Abstract
It is well recognised that there are pitfalls when defining the subcellular localisation of a protein with immunocytochemistry. Accurate protein localisation to particular cellular micro-architecture is crucial in defining its role within the cell. Huntingtin (HTT), the protein mutated in the neurodegenerative disorder Huntington’s disease (HD) is a large protein of ill-defined function. Bearing little resemblance to other proteins, its function has been difficult to assign, therefore localising this protein with precision within the cell may provide further clues as to its normal and pathological function. Lack of consistency between methods employed in different studies has resulted in varying conclusions as to its subcellular localisation. This technical review investigates the effects that different immunocytological methods can have upon the apparent subcellular localisation of the huntingtin protein, and discusses the implications this may have.
Introduction
Huntingtin (HTT) is the protein product of the HD gene which is the faulty gene in Huntington’s disease (HD). The precise subcellular localisation of HTT has been the subject of much debate since the generation of the first anti-HTT antibodies following the cloning of the HD gene [1] [2] [3]. Establishing localisation is important for several reasons. Firstly, to refine HTT’s role within the cell; secondly, to track any protein or subcellular interactions with confidence and thirdly, to monitor any effects of post-translational modifications on the localisation of this protein. Endogenous HTT has been detected and localised in various cell lines and in human, mouse, rat, rabbit and monkey tissue, using various antibodies (Table 1).
Table 1: Anti-HTT antibodies used to detect and localise huntingtin
| Huntingtin antibody | Type | Region against which antisera raised | Reference |
| Ab1356 | Affinity purified rabbit pAb IgG | 3114 – 3141 (aa) | 1 |
| Ab585 | Rabbit pAb IgG | 585 – 754 (aa) |
8, 10, 16, 22, 23 |
| Ab1 | Affinity purified rabbit pAb | 1 – 17 (aa) |
8, 10, 12, 23, 24 |
| Ab2911 | Affinity purified rabbit pAb | 2911 – 3140 (aa) |
8 |
| Ab2527 | Affinity purified rabbit pAb | 2527 – 2547 (aa) |
8, 22 |
| Ab1173 | Rabbit pAb | 1173 – 1196 (aa) |
8 |
| mAb4C8/mAb2166 | Mouse mAb | 181 - 810 (aa) |
2, 7, 14, 25, 26 |
| mAb4E6 | Mouse mAb | 4057 – 5253 (cDNA) |
2 |
| mAb2E8/mAb2168 | Mouse mAb | 2146 - 2541 (aa) |
2, 7 |
| mAb2C1 | Mouse mAb | 8366 – 9254 (cDNA) |
2 |
| AP78 | Affinity purified rabbit pAb | 1 – 17 (aa) |
11, 27 |
| AP81 | Affinity purified rabbit pAb | 650 – 663 (aa) |
11 |
| HDp549 | Affinity purified rabbit pAb | 549 – 679 (aa) |
3, 14, 28 |
| - | Rat mAb | 549 – 679 (aa) |
3 |
| IT15 antibody | Affinity purified rabbit pAb | 1188 – 1204 (aa) |
4 |
| GHM1 | Mouse mAb | 1765 – 2070 (aa) |
26, 27, 28 |
| BKP1 | Affinity purified rabbit pAb | 3 – 16 (aa) |
25, 26, 27 |
| BKP4 | Affinity purified rabbit pAb | 1593 – 1608 (aa) |
26 |
| mAb 2172 |
Mouse mAb | 2683 – 2979 (aa) |
26 |
| AP194 | Rabbit pAb | 1 – 17 (aa) |
7, 34 |
| HDB4E10 | Mouse mAb | 1844 – 2131 (aa) |
7, 17 |
| HDC8A4 | Mouse mAb | 2703 – 2911 (aa) |
7, 17 |
| HDA3E10 | Mouse mAb | 997 – 1276 (aa) |
17 |
| Antibody 1356 | Affinity purified rabbit pAb | 3114 – 3141 (aa) |
5, 6 |
| Antibody 1354 / 1358 | Affinity purified rabbit pAb | 3114 – 3141 (aa) |
5, 6 |
| Antibody 1359 | Affinity purified rabbit pAb | 701 – 744 (aa) |
5, 6 |
| Antibody 1495 | Affinity purified rabbit pAb | 701 – 744 (aa) |
5, 6 |
