in COS-7 cells indicated that recombinant HDC accumulates mainly in the endoplasmic reticulum (ER).31) However, since HDC lacks a canonical signal sequence at its N-terminus, it remained to be clarified as to how HDC is targeted to the ER. We found that the nascent 74-kDa HDC protein prepared in in vitro translation system with rabbit reticulocyte lysate was post-translationally targeted to exogenously-added microsomal membranes.31) Since there have been few reports that demonstrate the post-translational ER targeting of mammalian proteins, HDC appears to be targeted to the ER in a unique manner, the details of which remain unknown.
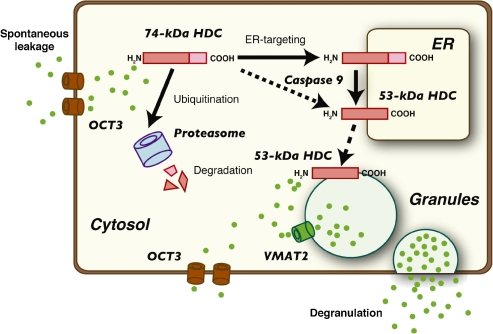
On the other hand, we also found that proteolytic cleavage of the recombinant 74-kDa protein results in its enzymatic activation.13) An increase in histamine synthesis in the stomach under repeated cold stress was accompanied by induction of proteolytic activity that could cause the post-translational processing of HDC in vitro. Recently, we identified caspase-9 as one of the processing enzymes responsible for enzymatic activation of mouse HDC in P-815 cells.32) Caspase-9-mediated cleavage of HDC was accompanied by formation of the 53-kDa species and drastic increases in its enzymatic activity. The regulation of histamine synthesis through the post-translational processing of HDC is summarized in Fig. 1 .
Figure 1.
Regulation of histamine synthesis by the post-translational processing of mouse HDC. HDC is initially translated as the precursor form, with a molecular weight of 74-kDa (74-kDa HDC). Since HDC lacks the canonical signal sequence at its N-terminus, HDC appears to be post-translationally targeted to the ER in an unidentified manner. One of the processing enzymes that are responsible for the post-translational processing of HDC was found to be caspase-9. The 53-kDa form of HDC is localized mainly in the granule fraction and possesses higher enzymatic activity. On the other hand, the 74-kDa form of HDC undergoes degradation through the ubiquitin–proteasome pathway. Histamine is synthesized in the cytosol and transported into the granules by vesicular monoamine transporter-2 (VMAT2). Stored histamine is released from the granules upon various stimuli, such as IgE-mediated antigen stimulation in mast cells. Excess amounts of histamine might be exported through organic cation transporter-3 (OCT3), which is known to be a transporter for organic cations with low affinity but high capacity. The 74-kDa HDC is the dominant form in the cells lacking histamine granules, such as macrophages, whereas the 53-kDa HDC is detected in the granule-containing cells, such as mast cells and neutrophils.
2.4. Immune modulation by histamine.
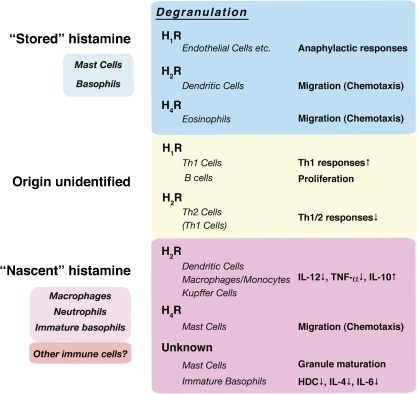
Histamine has a long history as a critical immune modulator; the histamine H1 receptor (H1R) mediates the proinflammatory effects of histamine, such as increases in vascular permeability and the expression of adhesion molecules in endothelial cells, whereas the H2R is involved in immune suppressive functions. Since a newly identified histamine receptor, the H4R, was found to be expressed specifically in leukocytes and to regulate the chemotactic responses of leukocytes, immune modulation by histamine has attracted further attention from researchers.33) Recent findings on histamine-mediated immune modulation are summarized in Fig. 2 .
Figure 2.
Immune modulation by histamine produced by various leukocytes. The actions of histamine can be classified into two categories; one mediated by stored histamine and the other by nascent histamine. In the former cases, histamine is produced and stored in the granules of mast cells and basophils and is liberated upon stimulation. In the latter case, HDC is induced at the transcriptional level upon various stimuli, and de novo synthesized histamine modulates the immune responses. Since several functions of histamine have been proposed through the experiments with exogenously-added histamine, the origin of histamine involved in such responses remains to be determined. Recent topics in this field are the identification of the H4R, which is expressed mainly in leukocytes, such as mast cells, eosinophils, and T cells.
2.4.1. H2R-mediated immune suppression.
Histamine is known to be a potent proinflammatory mediator, of which functions are largely mediated by the H1R. In addition to its H1R-mediated roles, accumulating evidence suggests that a wide variety of immune responses are modulated by histamine. A series of studies have indicated that histamine suppresses macrophage-mediated inflammatory responses by acting on the H2R. We focused on the role of the H2R in suppression of tumor immunity, since clinical trials indicated that several H2R antagonists, in particular cimetidine, has suppressive effects on tumor development. We demonstrated that local histamine synthesis in tumor tissues suppresses the local expression of cytokines, such as IFN-γ, TNF-α, and lymphotoxin-β, by acting on the H2R.34,35) Although it remains unknown as to what kinds of cells produce histamine in tumor tissues, tumor-infiltrating macrophages and neutrophils are potential candidates for the source of histamine. Establishment of gene targeted mouse strains for the H1R and H2R clarified the critical roles of histamine in helper T responses; the H1R is involved in augmentation of Th1 responses, whereas the H2R is involved in the suppression of both Th1 and Th2 responses.36)
2.4.2. IgE-mediated induction of histamine synthesis.
Histamine synthesis in mast cells is not always static but occasionally inducible. A wide variety of stimuli can augment histamine synthesis in mast cells, such as IL-3, stem cell factor, butyrate, phorbol ester, and glucocorticoid. We noticed that the amount of intracellular histamine is increased during sensitization of mast cells with IgE. In 2001, two groups reported that IgE can suppress apoptosis of IL-3-dependent bone marrow-derived mast cells (BMMCs) in the absence of its specific antigen. We demonstrated that a drastic increase in histamine synthesis occurs in murine BMMCs sensitized with IgE.37) Transcriptional activation of the HDC gene was induced by IgE in the absence of its antigen. Accumulating evidence indicated that the potency of IgE varied according to the clone, although this response was found to occur in the absence of its antigen. Potent IgE clones were found to induce cytokine production, such as IL-3, IL-4 and IL-6, histamine synthesis, migration, and adhesion. Induction of HDC in BMMCs was also observed upon IgE-mediated antigen stimulation or treatment with reagents that mobilize intracellular Ca2+ concentrations, such as thapsigargin and A23187.37,38) We demonstrated that the Ca2+ influx induced by IgE alone was mediated by pharmacologically different channels from that activated upon antigen stimulation.38) Absence of protein kinase C (PKC) βII did not affect Ca2+ mobilization upon antigen stimulation but abolished the Ca2+ mobilization induced by IgE alone, indicating the critical role of PKCβII in the IgE-mediated increase in histamine synthesis.39) Since hyper IgE syndrome has often been observed in chronic allergy diseases, such as hay fever and atopic dermatitis, and lowering of serum IgE levels with an anti-IgE antibody ameliorates disease severity, antigen-independent effects of IgE on mast cells might be involved in exacerbation of such chronic diseases.
2.5. Gene targeting of HDC.
Identification of the HDC gene provided us with the opportunity to establish gene-targeted mice. An international collaboration was carried out to prepare the HDC−/− mouse strain among three countries, Japan, Canada, and Hungary, including our group. Unexpectedly, HDC−/− mice were fertile and exhibited no gross abnormalities.40) No de novo synthesis of histamine was observed in the tissue homogenates of HDC−/− mice, indicating that HDC is the sole enzyme for histamine synthesis. Although tissue histamine levels were also drastically decreased in the HDC−/− mice, trace amounts of histamine were detectable in their tissues. Since enteric bacteria, such as lactobacillus, express the HDC ortholog and produce histamine, it was difficult to eliminate such trace amounts of residual histamine from HDC−/− mice. However, histamine content in the tissues of HDC−/− mice was controllable through dietary supplementation of histamine.41,42) We found that dietary histamine accumulated in the spleen of HDC−/− mice and that macrophages were responsible for this uptake.42)
It was anticipated that IgE-dependent immediate allergic responses were abolished in HDC−/− mice.41,43) However, we could not conclude that the resistance to anaphylactic reaction was due solely to the lack of histamine in these mice, since their tissue mast cells exhibited abnormal granule morphology and hence may have functional defects.40) Aberrant granule formation in HDC−/− mice suggested that histamine might affect mast cell maturation, but the functional roles of the histamine receptors in mast cells remains largely unknown. Although HDC−/− mice are a useful model for studying allergy and inflammation, it is critical to specifically characterize tissue mast cells that lack HDC.
