Summary
Present elimination strategies are based on recommendations derived during the Global Malaria Eradication Program of the 1960s. However, many countries considering elimination nowadays have high intrinsic transmission potential and, without the support of a regional campaign, have to deal with the constant threat of imported cases of the disease, emphasising the need to revisit the strategies on which contemporary elimination programmes are based. To eliminate malaria, programmes need to concentrate on identification and elimination of foci of infections through both passive and active methods of case detection. This approach needs appropriate treatment of both clinical cases and asymptomatic infections, combined with targeted vector control. Draining of infectious pools entirely will not be sufficient since they could be replenished by imported malaria. Elimination will thus additionally need identification and treatment of incoming infections before they lead to transmission, or, more realistically, embarking on regional initiatives to dry up importation at its source.
This is the third in a Series of four papers about malaria elimination
Introduction
The Roll Back Malaria strategy of Scaling Up for Impact through universal coverage with effective interventions,1 supported by an increase in malaria funding,2 has achieved low rates of malaria transmission in some areas and consequently a much reduced disease burden.3,4 Some countries, including several with historically medium-to-high transmission, are nearing a state of controlled low-endemic malaria,5 and policy makers have a decision to make: accept low rates of malaria transmission with a strategy of consolidation of control6 or redirect activities with the aim to eliminate malaria.
During the Global Malaria Eradication Program (GMEP), WHO Expert Committee reports described specific activities of an elimination programme through its defined phases, and provided advice based on years of experience from field campaigns.7–10 Since the 1970s, when WHO shifted the short-term strategic aim to control11 and relegated eradication to a long-term goal, there has been little scientific inquiry into or strategic thought about the theory, goals, and best approaches for national elimination. At the same time, many countries considering elimination nowadays have higher intrinsic transmission potential than do those that eliminated malaria during the GMEP and have to plan to maintain elimination despite continual importation of infections. Accordingly, the decision to move from controlled low-endemic malaria to elimination needs politicians, policy makers, and programme managers to have an informed understanding of the operational requirements for a contemporary elimination strategy so that they can set realistic goals and timelines that are relevant to malaria epidemiology nowadays.
The decision to convert a malaria programme that has successfully achieved a high level of control, such that malaria is no longer a major public health problem, into an elimination programme is complex12,13 and should take into account technical, operational, and financial feasibility.14 There is a broad consensus about the strategies that are needed to achieve controlled low-endemic malaria, which are based on universal coverage with prevention and treatment measures—all of which have a strong evidence base from empirical trials, observational studies, and routine monitoring and evaluation.1,3,15–18 However, elimination cannot be achieved by doing more of the same; transition from sustaining control to elimination demands additional activities. In the third paper in this Series, we review the activities needed to achieve and maintain malaria elimination in areas that have already reduced transmission to very low rates by identification of the essential operational changes that have to accompany a switch in focus from burden reduction to interruption of transmission. In doing so, several important knowledge gaps are identified that, in some cases, makes it challenging to provide evidence-based guidance about how to eliminate malaria.
Key messages.
-
•
The most important operational difference between a control and an elimination programme is the concentration of activities to identify and attack foci of clinical and asymptomatic infections that perpetuate transmission
-
•
Detection and curing of the high proportion of infections needed to interrupt transmission requires a robust surveillance system that combines passive and active case detection methods with rapid response, with radical treatment and targeted vector control
-
•
Most malaria-endemic countries considering elimination should aim to prevent importation of infections through proactive case detection at the border, screening of high-risk migrants, and implementation of cross-border and regional initiatives that can reduce transmission at the source of migration
-
•
Because elimination has a known quantitative goal to end endemic transmission and reduce the number of locally acquired cases to less than a specific threshold, monitoring systems incorporating extremely sensitive laboratory techniques such as PCR, genotyping, and serology have to be put in place to track progress
-
•
Malaria elimination cannot be business as usual, but needs a systemic and new programmatic approach supported by political and financial commitment, ideally throughout an entire region of nations
Differences between control and elimination
The programmatic focus of a country seeking to control malaria as a public health problem involves the effective treatment of clinical malaria that is detected through passive surveillance integrated into the public health infrastructure and prevention of disease through high coverage with vector control measures. The main determinant of an elimination campaign is that, by contrast with a programme designed to maintain controlled low-endemic malaria, it seeks to interrupt endemic transmission and prevent its re-establishment. Prerequisites for either state include scaling up and maintaining high rates of effective coverage of control measures such as longlasting insecticide-treated nets or indoor residual spraying, or both; rapidly detecting, diagnosing, and treating malaria cases with effective drugs; and securing sufficient funding to sustain the broader control programme. Only after low rates of malaria transmission have been achieved (a community parasite prevalence of around 1% or less for Plasmodium falciparum5,13) can activities of an elimination programme substantially differ from a programme that consolidates control. The most important difference between acceptance of low-parasite prevalence and seeking to interrupt endemic transmission is the concentration of activities towards identification of residual transmission foci and intensification of efforts to eliminate the last few infections. Such an active campaign of case detection and response, coupled with directed vector control efforts, should root out not only clinical cases but also asymptomatic infections that potentially perpetuate transmission.19,20
The strategy of the GMEP was to eradicate malaria everywhere, and guarding against imported malaria was given only little attention.21 Nowadays, however, as individual countries consider elimination, without accompanying reductions by their neighbours, this strategic reorientation from general scale-up of control measures to focused case detection and intervention has to be accompanied by the development of effective strategies to identify imported cases and prevent reintroduction of transmission. Imported malaria from outside the country could otherwise replenish the endemic reservoir of infections, and where a competent vector remains, imported infections can lead to indigenous (secondary) transmission and resurgence.5 Elimination therefore theoretically needs: (1) elimination of the mosquito vector so that transmission cannot re-emerge; (2) blockade of the flow of imported infections from endemic areas; or (3) reduction of the risks of infection at their origin. The first of these options is considered operationally unachievable and is not recommended.6 The second would necessitate either closing the borders of the country seeking elimination or setting up a system of border screening that could successfully identify and treat incoming infections. The third requires that all neighbouring countries from which substantial population movement into the eliminating country occurs also achieve transmission reductions to very low rates.
Methods and strategies to interrupt local transmission
Halting endemic transmission and draining the reservoir needs reduction of Rc, the basic reproduction number under control, to less than 1.22 To drain the reservoir within a reasonable timeframe, mathematical models suggest that Rc should be less than 0·5.23 Although reductions to such a rate might be possible on average throughout a country through the same scale-up of vector control activities that are necessary to achieve low parasite prevalence, foci will remain in which such reductions are not achieved.24,25 Interruption of transmission in these areas will require additional active measures, including identification of infections even if asymptomatic, effectively treating infections before onward transmission can occur, and intensification and adaption of focal vector control activities.
Detection of infections through surveillance
Although historically a strong emphasis was placed on surveillance, nowadays it is more often perceived as a monitoring and evaluation method. However, the GMEP,8,26 and recent mathematical modelling to assess the feasibility of malaria elimination on the islands of Zanzibar,14 show that surveillance is a pivotal component of any programme aiming to interrupt transmission completely. The surveillance package should always include the response, such as targeted vector control measures or radical treatment, which is triggered by case identification and thus directly contributes to the reduction of transmission. As such, surveillance becomes an essential elimination intervention in itself. However, surveillance methods and the different laboratory techniques used differ in their ability to detect all clinical and asymptomatic cases,27 and the pool of Plasmodium vivax and Plasmodium ovale infections cannot be directly defined because there is no known method to detect the hypnozoite stages in the liver.28
Passive case detection involves a system in which data are routinely received by a central health authority based on a set of rules and laws that need a health-care provider or health facility to report some diseases or disorders on an ongoing basis and at specific intervals (weekly, monthly, yearly).29 During the GMEP, health systems were generally considered to have little geographical coverage and thus generally to be insufficient for surveillance for elimination.27,30,31 Additionally, apart from limitations related to the precision of the diagnostic methods and completeness of treatment, passive case detection has other inherent weaknesses in detection of all new infections in the population (figure 1). For Zanzibar, taking into account the factors shown in figure 1, passive case detection is estimated to identify at best 40% of all new infections.14
Figure 1.
