Abstract
Nuclear export of mRNPs is mediated by transport factors such as NXF1 that bind mRNPs and mediate their translocation through the central channel of nuclear pores (NPC) using transient interactions with FG-nucleoporins. A number of nuclear factors enhance the efficiency of this process by concentrating mRNPs at the nuclear face of the pores. Although this enhancement has been explored mainly with the yeast TREX-2 complex, recent work has indicated that mammalian cells employ GANP (Germinal-centre Associated Nuclear Protein) for efficient mRNP nuclear export and for efficient recruitment of NXF1-containing mRNPs to NPCs. GANP is constructed from several domains that show local homology to FG-nucleoporins, the yeast mRNA export factor Sac3p and the mammalian MCM3 acetyltransferase. Whereas yeast TREX-2 is located primarily at nuclear pores, some GANP is located in the nuclear interior in addition to that found at the pores. GANP depletion inhibits bulk mRNA export, resulting in retention of mRNPs and NXF1 in punctate foci within the nucleoplasm, consistent with GANP's being an integral component of the mammalian mRNA export machinery. Here, we discuss the model for GANP function presented in our recent paper and its implications for the mechanism of mRNA export in mammalian cells.
Key words: GANP, mRNA export, nucleoporins
Introduction
Export of mRNA through nuclear pores (NPCs) is mediated by transport factors that bind both mRNPs and NPC proteins that contain characteristic FG sequence repeats (FG-nucleoporins). A conserved heterodimeric mRNA exporter, NXF1-p15, (Mex67p-Mtr2p in yeast) binds to mRNPs via adaptor proteins and facilitates translocation through the central channel of NPC's through transient interactions with nucleoporin FG repeats.1–8 These adaptor proteins include ALY (Yra1 in yeast)7,9,10 and the SR splicing factors,11 which can bind directly to both RNA and NXF1 and couple the splicing and export components of the gene expression machinery.5,12
Although the mRNA export machinery has been investigated extensively in yeast and is partly conserved in mammalian cells, there are important functional differences between the two divergent kingdoms. For example, the TREX (Transcription-Export) complex plays a central role in the transport of mRNA from the nucleus to the cytoplasm and is highly conserved between yeast and mammals. It is composed of ALY (Yra1 in yeast), UAP56 (Sub2 in yeast) and the multi-subunit THO complex.13 Recent work has indicated that human TREX is recruited to the 5′ end of mRNA.14 However, TREX is recruited by the transcription machinery in yeast15 whereas it is recruited by the splicing machinery in mammals.16 This fundamental difference may be due to the presence of introns in the majority of metazoan genes but only a minority of yeast genes. Furthermore, although Yra1 is essential for mRNA export in yeast,17 the metazoan homolog ALY, along with additional components of the exon junction complex, is dispensable for nuclear mRNA export.18 This suggests that additional adaptor proteins mediate the interaction between NXF1 and cellular mRNAs in metazoa.18
In yeast, Sac3 is a central component of the TREX-2 complex that facilitates gene gating, whereby actively-transcribing genes such as GAL1 are tethered to the nuclear face of NPC's.5,19–22 Gene gating facilitates integration of transcription, processing and mRNA nuclear export.19 It is not yet clear how similar mammalian cells are to yeast in this regard.23 Metazoan transcription and maturation of the majority of mRNPs occurs primarily in or around transcription factories, located deeper within the nucleoplasm24 that are distant from NPCs. Indeed, examination of the dynamics of single mRNPs in human U20S cells indicates that they accumulate in a particulate pattern in the nucleoplasm similar to that seen with the transcription factories.25,26 Importantly, the nuclear distribution of specific mRNPs is not influenced by the relative position of the transcription site in relation to the nuclear envelope.25,26 These different mechanisms for transcription, splicing and export of mRNPs could be correlates of the increased nuclear size and genome complexity of metazoans.