| Antibody 93 | Affinity purified rabbit pAb | 1929 – 2421 (aa) |
5, 6 |
| Antibody 97 | Rabbit pAb | 596 – 1030 (aa) |
6 |
| Antibody 98 | Rabbit pAb | 852 – 1193 (aa) |
6 |
| Antibody 7 | Affinity purified rabbit pAb | 1 – 19 (aa) |
6 |
| Antibody 10 | Affinity purified rabbit pAb | 1 – 19 (aa) |
6 |
| Antibody 1859 | Rabbit pAb | 1 – 19 (aa) |
6 |
| Antibody 1862 | Rabbit pAb | 1 – 19 (aa) |
6 |
| Antibody 2 | Rabbit pAb | 11 – 19 (aa) |
6 |
| Antibody 5 |
Rabbit pAb | 11 – 19 (aa) |
6 |
| Anti-HUNT3 | Affinity purified rabbit pAb | 2110 – 2121 (aa) |
29 |
| CAG53b | Rabbit pAb | 1 – 118 (with 51Q) (aa) |
24, 30 |
| HF1 | Rabbit pAb | 1981 – 2580 (aa) |
13, 14, 31, 32, 35 |
| - | Rabbit pAb | 3099 – 3130 (aa) |
13 |
| HP1 | Rabbit pAb | 80 – 113 | 31 |
| HP12 | Rabbit pAb | 2 – 17 | 31 |
| mAb 2170 | Mouse mAb | 1247 – 1645 (aa) |
8 |
| mAb 2168 | Mouse mAb | 2146 – 2541 (aa) |
8 |
| mAb 1574 | Mouse mAb | Expanded polyQ (TBP*) (aa) |
8, 34 |
| Ab78 | Rabbit pAb | 1 – 17 (aa) |
24 |
| HD1 | Rabbit pAb | 1 – 17 (aa) |
24 |
| HD48 | Rabbit pAb Mouse mAb |
1 – 82 (aa) |
32 |
| EM48 | Rabbit pAb | 1 – 256 (aa) |
28, 33, 35, 36 |
| 4h7h7 | Mouse mAb | 1-149(mouse) 1-171(human) [detects polyQ] |
9 |
| Ab675 | Affinity purified Rabbit pAb |
1 – 17 (aa) |
21 |
| P1874/3B5H10 | Mouse mAb | 1 – 171 with 65Q (aa) |
37 |
| mAb5492(2B4) | Mouse mAb | 1 – 82 (aa) |
38 |
| MW1 | Mouse mAb | DRPLA**-19Q (aa) |
39 |
| MW2 | Mouse mAb | DRPLA-35Q (aa) |
39 |
| MW3 | Mouse mAb | HD exon 1 with 67Q (aa) |
39 |
| MW4 | Mouse mAb | HD exon 1 with 67Q (aa) |
39 |
| MW5 | Mouse mAb | DRPLA-35Q (aa) |
39 |
| MW6 | Mouse mAb | HD exon 1 with 67Q (aa) |
39 |
| MW7 | Mouse mAb | HD exon 1 with 67Q (aa) |
39 |
| MW8 | Mouse mAb | HD exon 1 with 67Q (aa) |
39 |
Immunofluorescent studies with cells in culture have supported both a cytoplasmic and nuclear localisation of HTT [4] [5] [6] [7] [8]. The antibodies employed in these studies have spanned the entire length of the HTT protein (as shown in Table 1) thus indicating that the nuclear and cytoplasmic immunostaining seen does not appear to correspond to any particular HTT fragment, but rather the full length protein, or possibly a mixture of fragments such as those described in the recent study of Landles et al, 2010 [9]. Most of these reports have also employed the complementary technique of subcellular fractionation corroborating the presence of HTT within the cytoplasmic and nuclear fractions of the cells tested [5] [6] [7] [8]. Moreover, in a subgroup of cell types, an array of HTT cleavage products were detected in the nucleus, and in some instances in the cytoplasm, that appeared to be both cell type and species dependent [7] [8]. More recently, the nature of such fragments has been studied in further detail in the HdhQ150 knock-in mouse brain [9]. It is possible that HTT fragments could be differentially located within the cell and indeed, could have individual functions.
In tissue, HTT mainly presents as an exclusively cytoplasmic protein [2] [3] [10] [11] [12] [13] [14] [15], although a few immunohistochemical (IHC) reports also show HTT within nuclei [1] [8] [16] [17]. Factors that may influence the final immunostaining pattern include the antibody used, the fixation and permeabilisation employed, the specific tissues studied and the post-mortem intervals in IHC studies of human tissue. HTT may also adopt different conformations according to cell type or stage of cellular differentiation, thereby evading detection or presenting different epitopes under different conditions.