Effect of passive case detection on transmission is limited by a cascade of factors
The percentage of infections identified through passive case detection depends on the proportion of new infections that produce clinical symptoms, the proportion of clinical cases that seek treatment in a reporting facility, the proportion of treatment-seeking cases that are tested for malaria, and the sensitivity and quality (performance) of diagnostic tests. Furthermore, the effect of passive case detection on transmission will depend on the proportion of infections identified by diagnostics that are prescribed and receive appropriate treatment, the proportion of those receiving treatment that adhere to it, and the efficacy of the drug.
Additional effort needs to be made to optimise passive case detection, not only by ensuring access to malaria diagnosis and treatment, which should ideally be free in both public and private sectors, but also by improving health-seeking behaviour to reporting facilities and ensuring high testing rates with highly sensitive diagnostic tests (panel 1). Many of these conditions are equally important for controlled low-endemic malaria, but passive case detection alone is unlikely to be an adequate method as a pathway to elimination.42 In some settings, maintenance of elimination might be adequately supported only through routine passive case detection, as exemplified in the USA and large parts of Europe. However, case investigation remains a minimum requirement as long as a competent vector exists to perpetuate transmission (table).
Panel 1. Improving passive case detection for elimination.
Improving health-seeking behaviour for fever to increase the use of and reporting by private and public facilities with adequate diagnostic capacity and treatment
People take different actions when confronted with fever, varying from a wait-and-see attitude to immediate consultation with a health-care professional.32 The effectiveness of passive case detection is limited by the number of patients contacting the public health system, which shows the need to ensure financial access to care, both in the public and private sector, ideally with free malaria treatment as recommended by WHO.6 Health education, adapted to the local practices and the epidemiological context,33,34 should be an integral part of surveillance activities, not only to increase the acceptance of screening activities for active case detection but also to increase the efficiency of the passive system. Ideally, this approach would change attitudes in the community, making seeking testing for malaria the norm.
Ensuring high testing rates in fever and history of fever cases
Although Global Malaria Eradication Program documents insisted on testing all fevers,30 WHO guidelines recommend to test only fevers that have no other obvious cause.6 Clear and easy-to-follow testing algorithms are very important to ensure high testing rates, especially for low-level health workers. Additionally, in Zanzibar, regular supervision substantially improves testing rates in primary health-care facilities. Facilities participating in the newly set-up Malaria Early Detection System initially tested only around 15% of all people attending the outpatient department services, whereas around 30% of all attendees were estimated to present with fever. After intensive supervision, testing rates doubled and more malaria cases were reported.35 More research is needed to define evidence-based testing criteria for elimination settings that are cost effective.
Improving sensitivity of diagnostic tests
Light microscopy and quality rapid diagnostic tests are, when done well, sufficiently sensitive to detect malaria parasites for the parasitological confirmation of patients presenting with symptoms—a requirement for case management in all endemic settings.18 However, at low rates of endemicity, low-parasite-density infections are not only more common,36,37 but their detection is also more important because these sometimes asymptomatic carriers will continue to cause onward transmission. Both tests have limitations in detection of low-density infections,38,39 and quality assurance for microscopy is operationally challenging and labour intensive in elimination settings. Standardised protocols for quality assurance of rapid diagnostic tests, especially to verify potentially large numbers of negative results, are not available. Positive control wells or retesting negative samples with pooled DNA PCR techniques40 or loop-attenuated isothermal amplification41 are promising, but more research is needed to provide robust recommendations. Point-of-care DNA PCR would provide the desired sensitivity but this method is unlikely to be available in the near future. For the time being, DNA PCR seems most adapted to active case detection.
Table.
Malaria control measures in WHO-certified malaria-free countries and in endemic and non-certified malaria-free countries
|
WHO-certified malaria-free countries |
Endemic and non-certified malaria-free countries |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| USA | UK | Singapore | Mauritius | Oman | Sri Lanka | South Africa | Mexico | ||
| Dominant vector(s) | Anopheles quadrimaculatus, Anopheles freeborni, Anopheles albimanus | NA | Anopheles sundaicus, Anopheles maculatus | Anopheles gambiae sensu lato | Anopheles culicifacies, Anopheles fluviatlis | A culicifacies, Anopheles subpictus | Anopheles arabiensis | Anopheles pseudopunctipennis, A albimanus | |
| Total number of cases (year) | 1298 (2008) | 1495 (2009) | 152 (2008) | 27 (2008) | 898 (2009) | 670 (2008) | 5507 (2009)* | 2703 (2009) | |
| Local cases (year) | 0 (2008) | 0 (2009) | 1 (2008) | 0 (2008) | 0 (2009) | 604 (2008) | 2510 (2009) | 2698 (2009) | |
| Imported cases (year) | 1298 (2008) | 1495 (2009) | 151 (2008) | 27 (2008) | 898 (2009) | 66 (2008) | 1966 (2009) | 5 (2009) | |
| Plasmodium falciparum | 40·6% | 78·9% | 76·2% | 30% | 18% | 6% | 95% | 1% | |
| Plasmodium vivax | 14·6% | 13·7% | 21·2% | 70% | 80% | 94% | 5% | 99% | |
| Other (including mixed infections) | 3·6% | 7·4% | 2·6% | .. | 2% | .. | .. | .. | |
| Species unreported | 41·2% | .. | .. | .. | .. | .. | .. | .. | |
| Treatment | |||||||||
| P falciparum | AV+PG, AL, QN+D/T/CL | AV+PG, AL, QN+D/T/CL | QN+D/T/CL | AL, QN, AL,+PQ (if gametocytes identified) | AL | AL+PQ | AL | CQ+PQ | |
| P vivax | CQ+PQ | CQ+PQ | CQ+PQ | CQ+PQ | CQ+PQ | CQ+PQ | .. | CQ+PQ | |
| G6PD screening | Yes | Yes | Yes | No | Yes | Yes | No | No | |
| PCD | |||||||||
| Notification | All cases | All cases | All cases | All cases | All cases | All cases | All cases | All cases | |
| Diagnosis | Microscopy or PCR | Microscopy or PCR | Microscopy or PCR | Microscopy | Microscopy | Microscopy or RDT | Microscopy or RDT | Microscopy (some presumptive treatment) | |
| ACD | |||||||||
| Case investigation | Only if local transmission is suspected | .. | All cases | Yes | Yes | Yes | Yes | Yes | |
| Population screening | No | No | Yes (when local transmission is suspected) | Yes (outbreaks and migrant populations) | Yes (high-risk groups) | Yes (high-risk areas) | No | Yes (outbreaks) | |
| Border screening | No | No | Negative test needed for people applying for a work permit (rescreening for renewal) | Yes (port and airport) | Yes | Yes (starting 2010) | No | No | |
| Vector control | |||||||||
| Blanket | Policy set at the county level with blanket coverage in some counties | No | No | .. | No | No | IRS | No | |
| Targeted | Some counties augment blanket coverage with targeted interventions based on entomological surveilance | No | Environmental management | Larviciding | Larval control | IRS | No | Yes | |
| Outbreak response | Transfusion transmitted: test the involved units/donors, treat the donor, embargo remaining units; mosquito-transmitted malaria: active case detection, vector control, community education | .. | Entomological investigation, case investigation, search and destroy operations for both larvae and adult vectors | IRS, larviciding, environmental management, ACD, case investigation | .. | Vector control | IRS | ACD, supervised treatment of cases, MDA, IRS, breeding-site elimination | |
NA=not applicable. AV=atovaquone. PG=proguanil. AL=artemether-lumefantrine. QN=quinine. D=doxycycline. T=tetracycline. CL=clindamycine. PQ=primaquine. CQ=chloroquine. G6PD=glucose-6-phosphate dehydrogenase. PCD=passive case detection. RDT=rapid diagnostic test. ACD=active case detection. IRS=indoor residual spraying. MDA=mass drug administration.