GANP Functions in mRNA Export in Mammalian Cells
GANP (Germinal-center Associated Nuclear Protein) is a 210 kDa protein that is upregulated in germinal-centre B cells27 and also in a variety of lymphomas.28 GANP has been proposed to have a role in the immune response through generation of antigen-specific and high-affinity B cells during maturation in germinal-centres.29 GANP has also been reported to suppress DNA recombination.30 However, these observations do not explain why GANP is expressed in essentially all mammalian cells. Furthermore, because GANP also contains a region identical to MCM3AP (MCM3 acetylating protein), that interacts with and weakly acetylates MCM3,31,32 these proteins have often been confused in databases and the literature. The nucleotide sequence for MCM3AP is completely contained in the 3′ region of GANP, suggesting that MCM3AP is a splice variant33 and, furthermore, MCM3AP can be transcribed independently of GANP. Consequently, they should be referred to as independent, overlapping genes and not described interchangeably as at present in databases.
Recent work has identified GANP as an integral component of the mammalian bulk mRNA export machinery.34 Separate domains of GANP show local homology to FG-nucleoporins, the yeast mRNA export factor Sac3p and the mammalian MCM3 acetyltransferase. GANP therefore combines features from different components of the mRNA export machinery and NPCs, and so has the potential to coordinate key steps in the gene expression pathway. In support of this hypothesis, GANP is localized predominantly to the nuclear face of the nuclear envelope, albeit combined with weaker staining of the nuclear interior.34 Although it is not known precisely how GANP is targeted to the nuclear periphery, in yeast the CID motif (to which Sus1 and Cdc31 bind) targets Sac3 to the NPC.20,35 The CID motif appears to be conserved in GANP,20 suggesting it could have an analogous role for targeting GANP to NPCs. Also, the TREX-2 complex may be conserved in mammals, as Centrin-2, the human homolog of Cdc31, functions in mRNA export in vertebrates.36
GANP depletion inhibits mammalian mRNA export and causes nuclear accumulation of poly(A) + RNA with a concomitant decrease in levels in the cytoplasm.34 Poly(A) + RNA does not accumulate at the nuclear envelope of GANP depleted cells, but instead accumulates in a distinct punctate focal pattern throughout the nucleus, excluding nucleoli. A similar pattern of poly(A) + RNA localization is observed in cells depleted of NXF1, the major mRNA export factor in mammalian cells5,7 and resembles that seen for RNA polymerase II,37 suggesting that GANP depletion results in an accumulation of nuclear mRNP complexes localized at, or near, sites of transcription. Moreover, GANP is associated with nuclear mRNPs and inhibiting transcription causes GANP to accumulate in foci throughout the nucleus, with a corresponding decrease in levels at NPCs.34 Taken together, these findings suggest that GANP is mobile and that it shuttles between transcription factories and NPC's.
How might GANP facilitate efficient mRNA export in mammalian cells? NXF1 is considered to be the major exporter of bulk mRNA in metazoans5,7 and has a similar depletion phenotype to GANP. Yeast Sac3, which is homologous to a local region of GANP, interacts in vitro with Mex67, the yeast homologue of NXF1, although the molecular basis of this interaction has not been established.21 GANP interacts directly with NXF1 through its N-terminal fragment that contains FG motifs and overexpression of this fragment causes nuclear accumulation of both NXF1 and mRNA.34
GANP and NXF1 share several key features. Both associate with nuclear mRNPs, and both interact with NPCs. Furthermore, depletion of either protein from human cells results in retention of mRNPs in nuclear foci and not at NPCs.34 However, NXF1 localizes predominantly in nuclear foci, with less staining at NPCs, whereas GANP localizes predominantly at NPC's, with a corresponding decrease in the nuclear interior.34 Also, GANP interacts directly with the C-terminal domain of NXF1,34 that interacts with FG-repeat nucleoporins.2,3,6,38,39 Taken together, these results suggest that GANP may not fit the classical definition of an adaptor protein that recruits NXF1 to mRNPs. Adaptor proteins such as the SR proteins and ALY are localized predominantly in nuclear speckles and not at NPC's,10,40 in contrast to GANP and NXF1. Furthermore, the SR proteins and ALY bind to the N-terminal domains of NXF1,9,11 not the C-terminal NTF2-like and UBA-like domains that interact with FG-repeat nucleoporins and GANP. The in vivo localisation and interaction data favor GANP functioning in a later step in the export pathway, namely recruiting NXF1-containing mRNPs in the nuclear interior and delivering them to the NPC.