It is therefore apparent that numerous variables can affect the immunostaining result obtained [18] [19]. Sample preparation requires fixation, to maintain cell structure and prevent antigen leakage, and permeabilisation of cells to allow antibodies access to the specific antigens [20]. This study focuses on the effect that the immunocytological methods of fixation and permeabilisation have on the apparent subcellular localisation of the HTT protein. We examined both main types of fixation method. Organic solvents such as alcohols and acetone remove lipids and dehydrate the cells thereby precipitating the proteins on the cellular architecture. As this has a simultaneous permeabilisation action, the need for a further permeabilisation step is removed. Cross-linking reagents such as paraformaldehyde and glutaraldehyde form intermolecular bridges between proteins and create a network of linked antigens. These do not permeabilise the cell so a further permeabilisation step with either an organic solvent or a non-ionic detergent is used [20]. Here, five different methods of fixation/permeabilisation were compared to investigate the apparent localisation of the HTT protein in cells in culture.
Materials and methods
R6/1 mouse breeding and primary striatal neuron dissection
CBAxC57/BL6 female mice (Harlan Olac) were bred with R6/1 male mice (in-house colony). Pregnant female mice at gestational stage E13-15 were killed and the uterine horns removed and transferred to ice cold DMEM (Invitrogen). All experiments were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986, and local ethical review.
For the dissection of primary neurons, tissue was removed from the whole ganglionic eminence and transferred to eppendorf tubes containing sterile Hanks Buffered Salt Solution (HBSS). Dissected tissue was enzymatically digested using a solution of 0.1% trypsin (Worthington) and 0.05% DNase (Sigma-Aldrich) at 37oC for 20 minutes. Following two rinses in DMEM/F12 containing 0.05% DNase the tissue was dissociated to a single cell suspension by mechanical trituration using a Gilson pipette. Cell counts and percentage viable were assessed by dilution of cell suspensions in trypan blue and counted under an Olympus stereo microscope using a standard haemocytometer grid.
Cell culture
HeLa cells were grown on coverslips in 6 well plates in complete DMEM consisting of DMEM, penicillin/streptomycin (100unitsml-1/100μgml-1) (Invitrogen), glutamine (2mM) (Invitrogen) and 10% fetal bovine serum (FBS) (Invitrogen). IMR32 cells were grown on poly-Lysine coated coverslips in 6 well plates in complete RPMI medium consisting of RPMI medium (Invitrogen), penicillin/streptomycin (100unitsml-1/100μgml-1), glutamine (2mM) and 5% FBS.
To culture primary striatal cells from R6/1 offspring, 150,000 cells per 100μl were plated onto laminin-coated coverslips in 6 well plates. Standard growth media consisted of neurobasal medium (Invitrogen) supplemented with B27 nutrient matrix (1%) (Invitrogen), antibiotic/antimycotic solution (1%) (Invitrogen), Glucose (30mM) (Sigma-Aldrich), glutamine (1mM) and 1% FBS. Cells were allowed to adhere and grow for 7 days prior to processing.
Immunostaining
Five different fixation/permeabilisation methods were employed to assess the contribution of methodology to the immunostaining pattern of HTT observed. These are summarised in Table 2.
Table 2: Fixation and permeabilisation methods used to study the distribution of huntingtin with Ab 675
| Method | Fixation method | Permeabilisation method |
| A | 4% paraformaldehyde for 15 mins at room temperature | 0.1% Triton-X-100 for 30 mins at room temperature |
| B | -10oC methanol for 5 mins: Air dry (rehydrate in Phosphate Buffered Saline (PBS) before processing) | - |
| C | Ice cold Acetone for 2 mins: Air dry (rehydrate in PBS before processing) | - |
| D | 0.25% paraformaldehyde for 15mins followed by 70% ethanol for 1 hour | 0.1% Triton-X-100 for 30 mins at room temperature |
| E | 4% paraformaldehyde for 15 mins at room temperature | 0.5% Triton-X-100 for 2 mins at room temperature |
The primary antibody used to detect HTT was Ab 675, a rabbit polyclonal raised against the N-terminal region (N1-17) of HTT [21]. Following fixation and permeabilisation, cells were blocked in 10% goat serum at room temperature for 2 hours prior to incubation with rabbit polyclonal antibody 675 (1:100 dilution) for 1 hour at 37oC. Goat anti-rabbit secondary antibody alexafluor 568 (1:100 dilution) was subsequently incubated with the cells for 1 hour at 37oC, prior to overnight storage in PBS at 4oC and processing for microscopy. In order to control for false positive results, primary antibody was omitted and the secondary antibody only was added.