1031 cases are of unknown origin.
Pampana defined active case detection as “the search for fever cases […] performed by house-to-house visits at regular intervals in every locality of the malarious area”, pointing out that its major advantage is “total coverage in space”.10 Active case detection often served as the entry point to symptomatic and asymptotic treatment and radical cure. Strategies and methods for this detection can be broadly categorised under reactive or proactive case detection.
Reactive case detection is triggered whenever a case is identified by passive case detection. In the absence of a history of travel to an endemic area, local transmission could have occurred, and both the index case and any other locally acquired infections have to be identified, investigated, and treated to prevent additional onward transmission. Reactive case detection will involve visiting the household of the locally acquired case, screening family members, and screening neighbours within a defined radius. Screening around the index case is based on evidence from South America, Asia, and sub-Saharan Africa that malaria cases tend to cluster spatially.42–45 For example, in a low-endemic area in the Peruvian Amazon, actively seeking cases in a 100-m zone around a passively detected case yielded an increase in prevalence four to five times greater than that estimated by passive case detection.42 Determination of the appropriate radius within which to screen is a challenge and, because of a lack of evidence, has often been decided arbitrarily. Further scientific investigation in different eco-epidemiological settings is merited to lend support to spatial definitions of radii, sampling strategies, and numbers of people to be screened.
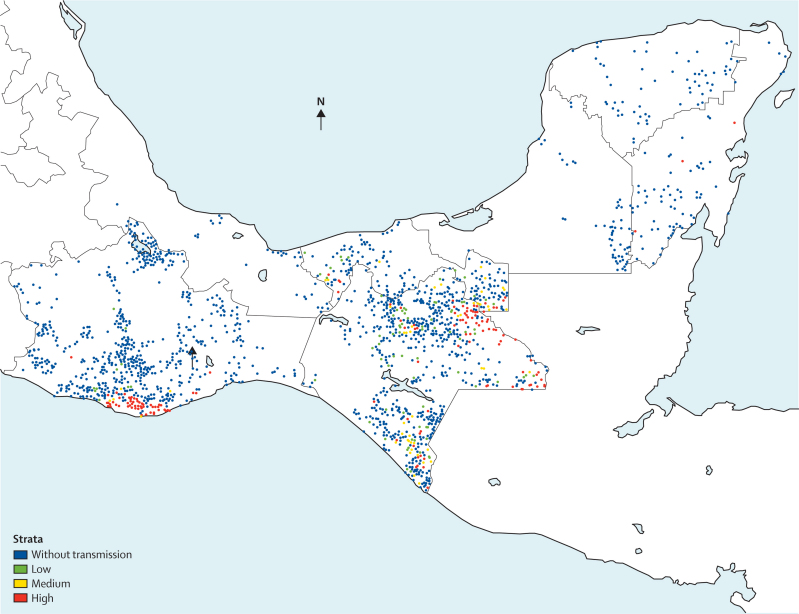
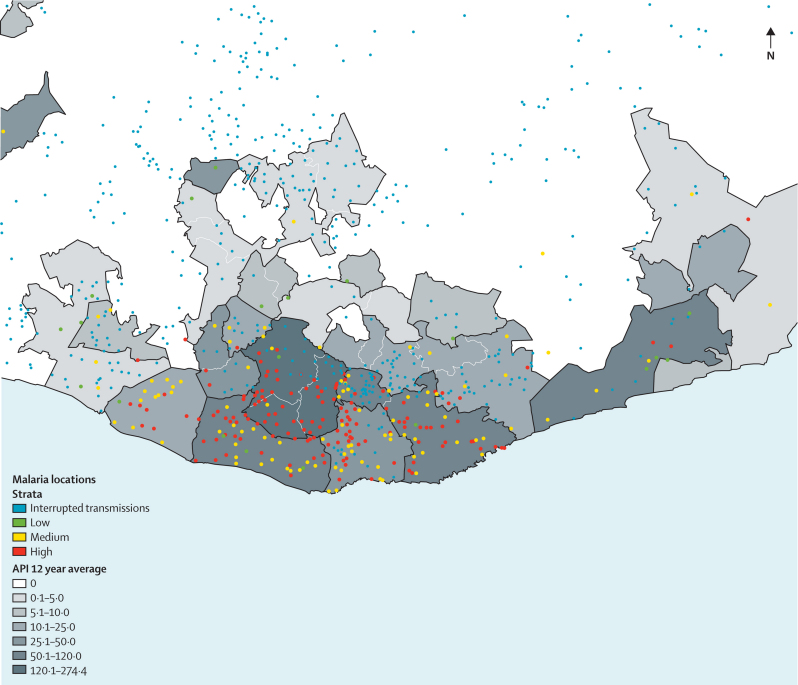
Data generated from passive case detection, case investigations, and reactive screenings can be used to map cases, identify risk factors for transmission, and target malaria control interventions. In Mexico, such an integrated epidemiologically driven system is used to identify residual transmission foci (figure 2) and to measure transmission risk within those foci (figure 3), allowing for targeted and context-specific malaria control interventions. Additionally, the ratio of locally acquired to imported cases from these case identification methods can be used to assess progress made in reduction of Rc, with a ratio of less than one locally acquired case for each imported case suggesting that the desired goal of Rc less than 0·5 has been reached.5
Figure 2.
Residual malaria transmission foci in the states of Oaxaca and Chiapas, Mexico
Localities are depicted as dots; blue dots indicate localities where transmission has been interrupted.
Figure 3.
Residual transmission focus in southern Oaxaca state, Mexico
The annual parasite index (API) is depicted by municipality. Localities are shown as dots with risk of transmission from low to high.
Proactive case detection involves the screening of focal populations without the trigger of a passively identified case. This approach is based on the knowledge that transmission is more likely during some periods of the year, in specific high-risk groups, or in target geographical areas. In Morocco, for example, the populations of some regions are screened routinely in what was historically the malaria transmission season.46 Mass blood screening has also been used in Taiwan,47 southern China,48 and Brazil.49 Proactive case detection is probably best suited for a circumscribed and non-mobile population, in geographically restricted settings (eg, islands), where transmission is seasonal, where mass surveys are socioculturally acceptable, and where treatment remains safe and effective for use in asymptomatic populations. Limitations to this approach include difficult logistics, testing fatigue in the population, population movements that restrict completeness of screening, and cost. Proactive case detection of the Brazilian Yanomami, which involved monthly screenings of about 10 000 people, was estimated to cost 2·3 times more than would passive case detection per positive smear.49
Once elimination is achieved, the need for continued active case detection ultimately depends on the risk of onward transmission from an imported case, as defined by the Rc of a specific area. For countries with low Rc such as the USA and UK, passive case detection, complemented by case investigation, has been enough to avoid resurgence (table). However, most countries that have recently eliminated or are planning elimination will most likely need to complement passive case detection with some form of rigorous active case detection (table). Further research is needed to establish the most cost-effective and context-adapted combinations and the systemic approaches that are needed for implementation.