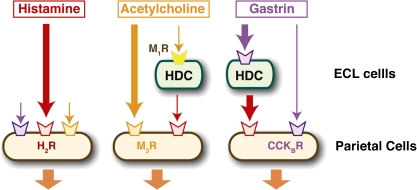
Histamine is one of the critical acid secretagogues in the stomach. We then investigated gastric acid secretion in HDC−/− mice.44) HDC−/− mice were found to be sensitive to exogenous histamine but were resistant to gastrin, which induces gastric acid secretion. Our study revealed the roles of histamine in gastric acid secretion induced by muscarinic stimulation and gastrin (Fig. 3 ). HDC−/− mice exhibited similar features to mice continuously treated with H2R antagonists, such as hypergastrinemia and acid hypersecretion in response to histamine. Although H2R−/− mice shared many features with HDC−/− mice, such as hypergastrinemia, a marked hypertrophy with enlarged folds of the gastric mucosa was observed only in the H2R−/− mice.45) These results suggested that histamine is involved in the maintenance of gastric mucosal cellular composition.
Figure 3.
Roles of histamine in gastric acid secretion. Histamine, acetylcholine (muscarinic), and gastrin are major secretagogues in gastric acid secretion. Accumulating evidence suggests that acetylcholine and gastrin stimulate histamine release from ECL cells, which synergistically enhance the acid secretion through direct stimulation of parietal cells by these secretagogues. Massive and prolonged acid secretion by gastrin was found to require transcriptional activation of the HDC gene. Investigation using the HDC−/− mice revealed that histamine plays a critical role in gastrin-mediated acid secretion but not in muscarinic acid secretion.
Accumulating evidence suggests that the HDC−/− strain is a useful tool in identification of novel physiological roles of histamine.46,47) The combinatorial use of gene targeted mice for HDC and histamine receptors should lead to great advancements in histamine research.
3. Biochemical and molecular characterization of prostaglandin (PG) receptors
Biochemical studies showed that the actions of PGs are mediated by G proteins, and ligand binding properties indicated that a variety of PGs cross-react with the other receptors, suggesting structural similarity of the receptors. It was repeatedly reported that PG actions are associated with changes in second messengers. Some PG actions were found to be associated with changes in cyclic AMP (cAMP) levels, phosphatidylinositol (PI) turnover or free calcium ion concentrations in the cell. However, none of the receptors had been isolated and cloned until the thromboxane (TX) A2 receptor was purified from human blood platelets and its cDNA cloned by Narumiya et al.48) This study revealed that the TXA2 receptor was a G-protein-coupled, rhodopsin-type receptor with seven transmembrane domains.
3.1. Cloning and expression of PG receptors.
In collaboration with Dr. Narumiya, we employed a homology screening strategy using mouse P-815, lung, kidney, and ovary cDNA libraries, and subsequently identified the structures of four subtypes of PGE receptors,49–52) the PGI2 receptor,53) and PGF2α receptor.54) These receptors were expressed and their ligand binding properties and signal transduction were examined in homogenous populations in heterologous expression systems (reviewed in Refs. 55, 56). In the following section, we summarize the structure and biochemical properties of the PGE receptor subtypes.
3.1.1. PGE receptor subtypes.
We isolated functional cDNAs of the mouse PGE receptor subtypes, EP1, EP2, EP3 and EP4, and characterized their structural and biochemical properties using recombinant receptors expressed in mammalian cells.49–52) EP1, EP2, EP3 and EP4 receptors consist of 405, 362, 365, and 513 amino acid residues with seven hydrophobic segments corresponding to putative transmembrane domains, respectively. The binding properties of the four PGE receptors were examined in detail with various synthetic PGE analogues, and found to be in good agreement with the results from the pharmacological experiments. For example, the cloned EP1 receptor shows about ten times higher affinity to PGE2 and iloprost than PGE1, while the other three receptors bind to PGE2 and PGE1 with equal affinities and to iloprost with much lower affinity. 17-Phenyl-PGE2 shows the highest affinity to EP1, while butaprost and M&B28767 are the best ligands to EP2 and EP3, respectively. Although AH23848B was used as a selective antagonist for EP4, this ligand showed moderate binding affinity for EP4. Signal transduction analyses demonstrated that these receptor subtypes couple to different pathways, as suggested by various biochemical studies: EP1 elicits the elevation of intracellular [Ca2+], EP2 and EP4 stimulates adenylate cyclase while EP3 inhibits the enzyme.
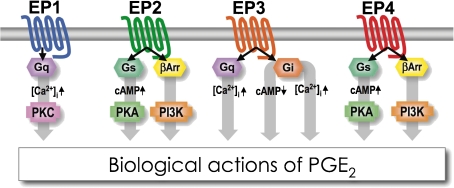
The EP1 receptor mediates the PGE2-induced elevation of free Ca2+ concentrations in CHO cells. This increase is dependent on extracellular Ca2+ and occurs without a detectable PI response, suggesting that EP1 regulates Ca2+ channel gating via an unidentified G protein.57) It was reported that EP1 expressed in Xenopus oocytes can couple to TRP5, a candidate for the receptor-activated Ca2+ channel (RACC), and this coupling is inhibited by an anti-sense oligonucleotide for Gq/G11 but not by one for Gi1 (Fig. 4 ).58) The EP2 and EP4 receptors couple to Gs and mediate increases in cAMP concentrations. The major signaling pathway of the EP3 receptor is inhibition of adenylate cyclase via Gi. It should be noted, however, that EP3 receptors do not couple exclusively to the pathways described but often to more than one G protein and signal transduction pathway. Hatae and Yamaoka found that when expressed in COS7 cells, the EP3 receptor elicits augmentation of EP2- or EP4-induced cAMP formation via Gq/11.59,60) Interestingly, this EP3-induced superactivation is observed irrespective of the C-terminal tail structure; EP3α, β, γ, which are the C-terminal splicing variants, and C-terminal-truncated mutant EP3 showed similar responses. Such coupling of EP3 to Gq/11 is dependent on lipid-raft structure. Localization of EP receptors to lipid raft microdomains may determine the cell-specific signal transduction pathway of EP3. In contrast, of interest is the presence of two EPs, EP2 and EP4, that are coupled to increases in cAMP. Nishigaki et al. found that EP4 shows higher sensitivity to agonist-induced desensitization than EP2.61) However, EP2 and EP4 apparently function redundantly in some processes. For example, both EP2 and EP4 mediate potentiation of Th17 expansion through cAMP–PKA signaling by PGE2, although the extent of the contribution by each receptor may be different.62) On the other hand, there are processes in which EP2 and EP4 play distinct roles. Some of these may be due to selective expression of either receptor in relevant cells such as the action of EP2 during cumulus expansion in ovulation and fertilization, and that of EP4 in closure of the ductus arteriosus (see 3.4.1).63,64) However, only EP4 stimulates IL-23 production from dendritic cells in mice, although both EP2 and EP4 are expressed in these cells.62) In 2003, Fujino et al. found that EP4 couples to PI3-kinase in addition to activation of adenylate cyclase.65) Later, Buchanan et al. demonstrated that EP4 activates PI3-K through activation of β-arrestin/Src pathway.66) Furthermore, we found that both EP2 and EP4 couple to PI3-kinase in naive T cells.62) Indeed, Chun et al. demonstrated that EP2 is also coupled to PI3-kinase via β-arrestin1 (Fig. 4).67)
Figure 4.
Signal transduction pathways of the four PGE receptor subtypes. EP1 is coupled to Gq/11 (Gq) and intracellular Ca2+ mobilization. EP2 and EP4 are coupled to the Gs/adenylyl cyclase/cAMP/protein kinase A (PKA) pathway. Recently, it was shown that the two receptors are also coupled to the β-arrestin (βArr)/Phosphoinositide 3-kinase (PI3-K) pathway. The EP3 receptor is mainly coupled to Gi, but in particular cell types, EP3 augments EP2/EP4-induced cAMP formation via the Gq/phospholipase C/Ca2+ pathway.