Fluorescence microscopy
Cells processed for immunocytochemical analysis were visualised using a Zeiss Axiovert-S100 TV fluorescence microscope (Zeiss) using an excitation 560/40 / emission 630/60 filter. Optimal magnification of the cell images was achieved using immersion oil and an apical zoom lens (x40).
Confocal laser scanning microscopy (clsm)
A 1024-MP laser scanning microscope (lsm) (BioRad Microscience) was used to obtain optical sections through different cells immunostained with antibody 675. The 1024 scan head was attached to a Zeiss Axiovert-S100 TV microscope. This instrument was used in two modes 1) EGFP confocal mode using a visible krypton-argon ion laser (AL) at 488nm and 2) ECFP-multiphoton mode using a pulsed infrared laser 5w Verdi-mira-900 (Coherent Ltd, UK) at 780nm. A selection of typical high-resolution (x,y) single frames (512 x 512; 0.035μm x 0.035μm pixel size) were collected with a x40 1.3 NA oil immersion lens.
Results
HTT immunostaining in HeLa cells with various methods of cell processing

Figure 1 : Immunolocalisation of endogenous huntingtin within HeLa cells using various methods of cell processing
Hela cells treated with different methods of fixation and permeabilisation (Table 2) displayed varying patterns of HTT localisation. Nuclear localisation was more prominent when cross-linking fixation methods were used, and diminished when more dehydrating methods were employed. Cells were incubated with Ab 675 (1:100) and a goat-anti-rabbit-568 secondary antibody (1:100) prior to visualisation by confocal microscopy (CLSM). Scale bar = 10µm.
Method A:
HeLa cells fixed with 4% paraformaldehyde and permeabilised with 0.1% Triton-X-100, exhibited HTT immunostaining with Ab 675 in both the cytoplasm and nucleus in approximately 85-90% of cells studied. The remaining 10-15% of cells exhibited an exclusively cytoplasmic stain (Figure 1A). The overall appearance was very granular with numerous bright punctae interspersed throughout the whole cell. The discrete punctae were more pronounced when studied by clsm due to the higher resolution. In the majority of cells, immunostaining was of equal intensity in both cytoplasmic and nuclear compartments, and within the nucleus, there was an intense labelling of punctate bodies.
Method B
HeLa cells fixed and permeabilised with 100% ice-cold methanol demonstrated a predominantly cytoplasmic HTT localisation and only faint staining of the nucleoplasm (Figure 1B). Brightly stained punctate bodies were noted within the nucleus. Dehydration of the cells was apparent given their shrunken appearance.
Method C
Treatment with ice-cold acetone also resulted in an apparent absence of HTT from the nucleoplasm and the localisation of HTT to punctate bodies within the nucleus. Cytoplasmic staining of HTT was intense and more diffuse compared with the nucleus (Figure 1C). However, cell morphology was adversely affected with enlarged nuclei and shrunken cytoplasm noted.
Method D
A combination of both detergent and solvent was used in an attempt to preserve cellular architecture. Cells were fixed in 0.25% paraformaldehyde for 15 mins at room temperature prior to a further fixation/permeabilisation in 70% ethanol for 1 hour and a subsequent permeabilisation step with 0.1% Triton-X-100. Cell morphology appeared less distorted than for methods B and C. There was an absence of diffuse HTT staining from the nucleoplasm yet it appeared lightly speckled and HTT localised to punctate nuclear bodies. The immunostain within the cytoplasm was granular and punctate (Figure 1D).
Method E
Cells fixed in 4% paraformaldehyde and permeabilised more vigorously with 0.5% Triton-X-100 for 2 minutes acquired a peculiar appearance. Some cells maintained a diffuse nuclear immunostain whereas other cells had nuclei completely devoid of HTT signal. HTT was seen to collapse into a thin perinuclear ring (Figure 1E).
It is noteworthy that in each case, immunostaining with secondary antibody alone produced no significant stain thereby excluding a contribution from the secondary antibody in the staining pattern produced (data not shown).
HTT immunostaining in IMR32 cells and mouse primary striatal neurons
To extend this investigation, the immunostaining of HTT within IMR32 cells that had been fixed and permeabilised by methods A and C respectively was performed. This compared a cross-linking method with a dehydration method.