GANP contains a C-terminal domain that is identical to MCM3AP (MCM3 acetylating protein), that interacts with and acetylates MCM3, albeit weakly, or histones even more weakly.31,32 As GANP contains the whole of MCM3AP within its sequence, it is possible that GANP might also possess acetyltransferase activity, though this has not been detected to date. It remains possible that acetylation might be a mechanism used during mRNA export to convert mRNPs from an export-inactive state to an export-active one or vice versa. Indeed, it has been shown that acetylation itself can result in functional chromatin reorganisation at mammalian nuclear pore complexes.23 Furthermore, the SAGA transcription activation complex, of which histone acetyltransferase Gcn5 is a member, generates a number of histone modifications including acetylation.41 Along with its role in transcription activation, SAGA is also required for mRNA export through its component Sus1/ENY2.42,43 Therefore acetylation as a mechanism for regulating mRNA export deserves further examination.
Does GANP have an Active Role in Recruitment of mRNPs in the Nucleoplasm for Transport to NPCs?
These observations have lead us to develop a model34 to account for the function of GANP in mammalian mRNA export, based on the hypothesis that GANP is recruited to mature mRNPs after NXF1 is attached, and functions to enhance delivery of mRNPs to NPCs (Fig. 1). Although GANP contains six putative FG repeats, many human nucleoporins such as Nup153 and 214 contain clusters of more than 20 such repeats. As GANP interacts with the same domain of NXF1 that binds to FG-nucleoporins, we proposed34 that when the mRNPs containing GANP and NXF1 reach NPCs, the higher concentration of FG motifs in FG-nucleoporins displaces GANP from NXF1 in the mRNP, freeing it to pass through the pores, mediated by transient interactions between NXF1 and the FG repeats that line the transport channel.
Figure 1.
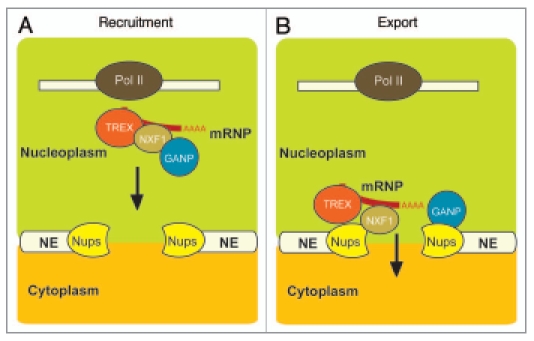
Model for GANP function in mammalian mRNA export. Following transcription and splicing in foci located deep in the nucleoplasm, it is hypothesized that GANP binds to mature mRNPs via NXF1 (A). Once GANP is bound to mRNP's, it functions as a courier to expedite delivery of mRNPs to the nuclear envelope, where the higher concentration of FG repeats in nucleoporins displaces GANP from mRNP's (B), enabling export through NPC's to the cytoplasm.
Although GANP contains a local region that is homologous to yeast Sac3, which is also involved in a late step of mRNP nuclear export, the results obtained with GANP indicate there are crucial differences in the way the two proteins function in mRNA export. In yeast, one function of Sac3 is to tether selected actively-transcribing genes such as GAL1 to NPCs in a process known as gene gating.5,19–22 In contrast, the transcription and maturation of the majority of metazoan mRNPs occur deeper within the nucleoplasm,24 in or around transcription factories that are distant from NPCs. Thus, in contrast to yeast Sac3, which is tethered to the nuclear face of NPCs, GANP is dynamic, moving between the inner face of the nuclear envelope and nuclear interior. The requirement for GANP to facilitate transport of mRNPs through the mammalian nucleus to NPCs is possibly a consequence of the increased nuclear size and genome complexity in metazoans.
Interestingly, two recent studies in Drosophila have suggested that nucleoporins such as Nups 98, 50 and 62 can associate with chromatin in the nucleoplasm to stimulate expression of developmentally regulated genes.44,45 These studies indicated that the subset of metazoan nucleoporins bound to chromatin predominantly associate with active genes in the nucleoplasm. Therefore, in addition to their integral role in nuclear transport, nucleoporins also play a role in gene expression in the nucleoplasm. The authors of these papers have speculated that the mobility of NPC components may establish a mechanism of communication between sites of production of mRNA and sites of its final exit.44 Although GANP itself is not classified as a “nucleoporin” (primarily because GANP was not identified in a large-scale screen for mammalian NPC components46), it possesses many similarities to these proteins, such as an N-terminal domain that contains extensive homology to nucleoporins over and above the FG repeats that it contains.34 Overall, the data obtained to date suggest that GANP functions to establish a mechanism of communication between sites of mRNA production and their export. Thus, recent work34 has established a role for GANP distinct from those proposed previously,27–30,33,47 which focussed on cells of the immune system in which GANP was discovered. Importantly, GANP is upregulated in a variety of lymphomas28 and so the newly identified association of GANP with mRNA export in mammalian cells has broad implications for understanding its possible role in cancer progression.