3.2. Identification and characterization of PG receptor isoforms.
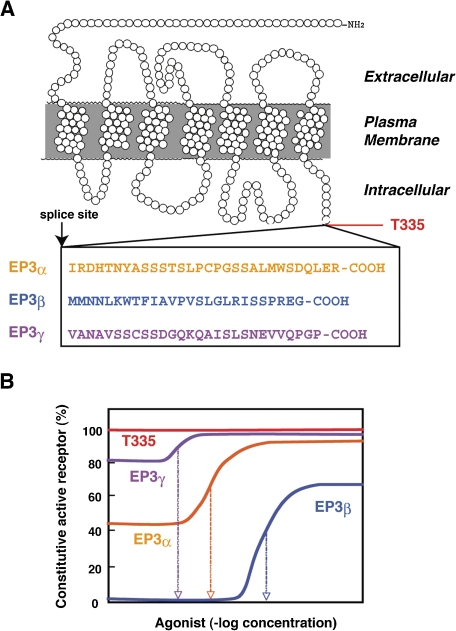
In addition to the receptor subtypes encoded by different genes, alternative splicing can create several isoforms of the EP3 receptor, which differ in their C-terminal sequence. There exist three isoforms in mouse,68,69) four isoforms in bovine,70) and six isoforms in human (Fig. 5A ).71) These isoforms displayed identical agonist binding properties, but were functionally different in their efficiency of G protein activation, specificity of G protein coupling, sensitivity to agonist-induced desensitization and leakage of agonist-insensitive constitutive activity.72,73) For example, mouse EP3α and EP3β both couple to Gi, but three orders lower concentration of agonists were required for EP3α than EP3β for activation of G protein. EP3α undergoes both short and long term agonist-induced desensitization, whereas EP3β undergoes neither short nor long term desensitization. On the other hand, bovine EP3A couples to Gi to induce the inhibition of adenylate cyclase, EP3B and C couple to Gs to increase cAMP, and EP3D couples to Gq, in addition to Gi and Gs, to evoke a pertussis toxin-insensitive PI response. RT-PCR analyses demonstrated that these mRNAs are expressed in various tissues in different relative amounts. In order to elucidate the role of the C-terminal domains of the EP3 isoforms, we constructed a mutant EP3 receptor in which the C-terminus was truncated at the splice site. The mutant receptor retained the ability to associate with Gi2, and its binding affinity showed sensitivity to guanine nucleotides. However, expression of the mutant receptor alone without agonist stimulation was enough to inhibit adenylate cyclase (Fig. 5B). Pertussis toxin treatment of cells expressing this mutant receptor raised forskolin-induced cAMP accumulation. These results indicate that the C-terminal domain of the EP3 receptor plays a role in preventing the leakage of agonist-independent constitutive activation of G proteins.
Figure 5.
Structure and constitutive activity of mouse EP3 receptor isoforms (EP3α/β/γ) and the C-terminus-truncated mutant receptor (T335). A. Sequences of the C-terminal domains created by alternative splicing are shown. T335 is a C-terminus-truncated mutant receptor. B. Different agonist-induced and constitutive Gi activity in mouse EP3 receptor isoforms (EP3α/β/γ) and the C-terminus-truncated mutant receptor (T335).
3.3. Distribution and expression regulation of PG receptors.
PG receptors have been suggested to exist in many tissues in the body, based on previous pharmacological and biochemical studies. The various therapeutic effects as well as side-effects of aspirin-like drugs also reflect their involvement in various physiological and pathophysiological processes. The exact distribution of each receptor and the identities of cells expressing each receptor, however, remain mostly unknown because of the relatively low expression levels of these receptors and the expression of multiple receptors in a single tissue. We approached this issue using molecular biological techniques such as Northern blot analysis and in situ hybridization, which provided detailed information on PG receptor distribution.74) These analyses have shown that each receptor is specifically distributed in the body, and that expression levels are variable among tissues.
3.3.1. Kidney.
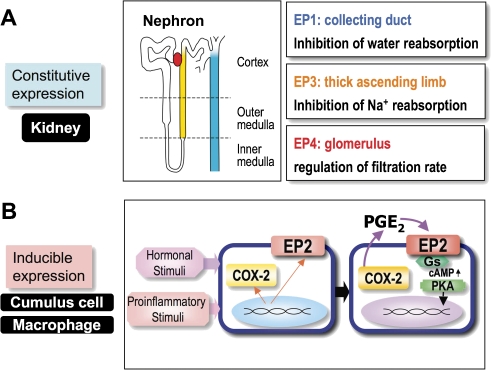
Among the four PGE receptors, EP3 and EP4 receptors are widely distributed throughout the body, with their mRNAs being expressed in almost all mouse tissues examined. In contrast, the distribution of EP1 mRNA is restricted to several organs such as the kidney, lung and stomach, and EP2 is the least abundant among the EP receptors. In general, the PGE receptor subtypes show ‘distinct’ cellular localizations. For example, in the kidney, we found that EP3 receptor is expressed in the tubular epithelium of the outer medulla, especially in the thick ascending limb and cortical collecting ducts, EP1 receptor in the papillary collecting ducts and EP4 receptor in the glomerulus (Fig. 6A ).75) The distribution pattern appears to correspond with the PGE2-mediated regulation of ion transport, water reabsorption and glomerular filtration, respectively. However, there was no expression of EP2 mRNA.
Figure 6.
Regulation of PGE receptor gene expressions. A. Constitutive expression of PGE receptor genes in different cell types of kidney. Schematic representation of the distribution of PGE receptors in the nephron (center panel). EP1 is expressed in the collecting duct (blue), EP3 is expressed in thick ascending limb (yellow), and EP4 is expressed in the glomerulus (red). EP1 and EP3 are involved in inhibition of water and ion transport, respectively. EP4 is involved in regulation of glomerular filtration rate. B. Inducible gene expression of COX-2 and the EP2 receptor in cumulus cells (hormonal stimuli) or macrophage (proinflammatory stimuli). In these cells, gene expression of COX-2 and EP2 is induced in a similar manner. As a result, COX-2-derived PGE2 acts on the EP2 receptor, and elicits functional changes via the Gs/cAMP/PKA pathway in an autocrine fashion.
3.3.2. Nervous system.
The differential expression of PGE receptors also occurs in the central nervous system. EP3 mRNA is widely expressed in the neurons of the cortex, hippocampus, thalamus, hypothalamus, midbrain and lower brain stem.76) Among these subregions, in the hypothalamus, EP3 mRNA is expressed in the neurons in the medial and median preoptic nuclei, especially in neurons surrounding the organum vasculosa lamina terminalis (OVLT). The OVLT has been regarded as a key structure with a poor blood-brain-barrier. Indeed, cyclooxygenase-2 (COX-2) is induced in this region in response to the peripheral administration of lipopolysaccharide (LPS). The EP3 receptor in this region may be involved in fever generation (see 3.4.2). In contrast, expression of EP4 and EP2 mRNA was reported in small groups of neuronal and non-neuronal cells.77) Zhang and Rivest further found that expression levels in some of these cells were increased upon the peripheral administration of LPS, which is an interesting finding in respect to the regulation of receptor gene expression by inflammatory stimuli (see 3.3.3). On the other hand, EP1, EP3 and EP4 mRNAs are expressed in neurons of the dorsal root ganglion (DRG).78) EP3 mRNA is expressed in half of the DRG neurons and these are in general small in size, suggesting the involvement of this receptor in PGE2-mediated hyperalgesia. However, pain modulation by PGs is also closely associated with IP expression in DRG neurons.78)
3.3.3. Transcriptional regulation of EP receptor gene expression.
COX-2 gene expression is known to be induced upon hormonal and proinflammatory stimuli. Such gene induction by hormonal and proinflammatory signals is also observed for the EP2 receptor (Fig. 6B). For example, macrophages produce large amounts of PGE2 in response to LPS treatment, and the resultant PGE2 at least in part act on the macrophages themselves, resulting in inhibition of cytokine release. This inhibitory effect of PGE2 is exerted through production of cAMP, suggesting the involvement of EP2 and/or EP4 receptors. We examined the details of expression of EP2 and EP4 receptors in macrophage cells, J774.1, and found that the former is effectively induced in response to LPS treatment in a time course similar to that of COX-2.79) We further demonstrated that the up-regulation of EP2 by LPS is completely inhibited by the simultaneous administration of interferon-γ. Induction of the EP2 receptor gene was also found in mouse peritoneal resident macrophages upon their exposure to LPS.80) In contrast, abundant expression of EP4 mRNA is present before the addition of LPS in these macrophages. Thus, EP2 may serve as an inducible type of PGE receptor in macrophages, being regulated in a manner similar to that proposed for COX-2.