Killing the parasite with appropriate treatment
Depletion of the parasite reservoir in the human host requires detection of both symptomatic and asymptomatic individuals and killing all forms of the parasite that they carry. Primaquine, which is recommended by WHO in elimination settings,18 and the related but unregistered 8-aminoquinoline tafenoquine,50,51 are very effective against the mature gametocytes of P falciparum52–54 but have a variable effect on the hypnozoites of P vivax and P ovale.55,56 Both drugs can cause haemolysis in individuals who are deficient in glucose-6-phosphate dehydrogenase (G6PD), with increasing risk when high-dose regimens or long treatment schedules are used.57 Even low-dose use in individuals with mild G6PD deficiency can induce transient haemolytic episodes.58 The need for pretreatment G6PD testing with high-quality rapid tests is evident, and large-scale trials have only recently been launched.59
Artemisinin-combination therapies kill developing gametocytes of P falciparum,60 thus reducing malaria transmissibility.61,62 The additional transmission-blocking effects of including primaquine, especially in passively detected symptomatic cases that seek prompt treatment, has not been confirmed.63 However, recrudescent or chronic infections detected by population screening are likely to be gametocytaemic,64 and there is most probably a potential benefit from addition of single-dose primaquine to kill mature gametocytes unaffected by the drugs used for therapy in such cases.65 More research in different epidemiological settings is needed to provide evidence of the benefit of single-dose primaquine in view of the risk of haemolytic events.66
Specific targeting of gametocytes is not needed to clear the blood stages of P vivax, P ovale, or Plasmodium malariae, which are susceptible to the recommended therapeutic treatment;67 however, primaquine is the only drug available as a hypnozoitocidal agent that can prevent the relapse infections that cause onward transmission.68 The standard treatment with 15 mg of primaquine daily for 14 days18,68 has not always proved effective at elimination of relapsing episodes.69,70 Alternative therapeutic regimens with increased doses69,71,72 or increased periods of administration are potentially more effective, although still not completely so,73,74 and safety and adherence to these regimens are a serious cause for concern.75
Primaquine, combined with chloroquine, is standard treatment policy in many countries endemic for P vivax.3 However, in most cases there is an important gap between policy and practice, probably because of health workers' safety concerns about primaquine use.66 For P falciparum, a few countries in the Americas use primaquine in combination with chloroquine (which is no longer recommended), but only three countries—Oman, Sri Lanka, and the Philippines—add it to their first-line artemisinin-based combination treatment.3 Safety concerns related to G6PD deficiency and the short half-life of primaquine make it difficult to make clear recommendations about best use of the only drug that is available for radical treatment, and alternatives are urgently needed because this remains the main obstacle to elimination of P vivax.28
Treatment of cases identified by passive case detection alone is unlikely to deplete the parasite reservoir or prevent onward transmission in most settings, even if radical treatment is given and there is near perfect adherence (which is difficult to achieve when primaquine is needed for 14 days or longer). Because additional active surveillance measures to detect asymptomatic carriers are expensive, alternative strategies such as mass drug administration (MDA) and mass screening and treatment have been proposed to reduce the parasite reservoir in human beings.18,76 Treatment regimens used for these schemes vary widely but almost always include primaquine for radical treatment. WHO does not encourage MDA,18 but, if applied in geographically defined regions or to specific target groups, might still have a role in containing or preventing outbreaks or reducing the risk of importation, especially for P falciparum. To lessen the risk of resistance developing, combinations of drugs other than those used for first-line treatment, including one partner drug that has a long half-life and, ideally, acts against sexual stages, should be used.77 MDA has been used for P vivax,78–80 but since hypnozoites in the liver can be unaffected, strategies to deal with relapses and to accompany focal infection need to be assessed further. More research is needed to define the potential role and operational feasibility of MDA and mass screening and treatment, including identification of which drugs should be used and how frequently, with assessments of safety, coverage, effectiveness, and acceptability.
Elimination-specific vector control activities
Vector control strategies targeted at specific and identifiable foci during an elimination programme might need different approaches from those routinely used in control programmes. Identification of areas of high risk, with use of geostatistical analysis of incident cases81 and rigorous entomological surveillance, are important to continuously reassess transmission potential and possibilities of emerging insecticide resistance,82 and replacement of the dominant vector species.83,84 Regular assessments of vector competence for transmission based on changes in dominant vector species and abundance will enable a decision about scaling down or changing approaches to vector control in existing or newly defined foci. In Morocco, entomological investigations of the last foci of transmission showed that larval control had reduced the vectorial capacity to such low rates that resurgence of malaria was unlikely despite the presence of gametocyte carriers in the human host population.85 Resistance to chemical agents that target adult and larval stages of the vectors poses a major threat to elimination, and mitigation of these risks demands intensive and regular molecular and bio-assay surveillance.6,86 In South Africa, for example, pyrethroids became largely ineffective and the pre-elimination stage was reached only after DDT was reintroduced.87 Similarly, in central Sudan, resistance to commonly used insecticides has prevented elimination of transmission foci in this low-endemic setting.88
Control programmes generally focus interventions on the most efficient vectors, which are usually indoor biting. Achievement of the complete cessation of endemic transmission might need programmes to target all vectors including those that rest or bite outdoors. At the start of the GMEP, outdoor biting and resting vectors were considered of minor importance because they were thought to be limited to few anopheles species.8 Later in the programme, vector behaviour, similar to the emerging resistance to DDT, was recognised to affect transmission.89 These outdoor biting vectors were not specifically targeted, however, and how far they contributed to the overall receptivity of transmission foci remains unclear. Although impregnated bednets have been shown to affect transmission by outdoor biting vectors,90 further research is needed to establish the most effective schemes for entomological surveillance and what additional vector control measures might be necessary to completely halt transmission.
One of the potential advantages of moving towards elimination is that over time some expensive and intensive vector control measures might be scaled back or stopped altogether. GMEP publications8,30,91 suggest that after the attack phase most vector control measures can be scaled down, but several examples from countries that have eliminated malaria clearly show that this approach is highly context dependent and largely defined by both the intrinsic transmission risk and the number of infections imported (table). Most malaria control programmes contemplating elimination will need to considerably strengthen their entomological activities and expertise not only to identify receptive foci but also to be able to make evidence-based decisions about vector control strategies and when to scale them down safely.
Reduction of the importation of infections
Even for countries that have long eliminated malaria, some importation is inevitable. In the USA, for example, 1298 cases were reported in 2008.92 However, intrinsic transmission potential is sufficiently low that only occasional cases of local transmission result from these importations. If comprehensive health-care systems and disease-reporting mechanisms exist, passive case detection can be sufficient to avoid resurgence from imported cases if the intrinsic transmission potential is low. In most malaria-endemic countries considering elimination the vectorial capacity is such that this rate of importation might result in substantial transmission.22 Elimination of malaria transmission will therefore need the stream of imported infections to be slowed through: proactive case detection and treatment in migrants and travellers before they lead to transmission; and cross-border and regional initiatives that can reduce endemicity in countries from where migrants originate. Achievement of malaria elimination will probably need a vertical approach, especially for surveillance and response, whereas the prevention of reintroduction measures can be integrated in larger communicable disease programmes, as shown in Mauritius and Singapore.93
Border screening
Border screening of immigrants is a specific form of proactive case detection that tries to restrict the importation of infections. Some contexts will be more conducive to monitoring and responding to importation risk than will others; islands such as Mauritius and Vanuatu, for example, have few means of entry, with boat and air travel quantifiable and easy to screen. Regions sharing large, poorly monitored, and sometimes inaccessible land borders will have great difficulty controlling importation risk, because of both the large number of potential entry routes and the less readily available data for numbers and characteristics of people using them.