Acknowledgements
This work was funded by the Medical Research Council (MRC) Cancer Research UK and the Wellcome Trust.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/12351
References
- 1.Herold A, Suyama M, Rodrigues JP, Braun IC, Kutay U, Carmo-Fonseca M, et al. TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol Cell Biol. 2000;20:8996–9008. doi: 10.1128/mcb.20.23.8996-9008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun IC, Herold A, Rode M, Izaurralde E. Nuclear export of mRNA by TAP/NXF1 requires two nucleoporin-binding sites but not p15. Mol Cell Biol. 2002;22:5405–5418. doi: 10.1128/MCB.22.15.5405-5418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RP, Hurt E, Neuhaus D, Stewart M. Structure of the C-terminal FG-nucleoporin binding domain of Tap/NXF1. Nat Struct Biol. 2002;9:247–251. doi: 10.1038/nsb773. [DOI] [PubMed] [Google Scholar]
- 4.Grant RP, Neuhaus D, Stewart M. Structural basis for the interaction between the Tap/NXF1 UBA domain and FG nucleoporins at 1 A resolution. J Mol Biol. 2003;326:849–858. doi: 10.1016/s0022-2836(02)01474-2. [DOI] [PubMed] [Google Scholar]
- 5.Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 6.Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung JU, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 1999;18:2593–2609. doi: 10.1093/emboj/18.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stutz F, Izaurralde E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 2003;13:319–327. doi: 10.1016/s0962-8924(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 8.Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, et al. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA. 2000;6:638–650. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407:401–405. doi: 10.1038/35030160. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Gattoni R, Stevenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell. 2003;11:837–843. doi: 10.1016/s1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 12.Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, et al. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature. 2001;413:644–647. doi: 10.1038/35098106. [DOI] [PubMed] [Google Scholar]
- 13.Reed R, Cheng H. TREX, SR proteins and export of mRNA. Curr Opin Cell Biol. 2005;17:269–273. doi: 10.1016/j.ceb.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell. 2006;127:1389–1400. doi: 10.1016/j.cell.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 16.Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 2005;19:1512–1517. doi: 10.1101/gad.1302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 2000;19:410–420. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatfield D, Izaurralde E. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol. 2002;159:579–588. doi: 10.1083/jcb.200207128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci USA. 1985;82:8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jani D, Lutz S, Marshall NJ, Fischer T, Kohler A, Ellisdon AM, et al. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol Cell. 2009;33:727–737. doi: 10.1016/j.molcel.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer T, Strasser K, Racz A, Rodriguez-Navarro S, Oppizzi M, Ihrig P, et al. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 2002;21:5843–5852. doi: 10.1093/emboj/cdf590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- 23.Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 2008;22:627–639. doi: 10.1101/gad.1632708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 25.Darzacq X, Singer RH, Shav-Tal Y. Dynamics of transcription and mRNA export. Curr Opin Cell Biol. 2005;17:332–339. doi: 10.1016/j.ceb.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, et al. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797–1800. doi: 10.1126/science.1099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwahara K, Yoshida M, Kondo E, Sakata A, Watanabe Y, Abe E, et al. A novel nuclear phosphoprotein, GANP, is upregulated in centrocytes of the germinal centre and associated with MCM3, a protein essential for DNA replication. Blood. 2000;95:2321–2328. [PubMed] [Google Scholar]
- 28.Fujimura S, Xing Y, Takeya M, Yamashita Y, Ohshima K, Kuwahara K, et al. Increased expression of germinal center-associated nuclear protein RNA-primase is associated with lymphomagenesis. Cancer Res. 2005;65:5925–5934. doi: 10.1158/0008-5472.CAN-04-3259. [DOI] [PubMed] [Google Scholar]