Hormonal stimuli can change both localization and expression level of PGE receptor subtypes. We found that the abundance and localization of mRNAs for EP2, EP3 and EP4 change considerably when mice undergo pseudopregnancy by treatment with PMSG and hCG.81) EP2 mRNA, which is hardly detectable before stimulation, is expressed considerably in luminal epithelial cells. This expression peaks on day 5 of pregnancy and disappears quickly thereafter. Because this induction occurs in parallel with that of COX-2 in this tissue, is coincident with the time of blastocyst implantation, and is sensitive to indomethacin treatment, EP2 may be involved in the implantation process. EP4 mRNA expression sharply increases on day 3 of pseudopregnancy and is maintained at a high level after day 5. In addition, EP2 expression is limited to luminal epithelial cells on day 0 but is further found in endometrial stromal cells and the glandular epithelium after induction. In contrast to these receptors expressed in the endometrium, EP3 receptor mRNA is expressed in smooth muscle layers. Its expression level is low on day 1, increases up to day 5 and then declines. The cellular localization of EP4 mRNA changes during pseudopregnancy; it is expressed in the longitudinal muscle layer before stimulation, but in circular smooth muscles on day 5. Thus, analysis of the uterine expression of EP receptors provides an interesting example of PG receptor induction under physiological conditions. It should be noted that gonadotropins also induce EP2 mRNA as well as COX-2 with a similar time course in the ovary (see 3.4.1). 63,82)
Since EP2 mRNA is expressed in luminal epithelial cells of the uterus, we hypothesized that the mouse EP2 gene may be regulated by the progesterone receptor (PR). In order to test this possibility, we investigated the transcriptional regulation of EP2 gene expression by reporter gene analysis using HeLa cells with or without expression of the PR.83) The 5′-flanking region (−3260 to −27, upstream of the translation initiation site) exhibited progesterone-induced promoter activation and basal promoter activity in the presence of PR. Using successive deletion analysis, we determined the six regulatory regions in the EP2 gene. Three regions were found to be involved in progesterone-induced promoter activation, whereas the other three regions were involved in basal promoter activity in the presence of PR. We identified a novel PR-binding sequence, 5′-G(G/A)CCGGA-3′, in two basal promoter regions and Sp1- and Sp3-binding sites in the other basal promoter region. Thus, we finally identified a novel PR-binding sequence, which may be involved in regulation of the basal promoter activity of the EP2 gene.
3.4. Physiological roles of PG receptor signaling.
PGs are presumed to play many important roles in a variety of physiological and pathophysiological processes in the body. These roles of PGs have been suggested both by examining the effects of aspirin-like drugs which inhibit PG production and by analyzing the in vitro and in vivo actions of each PG added exogenously. However, it is not necessarily clear as to which type of PG and which type of PG receptor are involved in each process. Neither is it clear as to how critical the action of PGs is in each process. In order to approach this issue, we employed targeted disruption of the PG receptor gene and generated EP1-, EP2-, EP3-, EP4-, and FP-deficient mice.63,64,84,85) Since recent progress on the roles of PG receptors in physiological and pathological conditions has been described elsewhere,86,87) in this review, we focused on the roles of EP2 signaling in fertilization as one of the representative physiological actions of PGs and the roles of EP3 signaling in fever generation as one of the pathological actions of PGs.
3.4.1. Impaired ovulation and fertilization in EP2-knockout mice.
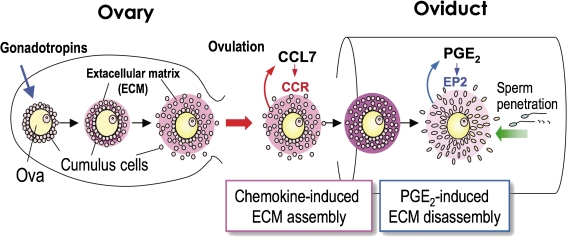
E- and F-type PGs are implicated in many aspects of reproductive functions. These include not only peripheral reproductive processes but also gonadotropin secretion in the central nervous system. To date, it has been accepted that luteinizing hormone-releasing hormone (LHRH) is secreted by the hypothalamus in response to PGE2. Although it was revealed that estradiol treatment is followed by increased expression of EP1 and EP3 mRNAs in LHRH neurons, no apparent changes were found in serum concentrations of LH and FSH in female mice of the COX-1- and COX-2-knockouts.88) In the ovary, the PG content of follicles increases as the follicles mature. Indomethacin abolishes LH-induced ovulation, and this effect is reversed by treatment with PGE2 or PGF2α.89) Studies on mice deficient in COX-2, but not COX-1, showed multiple reproductive failures in early pregnancy, such as ovulation, fertilization, implantation, and decidualization, suggesting that PGs play essential roles in these processes.90) Since IP-, EP1-, EP3-, EP4-, and TP-deficient females are fertile, these receptors may be dispensable in female reproduction. Likewise, ovulation, fertilization and implantation are normal in mice lacking the FP receptor gene, suggesting that PGF2α is not crucial for these processes.84) We reported a failure in early pregnancy in EP2-deficient female mice.63) We found that EP2-deficient female mice consistently deliver fewer pups than their wild-type counterparts irrespective of the genotypes of the mating males. We detected slightly impaired ovulation and dramatic reduction in fertilization in EP2-deficient mice and concluded that failure in early pregnancy in COX-2-deficient mice is at least in part due to dysfunction of the EP2 receptor. We further found that this phenotype is due to impaired expansion of the cumulus oophorus. To obtain insight into the mechanism causing fertilization failure in the EP2-deficient cumulus, we compared the gene expression profiles of wild-type and EP2-null cumuli isolated from the oviduct ampulla.91,92) We found increased gene expression of chemokines such as ccl2, ccl7 and ccl9 in EP2-deficient cumuli compared with those of wild-type. Furthermore, we molecularly dissected the functional consequences of enhanced chemokine signaling in EP2-mutated cumuli, and found that chemokine signaling facilitates both sperm attraction to the cumulus and compaction of the cumulus by integrin-mediated extracellular matrix assembly, the latter of which is down-regulated by PGE2-EP2 signaling to allow sperm penetration for successful fertilization (Fig. 7 ).
Figure 7.
Schematic model of the roles of PGE2–EP2 signaling in cumulus ECM status and successful fertilization. Gonadotropins stimulate secretion of extracellular matrix (ECM) components such as hyaluronan from cumulus cells. Ovulation-associated signals induce the gene expression and production of chemokines such as CCL7 in cumulus cells. CCL7 stimulates cumulus ECM protein assembly to protect the oocyte in an autocrine manner, and facilitates sperm migration to cumulus–oocyte complexes. Once the complexes reach the fertilization site, PGE2/EP2/cAMP signaling down-regulates such chemokine actions on the cumuli so that chemokine signaling does not interfere with sperm penetration.
3.4.2. Lack of fever generation in EP3-knockout mice.
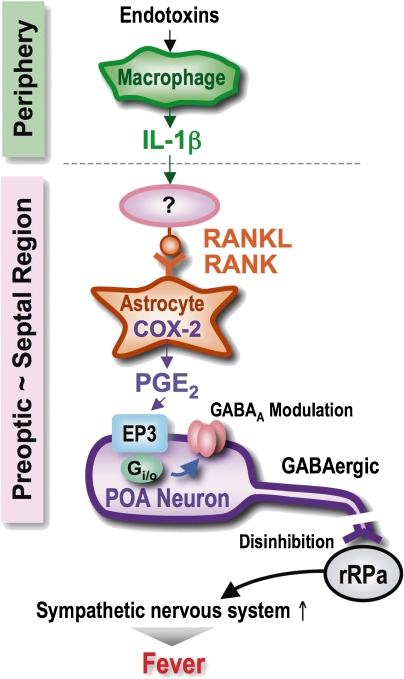
Fever is one of the most well-known phenotypes in which endogenous PGs play roles, because of the powerful anti-pyrogenic effects of aspirin-like drugs. A fever is elicited by cellular components of infectious organisms, such as LPS, as well as by non-infectious inflammatory insults. Both exogenous pyrogens and non-infectious insults stimulate the production of cytokines that work as endogenous pyrogens. These cytokines, IL-1 and IL-6 act on the preoptic area (POA), which then stimulates the neural pathways that raise body temperature. PGE2 has been suggested to work as a central mediator of fever93); E-type PG is a powerful inducer of fever when injected into the brain, and the level of PGE2 increases in the POA during LPS-induced fever, and indomethacin completely abolishes both LPS-induced fever and increased levels of PGE2 in the POA.94) On the other hand, Mitchell et al. argued against the role of PGE,95) based on the findings that some PGE antagonists failed to suppress cytokine-induced fever, and that aspirin-like drugs failed to inhibit macrophage inflammatory protein-1 (MIP-1)-induced and IL-8-induced fever. We used mice lacking each subtype of PGE receptor to address the above issues.85) In this study, mice lacking each of the four subtypes of PGE receptor, the EP1, EP2, EP3 and EP4 receptor, were generated by homologous recombination, and the fever responses to PGE2, IL-1β or LPS were examined. We found that only the EP3 receptor-deficient mice fail to show a febrile response to all of these stimuli. This study thus clearly demonstrated that PGE2 mediates fever generation in response to both exogenous and endogenous pyrogens by acting on the EP3 receptor (Fig. 8 ).