Historically, importation has only been quantified once elimination has been achieved and border screening is part of the country's strategy to avoid resurgence. The potential has been shown for alternative assessment of importation risk as an integral part of assessment of the feasibility of an elimination strategy; in Zanzibar, for example, importation risk was estimated with mobile phone data as a proxy for human population movement.94 Robust methods combining travel history data from health facilities and border surveys in geographical information systems will provide the necessary evidence base for understanding the magnitude of importation risk and designing appropriate strategies to control it. Additionally, border screening that is focused on specific, high-risk groups with high prevalence could be a more viable strategy than generalised population screening that is likely to prove extremely inefficient because of low prevalence. Border screening might not always be necessary once elimination has been achieved, notably in areas where receptivity is very low (table).
Cross-border and regional initiatives
Border screening is not always practical or desirable, especially in areas with artificial administrative borders and frequent border crossing by a large proportion of the population on a daily basis, sometimes even facilitated for economic reasons. Namibia and Angola, for example, have an agreement allowing free movement in an area of 60 km on either side of the border. Cross-border collaborations such as the Lubombo Spatial Development Initiative are potentially more effective in reducing importation than is border screening (webappendix). The political mechanisms, motivations, financing, and responsibilities for cross-border, regional approaches to elimination are likely to be complex. However, some have argued that this approach provides the only means of reducing importation risk between countries.13
Measurement of progress
One of the differences emphasised by the WHO Expert Committee between eradication and control programmes was that the administrative standard of progress for control was measurement of accomplishments, whereas for eradication it would change to measurement of what remains to be accomplished.9 Because elimination has a known quantitative goal of ending endemic transmission and reducing the number of locally acquired cases below a specific threshold,5,13 monitoring systems are essential to track progress towards that goal. Measures of the effect of control programmes typically include population-based surveys of parasite prevalence. At very low prevalence, however, the number of samples that has to be collected to find a positive result will probably be prohibitively high. WHO therefore proposes to use incidence measures collected through the routine data collection systems (passive and active).6 However, present and commonly used diagnostic methods for clinical management, microscopy, and rapid diagnostic tests are not ideal for surveillance because they have limited sensitivity for infections of low-parasite density, which are common in low-transmission settings.36,37
To improve precision of measurements of transmission, new diagnostic methods and approaches will therefore become increasingly important (panel 2). PCR methods provide a more sensitive means of testing than do microscopy or rapid diagnostic tests,103 but better standardised and field-applicable methods with robust quality-assurance mechanisms are needed. Genotyping the detected parasites for molecular epidemiology could potentially allow for the identification of the source of imported infections. Seroprevalence testing is an old technique that has recently been improved and standardised. It has been used in population-based surveys in Djibouti, Sudan, Swaziland, and Vanuatu,102 but needs to be validated as a routine surveillance method. Additionally, whereas sample collection (dry blood spots on filter paper) for these diagnostic techniques is easy, most malaria programmes are not equipped to analyse samples. A strong national or regional collaboration with research institutes will be needed not only for the analysis but also for the interpretation of results, especially in the absence of standardised quality-assured techniques.
Panel 2. Laboratory techniques to improve surveillance and monitoring of malaria infection and transmission.
In an elimination setting, detection of all infections, whether asymptomatic, low-parasite density, or imported, and measurement of transmission intensity, both to measure progress and to enable targeting foci, are key requirements to interrupt local transmission and avoid resurgence. Laboratory techniques detecting parasite DNA, specific molecular markers, and antibodies will therefore be needed to complement conventional diagnostic methods such as rapid diagnostic tests and microscopy.
DNA-PCR
Standard surveillance systems rely on diagnosis by microscopy or use of rapid detection tests that are not sufficiently specific or sensitive to detect low-parasite-density infections. PCR-based methods are simple to do, show greatly improved sensitivity, and are able to detect mixed infections. They are particularly valuable for screening large numbers of samples because analyses of dried blood spots can be done, and a single low-grade parasitaemic sample can be detected in a pool of 50 samples, providing a resource-saving and more cost-effective procedure.40,95
Genotyping
Elimination strategies need to know where infections originate; specifically, if they are autochthonous or imported and, if imported, from where. Malaria parasites are genetically highly variable, and genotyping has been used as a means to distinguish between indigenous and imported cases.96 Genetic variants can be measured from single drops of dried blood, and the assays are relatively cheap, rapid, and accurate. Molecular epidemiology can establish not only where infections originate, but also construct transmission networks in space and time to show relations between parasites in areas selected for elimination. Most studies so far have used molecular markers that are under selection (drug or immune) pressure, but there are alternative multilocus markers in Plasmodium falciparum and Plasmodium vivax that are not under selection but still show the variability that is needed.
Serology
Measurement of antimalarial antibodies in exposed populations is a valuable method because it integrates malaria exposure over time. The advent of recombinant antigen technology makes serology a robust and standardisable method. Antibodies can be detected in blood from a finger prick, and samples can be assayed quickly in large numbers. Detection of antibody responses is highly sensitive and specific, allowing an assessment of exposure to both P falciparum and P vivax (and potentially other forms of malaria) in the same sample.
For monitoring and assessment, seroprevalence rates can be used to define malaria endemicity97,98 and distinguish between areas of differential exposure when parasite rates are zero.99 In these areas, residual or potential foci of infection can be identified by geospatial analysis of individual or household level antibody response.99 Detailed examination of age-specific seroprevalence profiles can be used to monitor changes in transmission or to identify risk of exposure associated with behaviour such as travel or residence. Absence of antimalarial antibodies was used to show the success of elimination programmes in Mauritius,100 Greece,101 and on the island of Aneityum in Vanuatu.102
Conclusions
Politicians and policy makers need to understand that malaria elimination should not take a business-as-usual approach. The most notable change will involve the evolution of a surveillance system linked to rapid response, robust epidemiological data, and sustained vigilance over a long time. Most countries aiming for elimination do not yet have the surveillance systems required for an elimination effort and will need to invest substantially to improve disease notification and analysis.104 Furthermore, the challenges of reaching the highly efficient operational levels8,10 that are needed to stop transmission will be great. Even if such a programme is undertaken with great precision, its success within a country is likely to be contingent on a commitment throughout an entire region of countries.
Nationally, political leaders will need to create an environment within which strategies to support elimination would operate successfully. The factors that would contribute to this enabling environment—such as well functioning health systems, community participation, sufficient skilled human resources, sustainable financing, a national and regional legal framework to facilitate elimination, and political stability—have not been discussed here but are reviewed in broad terms in other papers in this Series.13,22 Nevertheless, even for the elimination of one disease, planning needs to be approached systemically to be successful.105 We have not addressed the complex issues related to the national certification process of malaria-free status.6 We do, however, recognise that this process remains imperfect and needs more investigative modelling, new methods to measure transmission (or its absence), and empirical research to improve the specificity of the present recommendation to prove the absence of local transmission “beyond reasonable doubt”.6
Systematic reviews of surveillance and response methods, and case studies from countries that have recently eliminated malaria, are essential to build a stronger evidence-base and generate practical, context-specific recommendations for future guidance. Research to improve available methods for diagnosis, treatment, and vector control is also needed. Long-term research needs have been discussed elsewhere;106 short-term priorities should include the development of methods to assess the overall feasibility of elimination strategies, understand the epidemiology of asymptomatic infections, quantify effect sizes of imported infection risks, and compare cost-effectiveness of different surveillance and response models.