- 29.Kuwahara K, Fujimura S, Takahashi Y, Nakagata N, Takemori T, Aizawa S, et al. Germinal center-associated nuclear protein contributes to affinity maturation of B cell antigen receptor in T cell-dependent responses. PNAS. 2004;101:1010–1015. doi: 10.1073/pnas.0307609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida M, Kuwahara K, Shimasaki T, Nakagata N, Matsuoka M, Sakaguchi N. GANP suppresses DNA recombination, measured by direct-repeat beta-galactosidase gene construct, but does not suppress the type of recombination applying to immunoglobulin genes in mammalian cells. Genes Cells. 2007;12:1205–1213. doi: 10.1111/j.1365-2443.2007.01119.x. [DOI] [PubMed] [Google Scholar]
- 31.Takei Y, Tsujimoto G. Identification of a Novel MCM3-associated Protein that Facilitates MCM3 Nuclear Localisation. J Biol Chem. 1998;273:22177–22180. doi: 10.1074/jbc.273.35.22177. [DOI] [PubMed] [Google Scholar]
- 32.Takei Y, Assenberg M, Tsujimoto G, Laskey R. The MCM3 acetylase MCM3AP inhibits initiation, but not elongation, of DNA replication via interaction with MCM3. J Biol Chem. 2002;277:43121–43125. doi: 10.1074/jbc.C200442200. [DOI] [PubMed] [Google Scholar]
- 33.Abe E, Kuwahara K, Yoshida M, Suzuki M, Terasaki H, Matsuo Y, et al. Structure, expression and chromosomal localisation of the human gene encoding a germinal centre-associated nuclear protein (GANP) that associates with MCM3 involved in the initiation of DNA replication. Gene. 2000;255:219–227. doi: 10.1016/s0378-1119(00)00336-x. [DOI] [PubMed] [Google Scholar]
- 34.Wickramasinghe VO, McMurtrie PI, Mills AD, Takei Y, Penrhyn-Lowe S, Amagase Y, et al. mRNA export from mammalian cell nuclei is dependent on GANP. Curr Biol. 2010;20:25–31. doi: 10.1016/j.cub.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer T, Rodriguez-Navarro S, Pereira G, Racz A, Schiebel E, Hurt E. Yeast centrin Cdc31 is linked to the nuclear mRNA export machinery. Nat Cell Biol. 2004;6:840–848. doi: 10.1038/ncb1163. [DOI] [PubMed] [Google Scholar]
- 36.Resendes KK, Rasala BA, Forbes DJ. Centrin 2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. Mol Cell Biol. 2008;28:1755–1769. doi: 10.1128/MCB.01697-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spector DL. Nuclear domains. J Cell Sci. 2001;114:2891–2893. doi: 10.1242/jcs.114.16.2891. [DOI] [PubMed] [Google Scholar]
- 38.Bachi A, Braun IC, Rodrigues JP, Pante N, Ribbeck K, von Kobbe C, et al. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA. 2000;6:136–158. doi: 10.1017/s1355838200991994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fribourg S, Braun IC, Izaurralde E, Conti E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol Cell. 2001;8:645–656. doi: 10.1016/s1097-2765(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 40.Custodio N, Carvalho C, Condado I, Antoniou M, Blencowe BJ, Carmo-Fonseca M. In vivo recruitment of exon junction complex proteins to transcription sites in mammalian cell nuclei. Rna. 2004;10:622–633. doi: 10.1261/rna.5258504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker SP, Grant PA. The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene. 2007;26:5329–5340. doi: 10.1038/sj.onc.1210603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurshakova MM, Krasnov AN, Kopytova DV, Shidlovskii YV, Nikolenko JV, Nabirochkina EN, et al. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J. 2007;26:4956–4965. doi: 10.1038/sj.emboj.7601901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, et al. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell. 2004;116:75–86. doi: 10.1016/s0092-8674(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 44.Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 140:372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell cycle genes inside the nucleoplasm. Cell. 140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuwahara K, Tomiyasu S, Fujimura S, Nomura K, Xing Y, Nishiyama N, et al. Germinal centre-associated nuclear protein (GANP) has a phosphorylation-dependent DNA-primase activity that is upregulated in germinal centre regions. Proc Natl Acad Sci USA. 2001;98:10279–10283. doi: 10.1073/pnas.181335698. [DOI] [PMC free article] [PubMed] [Google Scholar]