Figure 8.
Mechanism of inflammatory fever. Peripheral stimuli such as endotoxins initiate macrophage to produce IL-1β, which somehow induces RANKL expression in septal/POA region. RANKL, by acting on RANK on the astrocytes, initiates COX-2 expression and PGE2 production. PGE2 modifies the GABAA subunit composition via EP3-Gi/o pathway, and such a rapid modulation of GABAA channel may inhibit the GABAergic activity of EP3-expressing neurons, allowing the excitation of raphe (rRPa) neurons, leading to sympathetic nerve-mediated thermogenesis.
Receptor-activator of NF-κB ligand (RANKL) and its receptor RANK are essential regulators of bone remodeling, lymph node organogenesis and formation of a lactating mammary gland.96) Just recently, Penninger and colleague found the RANKL/RANK signaling in the septal to POA region plays a critical role in LPS-induced or cytokine-induced fever generation.97) Central administration of recombinant RANKL results in fever generation, which is alleviated by astrocyte-specific gene disruption of RANK. Peripheral administration of LPS or IL-1β augments RANKL/RANK expression in the septal/POA region. Intriguingly, RANKL injection induces COX-2 expression and PGE2 accumulation in the septal/POA region, and RANKL-induced fever generation was abolished by COX-2 inhibitor or gene disruption of EP3 receptor. These results indicate that RANKL/RANK signaling in the astrocyte of septal/POA region mediates the febrile response via induction of the COX-2/PGE2/EP3 receptor system (Fig. 8).
Which type of cell expressing EP3 receptor is the most critical for PGE2-induced fever generation? In our primary analysis,76) we found that the mRNA for the EP3 receptor is particularly abundant in the POA region. We further confirmed that the rat EP3 receptor protein is expressed in the cell bodies of these neurons with a distribution pattern similar to that of EP3 mRNA.98) Recent report by Lazarus et al. demonstrated that POA-specific EP3 gene disruption is enough to abolish PGE2-induced fever generation.99) Thus, the EP3 receptor expressed in neurons in the POA region works as an initial input of ‘pyrogenic’ PGE2 to alter the set point of thermal regulation.
Then, what occurs after EP3 activation in these neurons? Nakamura et al. revealed that most EP3-positive neurons in the POA (86%) are also positive for glutamate decarboxylase (GAD) 67 (gad67) gene expression and that injection of the GABAA agonist muscimol injection into the raphe pallidus nucleus blocks PGE2-induced fever.100) Based on these results, they proposed the mechanism of PGE2-induced fever as follows: the EP3-expressing neurons are GABAergic and exert tonic inhibition on raphe pallidus neurons which couples to stimulation of sympathetic nerves in the steady state (in the absence of PGE2), and when cytokine signals stimulate PG production within the POA, PGE2 somehow inhibits the GABAergic activity of EP3-expressing neurons, allowing the excitation of raphe neurons, leading to thermogenesis.100) Tsuchiya et al. found that particular subtypes of GABAA receptors and EP3 are co-expressed in the POA neurons, in which GABAA expression levels are altered in a fever-associated manner upon PGE2 administration.101) These results suggest that the EP3-expressing POA neurons are also susceptible to negative regulation by GABA. Indeed, local application of muscimol into the POA has been shown to induce hyperthermia in an NSAID-insensitive manner.102,103) This effect of muscimol may reflect the existence of a more universal thermal regulation mechanism by GABA within the POA. If this is true, PGE2–EP3 signaling may stimulate the susceptibility to GABA inhibition by affecting the GABAA channel properties such as sensitivity to GABA or GABA-elicited responses of the POA neurons (Fig. 8).
4. Perspective
Accumulating evidence indicates that nascent histamine plays critical roles in a wide variety of pathophysiological responses, which are regulated solely by HDC. Recently, histamine H3 and H4 receptors were identified and cloned, arousing considerable interest in the unknown functions of histamine in the central nervous and immune systems.104,105) It is increasingly recognized that where and when histamine is synthesized are the critical issues for better understanding of histamine-mediated responses. On the other hand, the mechanisms whereby PGE2 exerts its multipotent actions, once a mystery in physiology, have been clarified through the biochemical identification and cDNA cloning of the four EP subtype receptors. Furthermore, development of highly selective agonists and antagonists to each EP subtype and information obtained by studies on mice deficient in each EP receptor now provide opportunities to apply our knowledge to manipulate various PGE2-mediated pathological processes.106)
Our findings have revealed a comprehensive picture of the physiological roles of histamine and PGs by providing the missing pieces in their research fields. Comparison and combined use of gene-targeted mice for its synthesizing enzyme and its specific receptor have provided important insights into the role of histamine and PGs, respectively. Communication between different cells through ligand-receptor interactions is one of the fundamental components required for maintaining homeostasis in the local environment. The spatiotemporal regulation of ligand synthesis and receptor expression determines the impact of histamine and PGs. Co-ordinate progress in research on both the ligand and receptor is required for better understanding of the functions of autacoids.
Acknowledgements
We thank all the collaborators and the members of the Department of Physiological Chemistry, Graduate School of Pharmaceutical Sciences, Kyoto University. We also thank Dr. H. A. Popiel for careful reading of the manuscript.
Abbreviations
- ACTH
adrenocorticotropic hormone (corticotropin)
- cAMP
cyclic adenosine monophosphate
- COX
cyclooxygenase
- GABA
γ-aminobutyric acid
- HDC
l-histidine decarboxylase
- IL
interleukin
- LPS
lipopolysaccharide
- OVLT
organum vasculosa lamina terminalis
- PG
prostaglandin
- PPi
pyrophosphate
Profile
Atsushi Ichikawa was born in 1940 and started his research career in 1968 with studies on the molecular biology of histamine synthesis and prostaglandin actions in mast cells at the Faculty of Pharmaceutical Sciences, Graduate School of Kyoto University, after graduating from the Faculty of Pharmaceutical Sciences, Tokyo University. He performed pioneering works on; (1) the molecular characterization of histidine decarboxylase and novel functions of histamine in allergy, and (2) the structure and function of four subtypes of the prostaglandin E receptor participating in various pathophysiological actions. The later studies led to subsequent extensive studies of development of receptor-specific agonists and antagonists of prostaglandin E for clinical application in the world pharmaceutical manufactures. He is Director and professor, School of Pharmacy and Pharmaceutical Sciences, Institute for Biosciences, Mukogawa Women’s University and Professor Emeritus of Kyoto University. Prior to the current position, he was Professor, where he educated many undergraduate and graduate students in the field of physiological chemistry, and had served as the Dean at the Faculty of Pharmaceutical Sciences, Kyoto University (1993–1995), and the President of the Pharmaceutical Society of Japan (2001–2003) and the Biochemical Society of Japan (1999–2001). He received many awards including the Pharmaceutical Society of Japan Award (1999).