Although the GMEP has left a legacy of technical reports and guidelines, most were focused on epidemiological contexts in the Americas and Europe. The wealth of information available from the GMEP—one of WHO's best documented programmes—should be used to inform present and future elimination efforts, but a contemporary evidence base to support cost-effective decision making is only now beginning to be generated for the more complex transmission settings of sub-Saharan Africa and Asia. “The problem of finding an effective and economical method of [eliminating] malaria in tropical Africa has not yet been solved”, stated the WHO Expert Committee in 1957.9 With the subsequent shift away from elimination activities, the ensuing 50 years have not provided solutions, especially for sub-Saharan Africa where large-scale interventions have only been implemented for the first time in the past decade.22 In the absence of clear guidance, the decision to pursue malaria elimination might be made on a political basis without careful and rigorous assessment of the technical, operational, and financial feasibility of pursuing such a course.
Historically, it had been hoped that one approach could succeed everywhere as long as it was undertaken with “military precision”.107 An unfortunate consequence of this model was that it essentially replaced malariologists with technicians who were skilled in the logistics of directing insecticide spray campaigns but did not have a crucial understanding of the disease and its subtleties.108 Such a strategy was very successful in many settings, but the challenge of eliminating malaria in high-endemic regions will need more versatile and locally tailored, systemic approaches. Some have argued that elimination of malaria from a country can only be achieved through a global eradication effort109 since constant importation will otherwise make local elimination a precarious achievement. In the absence of such a global campaign, elimination in individual countries can be achieved and maintained only through robust surveillance and response combined with targeted vector control to eliminate residual or re-emerging foci. Although the GMEP needed great precision to achieve universal coverage with DDT, national elimination with the challenge of continued importation will require an equal level of precision for surveillance and response. The challenges nowadays are different from those faced by the GMEP and will need the development of new approaches, novel technologies, and sustainable financing to change the distribution of malaria prevalence progressively and to eventually eradicate the disease worldwide.
Acknowledgments
Acknowledgments
BM and JMC acknowledge support from the Global Health Group of the University of California, San Francisco, CA, USA, which is funded by the Bill & Melinda Gates Foundation and ExxonMobil. DLS, BM, JMC, and GT acknowledge support from the Bill & Melinda Gates Foundation. DLS also acknowledges funding from the RAPIDD program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health. RWS is a Wellcome Trust Principal Research Fellow (#079080) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). CD is supported by the Wellcome Trust (#078925). We thank Majed Al-Zjedali, Kee Tai Goh, Allison Tatarsky, and Peter Chiodini for providing updated information used in the table; Chris Cotter and Allison Phillips who assisted in the literature review; Bernard Nahlen, G Dennis Shanks, and the Malaria Elimination Group for extensive comments on the report; and Kevin Baird for insights into P vivax treatment and John Silver for insights into vector behaviour.
Contributors
BM, JMC, and DLS conceived the idea for the report. BM, JMC, RWS, and GAT wrote the report. BM and JMC did the literature review. BM, CD, MHR, RM, and GAT wrote the panels. LS, RRA, and MT contributed substantial technical input to the content of the report. All authors took part in the review, preparation, and final approval of the report.
Conflicts of interest
BM and JMC work within the malaria programme at the Clinton Health Access Initiative, which is supporting malaria elimination in southern Africa in collaboration with Global Health Group of the University of California, San Francisco, CA, USA. RA, MHR, and RM play leading roles in the elimination programmes of Sri Lanka, Mexico, and South Africa, respectively. MT, LS, and MHR are members of the Malaria Eradication Research Agenda project (MalERA) steering committee, of the Bill & Melinda Gates Foundation. MT is a scientific advisor to the Novartis Institute for Tropical Diseases board and is a board member of the UBS Optimus Foundation. BM, RWS, LS, DLS, RRA, MHR, RA, RM, MT, and GT serve as members of the Malaria Elimination Group. RWS has received funding from Novartis for chairing meetings of national control programmes in Africa and has received a research grant from Pfizer. CD declares that he has no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their employing organisations nor of the sources of funding.
Web Extra Material
References
- 1.Roll Back Malaria The Global Malaria Action Plan. 2008. http://www.rollbackmalaria.org/gmap/gmap.pdf (accessed April 1, 2010).
- 2.Global Partnership to Roll Back Malaria. Johansson EW, Cibulskis RE, Steketee RW. Malaria funding and resource utilization: the first decade of Roll Back Malaria. World Health Organization; Geneva: 2010. [Google Scholar]
- 3.WHO . World Malaria Report 2009. World Health Organization; Geneva: 2009. http://www.who.int/malaria/world_malaria_report_2009/en/index.html (accessed April 1, 2010). [Google Scholar]
- 4.Smith D, Hay S, Noor A, Snow R. Predicting changing malaria risk after expanded insecticide-treated net coverage in Africa. Trends Parasitol. 2009;25:511–516. doi: 10.1016/j.pt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J, Moonen B, Snow R, Smith D. How absolute is zero? An evaluation of historical and current definitions of malaria elimination. Malar J. 2010;9:213. doi: 10.1186/1475-2875-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Malaria elimination: a field manual for low and moderate endemic countries. World Health Organization; Geneva: 2007. [Google Scholar]
- 7.WHO . WHO Expert Committee on Malaria: fifth report. World Health Organization; Geneva: 1954. [PubMed] [Google Scholar]
- 8.WHO . The World Health Organization and malaria eradication. World Health Organization; Geneva: 1956. [Google Scholar]
- 9.WHO . WHO Expert Committee on Malaria: sixth report. World Health Organization; Geneva: 1957. [PubMed] [Google Scholar]
- 10.Pampana E. Textbook of malaria eradication. 2nd edn. Oxford University Press; Oxford: 1969. [Google Scholar]
- 11.WHO . Malaria control in countries where time-limited eradication is impracticable at present: report of a WHO interregional conference. World Health Organization; Geneva: 1974. [PubMed] [Google Scholar]
- 12.Moonen B, Barrett S, Tulloch J, Jamison DT. Shrinking the malaria map: a prospectus on malaria elimination. Global Health Group, Global Health Sciences, University of California, San Francisco; San Francisco: 2009. Making the decision. [Google Scholar]
- 13.Feachem RGA, Phillips AA, Hwang J. Shrinking the malaria map: progress and prospects. Lancet. 2010 doi: 10.1016/S0140-6736(10)61270-6. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanzibar Malaria Control Program Malaria elimination on Zanzibar: a feasibility assessment. 2009. http://www.malariaeliminationgroup.org/malaria-elimination-zanzibar-feasibility-assessment (accessed April 1, 2010).