This supported the finding of a fixation method-dependent effect upon the immunostaining pattern observed. Acetone fixation created much background debris and little nuclear staining (Figure 2B), whereas paraformaldehyde resulted in a nuclear and punctate cytoplasmic stain (Figure 2A).
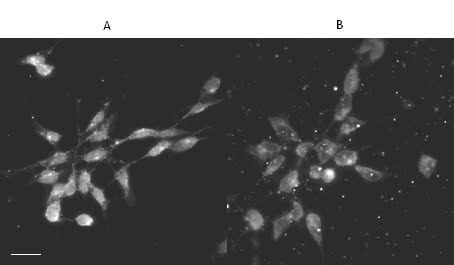
Figure 2 : Immunolocalisation of endogenous huntingtin within IMR32 cells fixed by two different methods
IMR32 cells were fixed in 4% paraformaldehyde and permeabilised in 0.1% Triton-X-100 for 30 mins (A) or fixed in ice cold acetone for 2 mins (B). Acetone fixation created much background debris and little nuclear staining (B), whereas paraformaldehyde fixation showed apparent localisation of HTT within the nucleus and a punctate pattern in the cytoplasm (A). Cells were incubated with Ab 675 (1:100) and a goat-anti-rabbit-568 secondary antibody (1:100) prior to visualisation on clsm. Scale bar = 20mm.
Similarly, in mouse primary striatal neurons, HTT immunostaining varied with the immunocytochemical method employed. The fixation protocol was critical for these cells to maintain their structure and adherence to the coverslip. Cells were fixed in 4% paraformaldehyde for 15 mins at room temperature in all cases, however, the subsequent permeabilisation method was varied. Cells were permeabilised with either Triton-X-100 (0.1%) for 30 mins at room temperature or 100% ethanol for 2 mins (Figure 3).

Figure 3 : Immunolocalisation of endogenous huntingtin within primary striatal neurons permeabilised by two different methods
Mouse primary striatal neurons were fixed in 4% paraformaldehyde and permeabilised with either 0.1% Triton-X-100 (A) or 100% ethanol (B). The HTT immunostaining pattern observed was similar for both permeabilisation reagents, as it appeared diffusely in both the cytoplasm and nucleus (A, B). However, it exhibited a more punctate pattern in axons when treated with Triton-X-100 (A). Cells were incubated with antibody 675 (1:100) in conjunction with the goat-anti-rabbit-568 secondary antibody (1:100) and visualised by clsm. Scale bar = 20mm.
The immunostaining pattern observed was similar when compared between different permeabilisation methods with HTT appearing in both the cytoplasm and nucleus, and the nuclear signal being more intense than that of the cytoplasm (Figure 3A, B). However, permeabilisation with Triton-X-100 led to a more punctate pattern within axons (Figure 3A) compared with the ethanol treatment (Figure 3B). This demonstrated that the choice of permeabilisation reagent can also have an effect on the apparent localisation of HTT observed, in addition to the fixative used.
Discussion
Details of the precise subcellular localisation of the HTT protein remain unclear and the varying methodologies employed to address this issue create more uncertainty. Most studies support the finding that HTT exists in both the cytoplasm and nucleus, although discrepancies do exist and the exact nature of the protein in each compartment remains ill defined. It is well established that the quality of the immunostaining pattern obtained is dependent upon numerous factors [19] and this brief study investigates the effect of fixation and permeabilisation methods on the resultant subcellular localisation of HTT. A significant effect was demonstrated whereby the nuclear localisation was more prominent when cross-linking fixation methods were used, and diminished when more dehydrating methods were employed. This was recognised for both HeLa and IMR32 cells and is consistent with previous immunostaining studies with HTT in purkinje cells of rabbit cerebellum [17] and in primary human fibroblasts [8]. Although the purpose of this study was not to compare HTT localisation between cell types, it is noteworthy that the pattern of distribution of HTT differed between cell types. A similar conclusion was drawn in the study of De Rooij et al., 1996 [5].
We have also shown that certain methods can lead to obvious cellular damage and therefore questionable validity of some of the patterns observed. The method of choice would therefore be a compromise between preservation of the cellular microarchitecture whilst retaining the epitopes of interest [18] [20].