References
- 1).Tomita K., Young J.W., Lardy H.A. (1968) Chemical structure and biological activity of thyroid hormones. Gunma Symp. Endocr. 5, 85–94 [Google Scholar]
- 2).Ichikawa A., Hayashi H., Minami M., Tomita K. (1972) An acute inflammation induced by inorganic pyrophosphate and adenosine triphosphate and its inhibition by cyclic 3′,5′-adenosine monophosphate. Biochem. Pharmacol. 21, 317–331 [DOI] [PubMed] [Google Scholar]
- 3).Lichtenstein L.M., Margolis S. (1968) Histamine release in vitro: Inhibition by catecholamines and methylxanthines. Science 161, 902–903 [DOI] [PubMed] [Google Scholar]
- 4).Hayashi H., Ichikawa A., Saito T., Tomita K. (1976) Inhibitory role of adenosine cyclic 3′,5′-monophosphate in histamine release from rat peritoneal mast cells in vitro. Biochem. Pharmacol. 25, 1907–1913 [DOI] [PubMed] [Google Scholar]
- 5).Yatsunami K., Ichikawa A., Tomita K. (1981) Accumulation of adenosine cyclic 3′,5′-monophosphate induced by prostaglandin E1 binding to mastocytoma P-815 cells. Biochem. Pharmacol. 30, 1325–1332 [DOI] [PubMed] [Google Scholar]
- 6).Kahlson G., Rosengren E. (1968) New approaches to the physiology of histamine. Physiol. Rev. 48, 155–196 [DOI] [PubMed] [Google Scholar]
- 7).Watanabe T., Taguchi Y., Shiosaka S., Tanaka J., Kubota H., Terano Y., et al. (1984) Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 295, 13–25 [DOI] [PubMed] [Google Scholar]
- 8).Ohmori E., Fukui T., Imanishi N., Yatsunami K., Ichikawa A. (1990) Purification and characterization of l-histidine decarboxylase from mouse mastocytoma P-815 cells. J. Biochem. 107, 834–839 [DOI] [PubMed] [Google Scholar]
- 9).Joseph D.R., Sullivan P.M., Wang Y.M., Kozak C., Fenstermacher D.A., Behrendsen M.E., et al. (1990) Characterization and expression of the complementary DNA encoding rat histidine decarboxylase. Proc. Natl. Acad. Sci. USA 87, 733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Yamamoto J., Yatsunami K., Ohmori E., Sugimoto Y., Fukui T., Katayama T., et al. (1990) cDNA-derived amino acid sequence of l-histidine decarboxylase from mouse mastocytoma P-815 cells. FEBS Lett. 276, 214–218 [DOI] [PubMed] [Google Scholar]
- 11).Watabe A., Fukui T., Ohmori E., Ichikawa A. (1992) Purification and properties of l-histidine decarboxylase from mouse stomach. Biochem. Pharmacol. 43, 587–593 [DOI] [PubMed] [Google Scholar]
- 12).Yamamoto J., Fukui T., Suzuki K., Tanaka S., Yatsunami K., Ichikawa A. (1993) Expression and characterization of recombinant mouse mastocytoma histidine decarboxylase. Biochim. Biophys. Acta 1216, 431–440 [DOI] [PubMed] [Google Scholar]
- 13).Tanaka S., Fukui T., Yamamoto J., Shima Y., Kume T., Ohgo M., et al. (1995) Processing and activation of recombinant mouse mastocytoma histidine decarboxylase in the particulate fraction of Sf9 cells by porcine pancreatic elastase. Biochim. Biophys. Acta 1253, 9–12 [DOI] [PubMed] [Google Scholar]
- 14).Yatsunami K., Tsuchikawa M., Kamada M. (1995) Comparative studies of human recombinant 74- and 54-kDa l-histidine decarboxylase. J. Biol. Chem. 270, 30813–30817 [DOI] [PubMed] [Google Scholar]
- 15).Safina F., Tanaka S., Inagaki M., Tsuboi K., Sugimoto Y., Ichikawa A. (2002) Expression of l-histidine decarboxylase in mouse male germ cells. J. Biol. Chem. 277, 14211–14215 [DOI] [PubMed] [Google Scholar]
- 16).Imanishi N., Ohmori E., Yatsunami K., Ichikawa A. (1988) Effect of hydrocortisone on histidine decarboxylase activity in rat stomach. Chem. Pharm. Bull. (Tokyo) 36, 4088–4094 [DOI] [PubMed] [Google Scholar]
- 17).Imanishi N., Nakayama T., Asano M., Yatsunami K., Tomita K., Ichikawa A. (1987) Induction of histidine decarboxylase by dexamethasone in mastocytoma P-815 cells. Biochim. Biophys. Acta 928, 227–234 [DOI] [PubMed] [Google Scholar]
- 18).Ohmori E., Imanishi N., Ohgoh M., Fukui T., Ichikawa A. (1991) Fluctuation of fetal rat hepatic histidine decarboxylase activity through the glucocorticoid–ACTH system. Biochem. Pharmacol. 41, 844–847 [DOI] [PubMed] [Google Scholar]
- 19).Kawai H., Ohgoh M., Emoto S., Ohmori E., Imanishi N., Yatsunami K., et al. (1992) Synergistic effects of 12-O-tetradecanoylphorbol-13-acetate and dexamethasone on de novo synthesis of histidine decarboxylase in mouse mastocytoma P-815 cells. Biochim. Biophys. Acta 1133, 172–178 [DOI] [PubMed] [Google Scholar]
- 20).Miyazaki T., Ohgoh M., Ohmori E., Yamamoto J., Emoto S., Yatsunami K., et al. (1992) Synergistic effects of cyclic AMP and Ca2+ ionophore A23187 on de novo synthesis of histidine decarboxylase in mastocytoma P-815 cells. Biochim. Biophys. Acta 1133, 179–186 [DOI] [PubMed] [Google Scholar]
- 21).Ohgoh M., Yamamoto J., Kawata M., Yamamura I., Fukui T., Ichikawa A. (1993) Enhanced expression of the mouse l-histidine decarboxylase gene with a combination of dexamethasone and 12-O-tetradecanoylphorbol-13-acetate. Biochem. Biophys. Res. Commun. 196, 1113–1119 [DOI] [PubMed] [Google Scholar]
- 22).Ohtsu H., Kuramasu A., Suzuki S., Igarashi K., Ohuchi Y., Sato M., et al. (1996) Histidine decarboxylase expression in mouse mast cell line P815 is induced by mouse peritoneal cavity incubation. J. Biol. Chem. 271, 28439–28444 [DOI] [PubMed] [Google Scholar]
- 23).Ai W., Liu Y., Langlois M., Wang T.C. (2004) Kruppel-like factor 4 (KLF4) represses histidine decarboxylase gene expression through an upstream Sp1 site and downstream gastrin responsive elements. J. Biol. Chem. 279, 8684–8693 [DOI] [PubMed] [Google Scholar]
- 24).Yatsunami K., Ohtsu H., Tsuchikawa M., Higuchi T., Ishibashi K., Shida A., et al. (1994) Structure of the l-histidine decarboxylase gene. J. Biol. Chem. 269, 1554–1559 [PubMed] [Google Scholar]
- 25).Nakagawa S., Okaya Y., Yatsunami K., Tanaka S., Ohtsu H., Fukui T., et al. (1997) Identification of multiple regulatory elements of human l-histidine decarboxylase gene. J. Biochem. 121, 935–940 [DOI] [PubMed] [Google Scholar]
- 26).Kuramasu A., Saito H., Suzuki S., Watanabe T., Ohtsu H. (1998) Mast cell-/basophil-specific regulation of human l-histidine decarboxylase gene by CpG methylation in the promoter region. J. Biol. Chem. 273, 31607–31614 [DOI] [PubMed] [Google Scholar]
- 27).Suzuki-Ishigaki S., Numayama-Tsuruta K., Kuramasu A., Sakurai E., Makabe Y., Shimura S., et al. (2000) The mouse l-histidine decarboxylase gene: structure and transcriptional regulation by CpG methylation in the promoter region. Nucleic Acids Res. 28, 2627–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Tanaka S., Nemoto K., Yamamura E., Ohmura S., Ichikawa A. (1997) Degradation of the 74 kDa form of l-histidine decarboxylase via the ubiquitin–proteasome pathway in a rat basophilic/mast cell line (RBL-2H3). FEBS Lett. 417, 203–207 [DOI] [PubMed] [Google Scholar]
- 29).Tanaka S., Nemoto K., Yamamura E., Ichikawa A. (1998) Intracellular localization of the 74- and 53-kDa forms of l-histidine decarboxylase in a rat basophilic/mast cell line, RBL-2H3. J. Biol. Chem. 273, 8177–8182 [DOI] [PubMed] [Google Scholar]
- 30).Tanaka S., Deai K., Konomi A., Takahashi K., Yamane H., Sugimoto Y., et al. (2004) Expression of l-histidine decarboxylase in granules of elicited mouse polymorphonuclear leukocytes. Eur. J. Immunol. 34, 1472–1482 [DOI] [PubMed] [Google Scholar]
- 31).Suzuki S., Tanaka S., Nemoto K., Ichikawa A. (1998) Membrane targeting and binding of the 74-kDa form of mouse l-histidine decarboxylase via its carboxyl-terminal sequence. FEBS Lett. 437, 44–48 [DOI] [PubMed] [Google Scholar]
- 32).Furuta K., Nakayama K., Sugimoto Y., Ichikawa A., Tanaka S. (2007) Activation of histidine decarboxylase through post-translational cleavage by caspase-9 in a mouse mastocytoma P-815. J. Biol. Chem. 282, 13438–13446 [DOI] [PubMed] [Google Scholar]
- 33).Jutel M., Akdis M., Akdis C.A. (2009) Histamine, hisatmine receptors and their role in immune pathology. Clin. Exp. Allergy 39, 1786–1800 [DOI] [PubMed] [Google Scholar]