- 15.Snow R, Marsh K. Malaria in Africa: progress and prospects in the decade since the Abuja Declaration. Lancet. 2010;376:137–139. doi: 10.1016/S0140-6736(10)60577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:1–77. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Beier J, Keating J, Githure J, Macdonald M, Impoinvil D, Novak R. Integrated vector management for malaria control. Malar J. 2008;7:S1–S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . Guidelines for the treatment of malaria. 2nd edn. World Health Organization; Geneva: 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html (accessed April 6, 2010). [PubMed] [Google Scholar]
- 19.Alves F, Gil L, Marrelli M. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- 20.Yekutiel P. Problems of epidemiology in malaria eradication. Bull World Health Organ. 1960;22:669–683. [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . WHO Expert Committee on Malaria: ninth report. World Health Organization; Geneva: 1962. [Google Scholar]
- 22.Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010 doi: 10.1016/S0140-6736(10)61301-3. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DL, Hay SI. Endemicity response timelines for Plasmodium falciparum elimination. Malar J. 2009;8:87. doi: 10.1186/1475-2875-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . WHO Expert Committee on Malaria: tenth report. World Health Organization; Geneva: 1964. [PubMed] [Google Scholar]
- 25.Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser RL. Epidemiology of malaria eradication. The role of surveillance in a malaria eradication program. Am J Public Health Nations Health. 1966;56:90–93. doi: 10.2105/ajph.56.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pull JH. Malaria surveillance methods, their uses and limitations. Am J Trop Med Hyg. 1972;21:651–657. doi: 10.4269/ajtmh.1972.21.651. [DOI] [PubMed] [Google Scholar]
- 28.Wells TNC, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26:145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Teutsch SM, Churchill RE. Principles and Practice of Public Health Surveillance. 2nd edn. Oxford University Press; Oxford: 2000. [Google Scholar]
- 30.WHO . WHO Expert Committee on Malaria: eighth report. World Health Organization; Geneva: 1961. [PubMed] [Google Scholar]
- 31.Yekutiel P. Newer surveillance methods in the control of communicable disease. I. Malaria. Basic principles. Milbank Mem Fund Q. 1965;43:375–385. [PubMed] [Google Scholar]
- 32.McCombie SC. Treatment seeking for malaria: a review of recent research. Soc Sci Med. 1996;43:933–945. doi: 10.1016/0277-9536(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 33.Tanner M, Vlassoff C. Treatment-seeking behaviour for malaria: a typology based on endemicity and gender. Soc Sci Med. 1998;46:523–532. doi: 10.1016/s0277-9536(97)00195-0. [DOI] [PubMed] [Google Scholar]
- 34.Cropley L. The effect of health education interventions on child malaria treatment-seeking practices among mothers in rural refugee villages in Belize, Central America. Health Promot Int. 2004;19:445–452. doi: 10.1093/heapro/dah406. [DOI] [PubMed] [Google Scholar]
- 35.Zanzibar Malaria Control Program . Zanzibar malaria early epidemic detection system biannual report, mid year 2009. Revolutionary Government of Zanzibar, Ministry of Health and Social Welfare; Zanzibar: 2009. [Google Scholar]
- 36.Elhassan IM, Hviid L, Jakobsen PH. High proportion of subclinical Plasmodium falciparum infections in an area of seasonal and unstable malaria in Sudan. Am J Trop Med Hyg. 1995;53:78–83. [PubMed] [Google Scholar]
- 37.Collins WE, Jeffery GM. A retrospective examination of the patterns of recrudescence in patients infected with Plasmodium falciparum. Am J Trop Med Hyg. 1999;61:44–48. doi: 10.4269/tropmed.1999.61-044. [DOI] [PubMed] [Google Scholar]
- 38.Coleman RE, Maneechai N, Rachaphaew N. Comparison of field and expert laboratory microscopy for active surveillance for asymptomatic Plasmodium falciparum and Plasmodium vivax in western Thailand. Am J Trop Med Hyg. 2002;67:141–144. doi: 10.4269/ajtmh.2002.67.141. [DOI] [PubMed] [Google Scholar]
- 39.Coleman RE, Maneechai N, Rachapaew N. Field evaluation of the ICT Malaria Pf/Pv immunochromatographic test for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Am J Trop Med Hyg. 2002;66:379–383. doi: 10.4269/ajtmh.2002.66.379. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SM, Juliano JJ, Trottman PA. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol. 2010;48:512–519. doi: 10.1128/JCM.01800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paris DH, Imwong M, Faiz AM. Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg. 2007;77:972–976. [PubMed] [Google Scholar]
- 42.Branch O, Casapia WM, Gamboa DV. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui F, Xu B, Chen Z. Spatio-temporal distribution of malaria in Yunnan Province, China. Am J Trop Med Hyg. 2009;81:503–509. [PubMed] [Google Scholar]
- 44.Gaudart J, Poudiougou B, Dicko A. Space-time clustering of childhood malaria at the household level: a dynamic cohort in a Mali village. BMC Public Health. 2006;6:286. doi: 10.1186/1471-2458-6-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooker S, Clarke S, Njagi JK. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop Med Int Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 46.El Khyari T Malaria elimination strategy in Morocco: plan and elements of evaluation. 1999. http://www.emro.who.int/RBM/MoroccoStrategicPlanEn.pdf (accessed April 6, 2010).
- 47.Yip K. Malaria eradication: the Taiwan experience. Parassitologia. 2000;42:117–126. [PubMed] [Google Scholar]
- 48.Zizhao L, Luoyuan S, Lian Z, Dongfang L, Yunpu S. Control strategies of malaria in Henan Province, China. Southeast Asian J Trop Med Public Health. 1999;30:240–242. [PubMed] [Google Scholar]
- 49.Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med. 2005;60:563–573. doi: 10.1016/j.socscimed.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Shanks GD, Oloo AJ, Aleman GM. A new primaquine analogue, tafenoquine (WR 238605), for prophylaxis against Plasmodium falciparum malaria. Clin Infect Dis. 2001;33:1968–1974. doi: 10.1086/324081. [DOI] [PubMed] [Google Scholar]
- 51.Elmes NJ, Nasveld PE, Kitchener SJ, Kocisko DA, Edstein MD. The efficacy and tolerability of three different regimens of tafenoquine versus primaquine for post-exposure prophylaxis of Plasmodium vivax malaria in the Southwest Pacific. Trans R Soc Trop Med Hyg. 2008;102:1095–1101. doi: 10.1016/j.trstmh.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Bjorkman A. Malaria eradication on islands. Lancet. 2000;356:1560–1564. doi: 10.1016/S0140-6736(00)03127-5. [DOI] [PubMed] [Google Scholar]
- 53.Shekalaghe S, Drakeley C, Gosling R. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One. 2007;2:e1023. doi: 10.1371/journal.pone.0001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baird JK. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev. 2009;22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 56.Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Beutler E, Duparc S, the G6PD Deficiency Working Group Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77:779–789. [PubMed] [Google Scholar]
- 58.Shekalaghe SA, ter Braak R, Daou M. Haemolysis after a single dose of primaquine co-administered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase (G6PD A-variant) deficient individuals in Tanzania. Antimicrob Agents Chemother. 2010;54:1762–1768. doi: 10.1128/AAC.01135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuwahata M, Wijesinghe R, Ho M. Population screening for glucose-6-phosphate dehydrogenase deficiencies in Isabel Province, Solomon Islands, using a modified enzyme assay on filter paper dried bloodspots. Malar J. 2010;9:223. doi: 10.1186/1475-2875-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Artemisinin Study Group Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 61.Price RN, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 62.Sutherland CJ, Ord R, Dunyo S. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawpoolsri S, Klein EY, Singhasivanon P. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malar J. 2009;8:159. doi: 10.1186/1475-2875-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 65.Chomcharn Y, Surathin K, Bunnag D, Sucharit S, Harinasuta T. Effect of a single dose of primaquine on a Thai strain of Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 1980;11:408–412. [PubMed] [Google Scholar]