This study confirms that immunostaining patterns should be interpreted with caution and related closely to the methodology employed where a lack of consistency is found between studies. Using additional methods for corroboration such as live cell overexpression studies and subcellular fractionation, may help support findings though these too have their own issues of validity. Accurate subcellular localisation of HTT is important to define its role within the cell both normally and pathologically and so an awareness of the effects of varying fixation and permeabilisation methods on this localisation is important. Methodology may disturb protein conformation and function and this could lead to false conclusions being drawn from artifactual findings.
Acknowledgements
We thank Lyn Elliston for technical assistance, Rachel Errington for assistance with confocal laser scanning microscopy and Rosemary Fricker-Gates for her help with primary striatal neuron preparation.
Funding information
This study was supported by a MRC studentship to ACH.
Competing interests
The authors have declared that no competing interests exist.
References
- Hoogeveen AT, Willemsen R, Meyer N, de Rooij KE, Roos RA, van Ommen GJ, Galjaard H. Characterization and localization of the Huntington disease gene product. Hum Mol Genet. 1993 Dec;2(12):2069-73.http://www.ncbi.nlm.nih.gov/pubmed/8111375 [DOI] [PubMed]
- Trottier Y, Devys D, Imbert G, Saudou F, An I, Lutz Y, Weber C, Agid Y, Hirsch EC, Mandel JL. Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat Genet. 1995 May;10(1):104-10.http://www.ncbi.nlm.nih.gov/pubmed/7647777 [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Levey AI, Heilman CJ, Whaley WL, Yi H, Nash NR, Rees HD, Madden JJ, Hersch SM. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8710-4. PubMed Central PMCID: PMC41036.http://www.ncbi.nlm.nih.gov/pubmed/7568002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessert DA, Gutridge KL, Dunbar JC, Carlock LR. The identification of a functional nuclear localization signal in the Huntington disease protein. Brain Res Mol Brain Res. 1995 Oct;33(1):165-73.http://www.ncbi.nlm.nih.gov/pubmed/8774958 [DOI] [PubMed] [Google Scholar]
- De Rooij KE, Dorsman JC, Smoor MA, Den Dunnen JT, Van Ommen GJ. Subcellular localization of the Huntington's disease gene product in cell lines by immunofluorescence and biochemical subcellular fractionation. Hum Mol Genet. 1996 Aug;5(8):1093-9.http://www.ncbi.nlm.nih.gov/pubmed/8842726 [DOI] [PubMed] [Google Scholar]
- Dorsman JC, Smoor MA, Maat-Schieman ML, Bout M, Siesling S, van Duinen SG, Verschuuren JJ, den Dunnen JT, Roos RA, van Ommen GJ. Analysis of the subcellular localization of huntingtin with a set of rabbit polyclonal antibodies in cultured mammalian cells of neuronal origin: comparison with the distribution of huntingtin in Huntington's disease autopsy brain. Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1061-7. PubMed Central PMCID: PMC1692596.http://www.ncbi.nlm.nih.gov/pubmed/10434306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Tartakoff AM. Nuclear relocation of normal huntingtin. Traffic. 2001 Jun;2(6):385-94.http://www.ncbi.nlm.nih.gov/pubmed/11389766 [DOI] [PubMed] [Google Scholar]
- Kegel KB, Meloni AR, Yi Y, Kim YJ, Doyle E, Cuiffo BG, Sapp E, Wang Y, Qin ZH, Chen JD, Nevins JR, Aronin N, DiFiglia M. Huntingtin is present in the nucleus, interacts with the transcriptional corepressor C-terminal binding protein, and represses transcription. J Biol Chem. 2002 Mar 1;277(9):7466-76. Epub 2001 Dec 5.http://www.ncbi.nlm.nih.gov/pubmed/11739372 [DOI] [PubMed] [Google Scholar]