- 34).Takahashi K., Tanaka S., Ichikawa A. (2001) Effect of cimetidine on intratumoral cytokine expression in an experimental tumor. Biochem. Biophys. Res. Commun. 281, 1113–1119 [DOI] [PubMed] [Google Scholar]
- 35).Takahashi K., Tanaka S., Furuta K., Ichikawa A. (2002) Histamine H2 receptor-mediated modulation of local cytokine expression in a mouse experimental tumor model. Biochem. Biophys. Res. Commun. 297, 1205–1210 [DOI] [PubMed] [Google Scholar]
- 36).Jutel M., Watanabe T., Klunker S., Akdis M., Thomot O.A.R., Malolepszy J., et al. (2001) Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413, 420–425 [DOI] [PubMed] [Google Scholar]
- 37).Tanaka S., Takasu Y., Mikura S., Satoh N., Ichikawa A. (2002) Antigen-independent induction of histamine synthesis by immunoglobulin E in mouse bone marrow-derived mast cells. J. Exp. Med. 196, 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Tanaka S., Mikura S., Hashimoto E., Sugimoto Y., Ichikawa A. (2005) Ca2+ influx-mediated histamine synthesis and IL-6 release in mast cells activated by monomeric IgE. Eur. J. Immunol. 35, 460–468 [DOI] [PubMed] [Google Scholar]
- 39).Liu Y., Furuta K., Teshima R., Shirata N., Sugimoto Y., Ichikawa A., et al. (2005) Critical role of PKCβII in activation of mast cells by monomeric IgE. J. Biol. Chem. 280, 38976–38981 [DOI] [PubMed] [Google Scholar]
- 40).Ohtsu H., Tanaka S., Terui T., Hori Y., Makabe-Kobayashi Y., Pejler G., et al. (2001) Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 502, 53–56 [DOI] [PubMed] [Google Scholar]
- 41).Ohtsu H., Kuramasu A., Tanaka S., Terui T., Hirasawa N., Hara M., et al. (2002) Plasma extravasation induced by dietary supplemented histamine in histamine-free mice. Eur. J. Immunol. 32, 1698–1708 [DOI] [PubMed] [Google Scholar]
- 42).Tanaka S., Deai K., Inagaki M., Ichikawa A. (2003) Uptake of histamine by mouse peritoneal macrophages and a macrophage cell line, RAW264.7. Am. J. Physiol. Cell Physiol. 285, C592–C598 [DOI] [PubMed] [Google Scholar]
- 43).Makabe-Kobayashi Y., Hori Y., Adachi T., Ishigaki-Suzuki S., Kikuchi Y., Kagaya Y., et al. (2002) The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 110, 298–303 [DOI] [PubMed] [Google Scholar]
- 44).Tanaka S., Hamada K., Yamada N., Sugita Y., Tonai S., Hunyady B., et al. (2002) Gastric acid secretion in l-histidine decarboxylase-deficient mice. Gastroenterology 122, 145–155 [DOI] [PubMed] [Google Scholar]
- 45).Kobayashi T., Tonai S., Ishihara Y., Koga R., Okabe S., Watanabe T. (2000) Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J. Clin. Invest. 105, 1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Beghdadi W., Porcherie A., Schneider B.S., Dubayle D., Peronet R., Huerre M., et al. (2008) Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J. Exp. Med. 205, 395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Musio S., Gallo B., Scabeni S., Lapilla M., Poliani P.L., Matarese G., et al. (2006) A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J. Immunol. 176, 17–26 [DOI] [PubMed] [Google Scholar]
- 48).Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., et al. (1991) Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 349, 617–620 [DOI] [PubMed] [Google Scholar]
- 49).Sugimoto Y., Namba T., Honda A., Hayashi Y., Negishi M., Ichikawa A., et al. (1992) Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J. Biol. Chem. 267, 6463–6466 [PubMed] [Google Scholar]
- 50).Watabe A., Sugimoto Y., Honda A., Irie A., Namba T., Negishi M., et al. (1993) Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J. Biol. Chem. 268, 20175–20178 [PubMed] [Google Scholar]
- 51).Honda A., Sugimoto Y., Namba T., Watabe A., Irie A., Negishi M., et al. (1993) Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J. Biol. Chem. 268, 7759–7762 [PubMed] [Google Scholar]
- 52).Katsuyama M., Nishigaki N., Sugimoto Y., Morimoto K., Negishi M., Narumiya S., et al. (1995) The mouse prostaglandin E receptor EP2 subtype: cloning, expression, and Northern blot analysis. FEBS Lett. 372, 151–156 [DOI] [PubMed] [Google Scholar]
- 53).Namba T., Oida H., Sugimoto Y., Kakizuka A., Negishi M., Ichikawa A., et al. (1994) cDNA cloning of a mouse prostacyclin receptor; multiple signaling pathways and expression in thymic medulla. J. Biol. Chem. 269, 9986–9992 [PubMed] [Google Scholar]
- 54).Sugimoto Y., Hasumoto K., Namba T., Irie A., Katsuyama M., Negishi M., et al. (1994) Cloning and expression of a cDNA for mouse prostaglandin F receptor. J. Biol. Chem. 269, 1356–1360 [PubMed] [Google Scholar]
- 55).Narumiya S., Sugimoto Y., Ushikubi F. (1999) Prostanoid receptors; structures, properties and functions. Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 56).Sugimoto Y., Narumiya S. (2007) Prostaglandin E receptors. J. Biol. Chem. 282, 11613–11617 [DOI] [PubMed] [Google Scholar]
- 57).Katoh H., Watabe A., Sugimoto Y., Ichikawa A., Negishi M. (1995) Characterization of the signal transduction of prostaglandin E receptor EP1 subtype in cDNA-transfected Chinese hamster ovary cells. Biochim. Biophys. Acta 1244, 41–48 [DOI] [PubMed] [Google Scholar]
- 58).Tabata H., Tanaka S., Sugimoto Y., Kanki H., Kaneko S., Ichikawa A. (2002) Possible coupling of prostaglandin E receptor EP1 to TRP5 expressed in X. laevis oocytes. Biochem. Biophys. Res. Commun. 298, 398–402 [DOI] [PubMed] [Google Scholar]
- 59).Hatae N., Yamaoka K., Sugimoto Y., Negishi M., Ichikawa A. (2002) Augmentation of receptor-mediated adenylyl cyclase activity by Gi-coupled prostaglandin receptor subtype EP3 in a Gβγ subunit-independent manner. Biochem. Biophys. Res. Commun. 290, 162–168 [DOI] [PubMed] [Google Scholar]
- 60).Yamaoka K., Yano A., Kuroiwa K., Morimoto K., Inazumi T., Hatae N., et al. (2009) Prostaglandin EP3 receptor superactivates adenylyl cyclase via the Gq/PLC/Ca2+ pathway in a lipid raft-dependent manner. Biochem. Biophys. Res. Commun. 389, 678–682 [DOI] [PubMed] [Google Scholar]
- 61).Nishigaki N., Negishi M., Ichikawa A. (1996) Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol. Pharmacol. 50, 1031–1037 [PubMed] [Google Scholar]
- 62).Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., et al. (2009) Prostaglandin E2-EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat. Med. 15, 633–640 [DOI] [PubMed] [Google Scholar]
- 63).Hizaki H., Segi E., Sugimoto Y., Hirose M., Ushikubi F., Matsuoka T., et al. (1999) Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP2. Proc. Natl. Acad. Sci. USA 96, 10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Segi E., Sugimoto Y., Yamasaki A., Aze Y., Oida H., Nishimura T., et al. (1998) Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem. Biophys. Res. Commun. 246, 7–12 [DOI] [PubMed] [Google Scholar]
- 65).Fujino H., Xu W., Regan J.W. (2003) Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J. Biol. Chem. 278, 12151–12156 [DOI] [PubMed] [Google Scholar]
- 66).Buchanan F.G., Gorden D.L., Matta P., Shi Q., Matrisian L.M., DuBois R.N. (2006) Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc. Natl. Acad. Sci. USA 103, 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Chun K.S., Lao H.C., Trempus C.S., Okada M., Langenbach R. (2009) The prostaglandin receptor EP2 activates multiple signaling pathways and β-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis 30, 1620–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Sugimoto Y., Negishi M., Hayashi Y., Namba T., Honda A., Watabe A., et al. (1993) Two isoforms of EP3 receptor with different C-terminal domains; Identical ligand binding properties and different coupling properties with Gi proteins. J. Biol. Chem. 268, 2712–2718 [PubMed] [Google Scholar]