- 66.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7(suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pukrittayakamee S, Imwong M, Singhasivanon P, Stepniewska K, Day NJ, White NJ. Effects of different antimalarial drugs on gametocyte carriage in P. vivax malaria. Am J Trop Med Hyg. 2008;79:378–384. [PubMed] [Google Scholar]
- 68.Galappaththy GNL, Omari AAA, Tharyan P. Primaquine for preventing relapses in people with Plasmodium vivax malaria. Cochrane Database Syst Rev. 2007;7 doi: 10.1002/14651858.CD004389.pub2. CD004389. [DOI] [PubMed] [Google Scholar]
- 69.Bunnag D, Karbwang J, Thanavibul A. High dose of primaquine in primaquine resistant vivax malaria. Trans R Soc Trop Med Hyg. 1994;88:218–219. doi: 10.1016/0035-9203(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 70.Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White NJ. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis. 1994;169:932–935. doi: 10.1093/infdis/169.4.932. [DOI] [PubMed] [Google Scholar]
- 71.Pukrittayakamee S, Imwong M, Chotivanich K, Singhasivanon P, Day NPJ, White NJ. A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg. 2010;82:542–547. doi: 10.4269/ajtmh.2010.09-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krudsood S, Tangpukdee N, Wilairatana P. High-dose primaquine regimens against relapse of Plasmodium vivax malaria. Am J Trop Med Hyg. 2008;78:736–740. [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips EJ, Keystone JS, Kain KC. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis. 1996;23:1171–1173. doi: 10.1093/clinids/23.5.1171. [DOI] [PubMed] [Google Scholar]
- 74.Yeshiwondim AK, Tekle AH, Dengela DO, Yohannes AM, Teklehaimanot A. Therapeutic efficacy of chloroquine and chloroquine plus primaquine for the treatment of Plasmodium vivax in Ethiopia. Acta Trop. 2010;113:105–113. doi: 10.1016/j.actatropica.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Baird JK, Rieckmann KH. Can primaquine therapy for vivax malaria be improved? Trends Parasitology. 2003;19:115–120. doi: 10.1016/s1471-4922(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 76.Song J, Socheat D, Tan B. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J. 2010;9:57. doi: 10.1186/1475-2875-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.von Seidlein L, Greenwood BM. Mass administrations of antimalarial drugs. Trends Parasitol. 2003;19:452–460. doi: 10.1016/j.pt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Garfield RM, Vermund SH. Changes in malaria incidence after mass drug administration in Nicaragua. Lancet. 1983;322:500–503. doi: 10.1016/s0140-6736(83)90523-8. [DOI] [PubMed] [Google Scholar]
- 79.Kaneko A. A community-directed strategy for sustainable malaria elimination on islands: short-term MDA integrated with ITNs and robust surveillance. Acta Tropica. 2010;114:177–183. doi: 10.1016/j.actatropica.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 80.WHO/WPRO . International Workshop on the Control of Vivax Malaria in East Asia. World Health Organization, Regional Office for the Western Pacific; 2004. http://www.wpro.who.int/internet/resources.ashx/MVP/Vivax_Mal_East+Asia.pdf (accessed April 1, 2010). [Google Scholar]
- 81.Hernández-Avila JE, Rodrıguez MH, Betanzos-Reyes AF. Determinant factors for malaria transmission on the coast of Oaxaca State, the main residual transmission focus in Mexico. Salud Publica Mex. 2006;48:405–417. doi: 10.1590/s0036-36342006000500007. [DOI] [PubMed] [Google Scholar]
- 82.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–389. doi: 10.1016/S1473-3099(08)70045-8. [DOI] [PubMed] [Google Scholar]
- 83.Aitken T, Trapido H. Replacement phenomenon observed amongst Sardinian anopheline mosquitoes following eradication measures. E J Brill; Leiden: 1961. [Google Scholar]
- 84.Gillies MT, Smith A. The effect of a residual house-spraying campaign in east Africa on species balance in the Anopheles funestus group. The replacement of A. funestus giles by A. rivulorum leeson. Bull Entomol Res. 1960;51:243–252. [Google Scholar]
- 85.Faraj C, Adlaoui E, Ouahabi S, Rhajaoui M, Fontenille D, Lyagoubi M. Entomological investigations in the region of the last malaria focus in Morocco. Acta Trop. 2009;109:70–73. doi: 10.1016/j.actatropica.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 86.WHO/EMRO . Guidelines on prevention of the reintroduction of malaria. World Health Organization Regional Office for the Eastern Mediterranean; Geneva: 2007. [Google Scholar]
- 87.Maharaj R, Mthembu DJ, Sharp BL. Impact of DDT re-introduction on malaria transmission in KwaZulu-Natal. S Afr Med J. 2005;95:871–874. [PubMed] [Google Scholar]
- 88.Abdalla H, Matambo T, Koekemoer L, Mnzava A, Hunt R, Coetzee M. Insecticide susceptibility and vector status of natural populations of Anopheles arabiensis from Sudan. Tran R Soc Trop Med Hyg. 2008;102:263–271. doi: 10.1016/j.trstmh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Elliott R. The influence of vector behavior on malaria transmission. Am J Trop Med Hyg. 1972;21:755–763. doi: 10.4269/ajtmh.1972.21.755. [DOI] [PubMed] [Google Scholar]
- 90.Govella NJ, Okumu FO, Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82:415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.WHO . WHO Expert Committee on Malaria: seventh report. World Health Organization; Geneva: 1959. [PubMed] [Google Scholar]
- 92.Mali S, Steele S, Slutsker L, Arguin PM. Malaria surveillance—United States, 2008. MMWR Surveill Summ. 2010;59:1–15. [PubMed] [Google Scholar]
- 93.Lee YCA, Tang CS, Ang LW, Han HK, James L, Goh KT. Epidemiological characteristics of imported and locally-acquired malaria in Singapore. Ann Acad Med Singap. 2009;38:840–849. [PubMed] [Google Scholar]
- 94.Tatem AJ, Qiu Y, Smith DL, Sabot O, Ali AS, Moonen B. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J. 2009;8:287. doi: 10.1186/1475-2875-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsiang M, Lin M, Kemere J, Pilcher CD, Dorsey G, Greenhouse B. PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children. J Clin Microbiol (in press). [DOI] [PMC free article] [PubMed]
- 96.Osorio L, Todd J, Pearce R, Bradley DJ. The role of imported cases in the epidemiology of urban Plasmodium falciparum malaria in Quibdó, Colombia. Trop Med Int Health. 2007;12:331–341. doi: 10.1111/j.1365-3156.2006.01791.x. [DOI] [PubMed] [Google Scholar]
- 97.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 98.Stewart L, Gosling R, Griffin J. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bousema T, Youssef RM, Cook J. Serologic markers for detecting malaria in areas of low endemicity, Somalia, 2008. Emerg Infect Dis. 2010;16:392–399. doi: 10.3201/eid1603.090732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruce-Chwatt L, Draper CC, Konfortion P. Serioepidemiological evidence of eradication of malaria from Mauritius. Lancet. 1973;302:547–551. doi: 10.1016/s0140-6736(73)92361-1. [DOI] [PubMed] [Google Scholar]
- 101.Bruce-Chwatt L, Draper CC, Avramidis D, Kazandzoglou O. Sero-epidemiological surveillance of disappearing malaria in Greece. J Trop Med Hyg. 1975;78:194–200. [PubMed] [Google Scholar]
- 102.Cook J, Reid H, Iavro J. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 2010;9:169. doi: 10.1186/1475-2875-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ndao M, Bandyayera E, Kokoskin E, Gyorkos TW, MacLean JD, Ward BJ. Comparison of blood smear, antigen detection, and nested-PCR methods for screening refugees from regions where malaria is endemic after a malaria outbreak in Quebec, Canada. J Clin Microbiol. 2004;42:2694–2700. doi: 10.1128/JCM.42.6.2694-2700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Breman JG, Holloway CN. Malaria surveillance counts. Am J Trop Med Hyg. 2007;77:36–47. [PubMed] [Google Scholar]
- 105.de Savigny D, Adam T. Systems thinking for health systems strengthening. World Health Organization; Geneva: 2009. http://www.who.int/alliance-hpsr/resources/9789241563895/en/index.html (accessed April 1, 2010). [Google Scholar]
- 106.Enserink M. 2010. As challenges change, so does science. Science. 2010;328:843. doi: 10.1126/science.328.5980.843. [DOI] [PubMed] [Google Scholar]
- 107.Faird MA. The malaria campaign—why not eradication? World Health Forum. 1998;19:417–427. [PubMed] [Google Scholar]
- 108.Jeffery GM. Malaria control in the twentieth century. Am J Trop Med Hyg. 1976;25:361–371. doi: 10.4269/ajtmh.1976.25.361. [DOI] [PubMed] [Google Scholar]
- 109.Litsios S. Criticism of WHO's revised malaria eradication strategy. Parassitologia. 2000;42:167–172. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.