- Landles C, Sathasivam K, Weiss A, Woodman B, Moffitt H, Finkbeiner S, Sun B, Gafni J, Ellerby LM, Trottier Y, Richards WG, Osmand A, Paganetti P, Bates GP. Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease. J Biol Chem. 2010 Mar 19;285(12):8808-23. Epub 2010 Jan 19.http://www.ncbi.nlm.nih.gov/pubmed/20086007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel JP, Carraway R, Reeves SA, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995 May;14(5):1075-81.http://www.ncbi.nlm.nih.gov/pubmed/7748555 [DOI] [PubMed] [Google Scholar]
- Sharp AH, Loev SJ, Schilling G, Li SH, Li XJ, Bao J, Wagster MV, Kotzuk JA, Steiner JP, Lo A, et al. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron. 1995 May;14(5):1065-74.http://www.ncbi.nlm.nih.gov/pubmed/7748554 [DOI] [PubMed] [Google Scholar]
- Bhide PG, Day M, Sapp E, Schwarz C, Sheth A, Kim J, Young AB, Penney J, Golden J, Aronin N, DiFiglia M. Expression of normal and mutant huntingtin in the developing brain. J Neurosci. 1996 Sep 1;16(17):5523-35.http://www.ncbi.nlm.nih.gov/pubmed/8757264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski CM, Cha JH, Young AB, Persichetti F, MacDonald M, Gusehttp://www.ncbi.nlm.nih.gov/pubmed/7647777Edit Delete lla JF, Penney JB Jr, Standaert DG. Huntingtin immunoreactivity in the rat neostriatum: differential accumulation in projection and interneurons. Exp Neurol. 1997 Apr;144(2):239-47.http://www.ncbi.nlm.nih.gov/pubmed/9168825 [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Gutekunst CA, Persichetti F, McNeil SM, Kowall NW, Gusella JF, MacDonald ME, Beal MF, Hersch SM. Heterogeneous topographic and cellular distribution of huntingtin expression in the normal human neostriatum. J Neurosci. 1997 May 1;17(9):3052-63.http://www.ncbi.nlm.nih.gov/pubmed/9096140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997 Sep 26;277(5334):1990-3.http://www.ncbi.nlm.nih.gov/pubmed/9302293 [DOI] [PubMed] [Google Scholar]
- Sapp E, Schwarz C, Chase K, Bhide PG, Young AB, Penney J, Vonsattel JP, Aronin N, DiFiglia M. Huntingtin localization in brains of normal and Huntington's disease patients. Ann Neurol. 1997 Oct;42(4):604-12.http://www.ncbi.nlm.nih.gov/pubmed/9382472 [DOI] [PubMed] [Google Scholar]
- Wilkinson FL, Nguyen TM, Manilal SB, Thomas P, Neal JW, Harper PS, Jones AL, Morris GE. Localization of rabbit huntingtin using a new panel of monoclonal antibodies. Brain Res Mol Brain Res. 1999 May 21;69(1):10-20.http://www.ncbi.nlm.nih.gov/pubmed/10350633 [DOI] [PubMed] [Google Scholar]
- Fritschy JM. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur J Neurosci. 2008 Dec;28(12):2365-70. Review.http://www.ncbi.nlm.nih.gov/pubmed/19087167 [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci. 2008 Sep 10;28(37):9083-6.http://www.ncbi.nlm.nih.gov/pubmed/18784286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melan MA. Overview of cell fixatives and cell membrane permeants. Methods Mol Biol. 1999;115:45-55. Review.http://www.ncbi.nlm.nih.gov/pubmed/10098165 [DOI] [PubMed] [Google Scholar]
- Boutell JM, Wood JD, Harper PS, Jones AL. Huntingtin interacts with cystathionine beta-synthase. Hum Mol Genet. 1998 Mar;7(3):371-8.http://www.ncbi.nlm.nih.gov/pubmed/9466992 [DOI] [PubMed] [Google Scholar]
- Velier J, Kim M, Schwarz C, Kim TW, Sapp E, Chase K, Aronin N, DiFiglia M. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp Neurol. 1998 Jul;152(1):34-40.http://www.ncbi.nlm.nih.gov/pubmed/9682010 [DOI] [PubMed] [Google Scholar]
- Laforet GA, Sapp E, Chase K, McIntyre C, Boyce FM, Campbell M, Cadigan BA, Warzecki L, Tagle DA, Reddy PH, Cepeda C, Calvert CR, Jokel ES, Klapstein GJ, Ariano MA, Levine MS, DiFiglia M, Aronin N. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J Neurosci. 2001 Dec 1;21(23):9112-23.http://www.ncbi.nlm.nih.gov/pubmed/11717344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997 Aug 8;90(3):537-48.http://www.ncbi.nlm.nih.gov/pubmed/9267033 [DOI] [PubMed] [Google Scholar]