- 69).Irie A., Sugimoto Y., Namba A., Harazono A., Honda A., Watabe A., et al. (1993) Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur. J. Biochem. 217, 313–318 [DOI] [PubMed] [Google Scholar]
- 70).Namba T., Sugimoto Y., Negishi M., Irie A., Ushikubi F., Kakizuka A., et al. (1993) Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 365, 166–170 [DOI] [PubMed] [Google Scholar]
- 71).Kotani M., Tanaka I., Ogawa Y., Usui T., Tamura N., Mori K., et al. (1997) Structural organization of the human prostaglandin EP3 receptor subtype gene (PTGER3). Genomics 40, 425–434 [DOI] [PubMed] [Google Scholar]
- 72).Negishi M., Sugimoto Y., Irie A., Narumiya S., Ichikawa A. (1993) Two isoforms of prostaglandin E receptor EP3 subtype; Different C-terminal domains determine sensitivity to agonist-induced desensitization. J. Biol. Chem. 268, 9517–9521 [PubMed] [Google Scholar]
- 73).Ichikawa A., Negishi M., Hasegawa H. (1997) Three isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive Gi activity and agonist-dependent Gs activity. Adv. Exp. Med. Biol. 433, 239–242 [DOI] [PubMed] [Google Scholar]
- 74).Sugimoto Y., Narumiya S., Ichikawa A. (2000) Distribution and function of prostanoid receptors: studies from knockout mice. Prog. Lipid Res. 39, 289–314 [DOI] [PubMed] [Google Scholar]
- 75).Sugimoto Y., Namba T., Shigemoto R., Negishi M., Ichikawa A., Narumiya S. (1994) Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am. J. Physiol. 266, F823–F828 [DOI] [PubMed] [Google Scholar]
- 76).Sugimoto Y., Shigemoto R., Namba T., Negishi M., Mizuno M., Narumiya S., et al. (1994) Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience 62, 919–928 [DOI] [PubMed] [Google Scholar]
- 77).Zhang J., Rivest S. (1999) Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur. J. Neurosci. 11, 2651–2668 [DOI] [PubMed] [Google Scholar]
- 78).Oida H., Namba T., Sugimoto Y., Ushikubi F., Ohishi H., Ichikawa A., et al. (1995) In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br. J. Pharmacol. 116, 2828–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Katsuyama M., Ikegami R., Karahashi H., Amano F., Sugimoto Y., Ichikawa A. (1998) Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem. Biophys. Res. Commun. 251, 727–731 [DOI] [PubMed] [Google Scholar]
- 80).Ikegami R., Sugimoto Y., Segi E., Katsuyama M., Karahashi H., Amano F., et al. (2001) The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by LPS in C3H/HeN peritoneal macrophages. J. Immunol. 166, 4689–4696 [DOI] [PubMed] [Google Scholar]
- 81).Katsuyama M., Sugimoto Y., Morimoto K., Hasumoto Y., Fukumoto M., Negishi M., et al. (1997) Distinct cellular localization of the messenger ribonucleic acid for prostaglandin E receptor subtypes in the mouse uterus during pseudopregnancy. Endocrinology 138, 344–350 [DOI] [PubMed] [Google Scholar]
- 82).Segi E., Haraguchi K., Sugimoto Y., Tsuji M., Tsunekawa H., Tamba S., et al. (2003) Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol. Reprod. 68, 804–811 [DOI] [PubMed] [Google Scholar]
- 83).Tsuchiya S., Tanaka S., Sugimoto Y., Katsuyama M., Ikegami R., Ichikawa A. (2003) Identification and characterization of a novel progesterone receptor-binding element in the mouse prostaglandin E receptor subtype EP2 gene. Genes Cells 8, 747–758 [DOI] [PubMed] [Google Scholar]
- 84).Sugimoto Y., Yamasaki A., Segi E., Tsuboi K., Aze Y., Nishimura T., et al. (1997) Failure of parturition in mice lacking the prostaglandin F receptor. Science 277, 681–683 [DOI] [PubMed] [Google Scholar]
- 85).Ushikubi F., Segi E., Sugimoto Y., Murata T., Matsuoka T., Kobayashi T., et al. (1998) Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395, 281–284 [DOI] [PubMed] [Google Scholar]
- 86).Matsuoka T., Narumiya S. (2008) The roles of prostanoids in infection and sickness behaviors. J. Infect. Chemother. 14, 270–278 [DOI] [PubMed] [Google Scholar]
- 87).Narumiya S. (2009) Prostanoids and inflammation: a new concept arising from receptor knockout mice. J. Mol. Med. 87, 1015–1022 [DOI] [PubMed] [Google Scholar]
- 88).Davis B.J., Lennard D.E., Lee C.A., Tiano H.F., Morham S.G., Wetsel W.C., et al. (1999) Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140, 2685–2695 [DOI] [PubMed] [Google Scholar]
- 89).Murdoch W.J., Hansen T.R., Mcpherson L.A. (1993) Role of eicosanoids in vertebrate ovulation. Prostaglandins 46, 85–115 [DOI] [PubMed] [Google Scholar]
- 90).Lim H., Paria B.C., Das S.K., Dinchuck J.E., Langenbach R., Trzaskos J.M., et al. (1997) Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208 [DOI] [PubMed] [Google Scholar]
- 91).Tamba S., Yodoi R., Segi-Nishida E., Ichikawa A., Narumiya S., Sugimoto Y. (2008) Timely interaction between prostaglandin and chemokine signaling is a prerequisite for successful fertilization. Proc. Natl. Acad. Sci. USA 105, 14539–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Yodoi R., Tamba S., Morimoto K., Segi-Nishida E., Nishihara M., Ichikawa A., et al. (2009) RhoA/Rho kinase signaling in the cumulus mediates extracellular matrix assembly. Endocrinology 150, 3345–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Milton A.S., Wendlandt S. (1970) A possible role for prostaglandin E1 as a modulator for temperature regulation in the central nervous system of the cat. J. Physiol. 207, 76P–77P [PubMed] [Google Scholar]
- 94).Stitt J.T. (1986) Prostaglandin E as the neural mediator of the febrile response. Yale J. Biol. Med. 59, 137–149 [PMC free article] [PubMed] [Google Scholar]
- 95).Mitchell D., Laburn H.P., Cooper K.E., Hellon R.F., Cranston W.I., Townsend Y. (1986) Is prostaglandin E the neural mediator of the febrile response? The case against a proven obligatory role. Yale J. Biol. Med. 59, 159–168 [PMC free article] [PubMed] [Google Scholar]
- 96).Leibbrandt A., Penninger J.M. (2008) RANK/RANKL: regulators of immune responses and bone physiology. Ann. N. Y. Acad. Sci. 1143, 123–150 [DOI] [PubMed] [Google Scholar]
- 97).Hanada R., Leibbrandt A., Hanada T., Kitaoka S., Furuyashiki T., Fujihara H., et al. (2009) Central control of fever and female body temperature by RANKL/RANK. Nature 462, 505–509 [DOI] [PubMed] [Google Scholar]
- 98).Nakamura K., Kaneko T., Yamashita Y., Hasegawa H., Katoh H., Ichikawa A., et al. (1999) Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci. Lett. 260, 117–120 [DOI] [PubMed] [Google Scholar]
- 99).Lazarus M., Yoshida K., Coppari R., Bass C.E., Mochizuki T., Lowell B.B., et al. (2007) EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 10, 1131–1133 [DOI] [PubMed] [Google Scholar]
- 100).Nakamura K., Matsumura K., Kaneko T., Kobayashi S., Katoh H., Negishi M. (2002) The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 22, 4600–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Tsuchiya H., Oka T., Nakamura K., Ichikawa A., Saper C.B., Sugimoto Y. (2008) Prostaglandin E2 attenuates preoptic expression of GABAA receptors via EP3 receptors. J. Biol. Chem. 283, 11064–11071 [DOI] [PubMed] [Google Scholar]
- 102).Osborne P.G., Onoe H., Watanabe Y. (1994) GABAergic system inducing hyperthermia in the rat preoptic area: its independence of prostaglandin E2 system. Brain Res. 661, 237–242 [DOI] [PubMed] [Google Scholar]
- 103).Osaka T. (2004) Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R306–R313 [DOI] [PubMed] [Google Scholar]
- 104).Gemkow M.J., Davenport A.J., Harich S., Ellenbroek B.A., Cesura A., Hallett D. (2009) The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov. Today 14, 509–515 [DOI] [PubMed] [Google Scholar]
- 105).Thurmond R.L., Gelfand E.W., Dunford P.J. (2008) The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat. Rev. Drug Discov. 7, 41–53 [DOI] [PubMed] [Google Scholar]
- 106).Jones R.L., Giembycz M.A., Woodward D.F. (2009) Prostanoid receptor antagonists: development strategies and therapeutic applications. Br. J. Pharmacol. 158, 104–145 [DOI] [PMC free article] [PubMed] [Google Scholar]