- Hackam AS, Singaraja R, Wellington CL, Metzler M, McCutcheon K, Zhang T, Kalchman M, Hayden MR. The influence of huntingtin protein size on nuclear localization and cellular toxicity. J Cell Biol. 1998 Jun 1;141(5):1097-105.http://www.ncbi.nlm.nih.gov/pubmed/9606203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D, Hackam A, Wieczorek A, Ellerby L, Wellington C, McCutcheon K, Singaraja R, Kazemi-Esfarjani P, Devon R, Kim SU, Bredesen DE, Tufaro F, Hayden MR. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nat Genet. 1998 Feb;18(2):150-4.http://www.ncbi.nlm.nih.gov/pubmed/9462744 [DOI] [PubMed] [Google Scholar]
- Kalchman MA, Graham RK, Xia G, Koide HB, Hodgson JG, Graham KC, Goldberg YP, Gietz RD, Pickart CM, Hayden MR. Huntingtin is ubiquitinated and interacts with a specific ubiquitin-conjugating enzyme. J Biol Chem. 1996 Aug 9;271(32):19385-94.http://www.ncbi.nlm.nih.gov/pubmed/8702625 [DOI] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999 May;23(1):181-92.http://www.ncbi.nlm.nih.gov/pubmed/10402204 [DOI] [PubMed] [Google Scholar]
- Wood JD, MacMillan JC, Harper PS, Lowenstein PR, Jones AL. Partial characterisation of murine huntingtin and apparent variations in the subcellular localisation of huntingtin in human, mouse and rat brain. Hum Mol Genet. 1996 Apr;5(4):481-7.http://www.ncbi.nlm.nih.gov/pubmed/8845840 [DOI] [PubMed] [Google Scholar]
- Martín-Aparicio E, Avila J, Lucas JJ. Nuclear localization of N-terminal mutant huntingtin is cell cycle dependent. Eur J Neurosci. 2002 Jul;16(2):355-9.http://www.ncbi.nlm.nih.gov/pubmed/12169117 [DOI] [PubMed] [Google Scholar]
- Persichetti F, Ambrose CM, Ge P, McNeil SM, Srinidhi J, Anderson MA, Jenkins B, Barnes GT, Duyao MP, Kanaley L, et al. Normal and expanded Huntington's disease gene alleles produce distinguishable proteins due to translation across the CAG repeat. Mol Med. 1995 May;1(4):374-83.http://www.ncbi.nlm.nih.gov/pubmed/8521295 [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Williams M, Charles V, Garrett L, Pike-Buchanan L, Whetsell WO Jr, Miller G, Tagle DA. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet. 1998 Oct;20(2):198-202.http://www.ncbi.nlm.nih.gov/pubmed/9771716 [DOI] [PubMed] [Google Scholar]
- Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington's disease mice. J Neurosci. 2001 Nov 1;21(21):8473-81.http://www.ncbi.nlm.nih.gov/pubmed/11606636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999 Mar;8(3):397-407. Erratum in: Hum Mol Genet 1999 May;8(5):943.http://www.ncbi.nlm.nih.gov/pubmed/9949199 [DOI] [PubMed] [Google Scholar]
- Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li XJ, Li SH, Yi H, Vonsattel JP, Gusella JF, Hersch S, Auerbach W, Joyner AL, MacDonald ME. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet. 2000 Mar 1;9(4):503-13.http://www.ncbi.nlm.nih.gov/pubmed/10699173 [DOI] [PubMed] [Google Scholar]
- Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, Ren S, Li XJ, Albin RL, Detloff PJ. Neurological abnormalities in a knock-in mouse model of Huntington's disease. Hum Mol Genet. 2001 Jan 15;10(2):137-44.http://www.ncbi.nlm.nih.gov/pubmed/11152661 [DOI] [PubMed] [Google Scholar]
- Legleiter J, Lotz GP, Miller J, Ko J, Ng C, Williams GL, Finkbeiner S, Patterson PH, Muchowski PJ. Monoclonal antibodies recognize distinct conformational epitopes formed by polyglutamine in a mutant huntingtin fragment. J Biol Chem. 2009 Aug 7;284(32):21647-58. Epub 2009 Jun 2.http://www.ncbi.nlm.nih.gov/pubmed/19491400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon ES, Hladik CL, Shang P, Burns DK, Raisanen J, White CL 3rd. Neuroanatomic profile of polyglutamine immunoreactivity in Huntington disease brains. J Neuropathol Exp Neurol. 2009 Mar;68(3):250-61.http://www.ncbi.nlm.nih.gov/pubmed/19225411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Ou S, Patterson PH. New anti-huntingtin monoclonal antibodies: implications for huntingtin conformation and its binding proteins. Brain Res Bull. 2001 Oct-Nov 1;56(3-4):319-29.http://www.ncbi.nlm.nih.gov/pubmed/11719267 [DOI] [PubMed] [Google Scholar]


