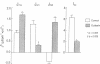Abstract
The role of apical and basolateral membranes in aldosterone-induced active potassium (K) secretion in rat distal colon was investigated by measuring mucosal-to-serosal (Jms) and serosal-to-mucosal (Jsm) 42K fluxes (mueq.h-1.cm-2) across isolated stripped mucosa under short-circuit conditions in normal and secondary-hyperaldosterone animals. In normal colons mucosal tetraethylammonium (TEA; 30 mM) or barium (Ba; 5 mM), but not cesium (Cs; 15 mM), reduced Jsm without affecting Jms. In aldosterone animals (a) net K secretion (-0.54 +/- 0.11) was converted to net K absorption (0.63 +/- 0.15) by mucosal TEA, which produced a marked reduction in Jsm (0.82 +/- 0.07) and an increase in Jms (0.35 +/- 0.07). In contrast mucosal Ba resulted in a relatively smaller reduction in JK(sm) without altering JK(ms), whereas mucosal Cs was ineffective; (b) serosal bumetanide or the removal of serosal Na or Cl markedly inhibited JK(sm and abolished net K secretion; and (c) serosal ouabain (1 mM) produced qualitatively similar effects to those of serosal bumetanide. These results demonstrate that (a) normal rat distal colon contains apical TEA- and Ba-sensitive K channels; (b) aldosterone induces TEA-sensitive and Ba-sensitive apical K channels; (c) aldosterone-induced K secretion requires both the Na,K-pump and Na-K-2Cl cotransport for K uptake across the basolateral membrane; and (d) alteration of any of these processes results in inhibition of aldosterone-induced active K secretion simultaneously with stimulation of K absorption.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demarest J. R., Finn A. L. Characterization of the basolateral membrane conductance of Necturus urinary bladder. J Gen Physiol. 1987 Apr;89(4):541–562. doi: 10.1085/jgp.89.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest J. R., Finn A. L. Interaction between the basolateral K+ and apical Na+ conductances in Necturus urinary bladder. J Gen Physiol. 1987 Apr;89(4):563–580. doi: 10.1085/jgp.89.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Mandel K. G., Masui H., McRoberts J. A. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl- cotransport system. J Clin Invest. 1985 Feb;75(2):462–471. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of potassium by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):603–617. doi: 10.1113/jphysiol.1967.sp008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E. S., Hayslett J. P., Binder H. J. Mechanism of active potassium absorption and secretion in the rat colon. Am J Physiol. 1984 May;246(5 Pt 1):G611–G617. doi: 10.1152/ajpgi.1984.246.5.G611. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Jones W. J., Hayslett J. P., Binder H. J. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology. 1985 Jan;88(1 Pt 1):41–46. doi: 10.1016/s0016-5085(85)80130-x. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Sandle G. I., Hayslett J. P., Binder H. J. Dietary potassium modulates active potassium absorption and secretion in rat distal colon. Am J Physiol. 1986 Nov;251(5 Pt 1):G619–G626. doi: 10.1152/ajpgi.1986.251.5.G619. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Zimmerman T. W., Hayslett J. P., Binder H. J. Corticosteroid alteration of active electrolyte transport in rat distal colon. Am J Physiol. 1983 Nov;245(5 Pt 1):G668–G675. doi: 10.1152/ajpgi.1983.245.5.G668. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Lang F. Evidence for electroneutral sodium chloride cotransport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983 Mar;396(4):308–314. doi: 10.1007/BF01063936. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Schultz S. G. Potassium transport and intracellular potassium activities in rabbit gallbladder. J Membr Biol. 1982;65(1-2):41–47. doi: 10.1007/BF01870467. [DOI] [PubMed] [Google Scholar]
- Halevy J., Budinger M. E., Hayslett J. P., Binder H. J. Role of aldosterone in the regulation of sodium and chloride transport in the distal colon of sodium-depleted rats. Gastroenterology. 1986 Nov;91(5):1227–1233. doi: 10.1016/s0016-5085(86)80021-x. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol. 1986 Aug;251(2 Pt 1):C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Ishida H., Suzuki Y. Potassium secretion in the guinea pig distal colon. Jpn J Physiol. 1987;37(1):33–48. doi: 10.2170/jjphysiol.37.33. [DOI] [PubMed] [Google Scholar]
- Kashgarian M., Taylor C. R., Binder H. J., Hayslett J. P. Amplification of cell membrane surface in potassium adaptation. Lab Invest. 1980 Jun;42(6):581–588. [PubMed] [Google Scholar]
- Kawahara K., Hunter M., Giebisch G. Potassium channels in Necturus proximal tubule. Am J Physiol. 1987 Sep;253(3 Pt 2):F488–F494. doi: 10.1152/ajprenal.1987.253.3.F488. [DOI] [PubMed] [Google Scholar]
- Kinne R., Hannafin J. A., König B. Role of the NaCl-KCl cotransport system in active chloride absorption and secretion. Ann N Y Acad Sci. 1985;456:198–206. doi: 10.1111/j.1749-6632.1985.tb14865.x. [DOI] [PubMed] [Google Scholar]
- Martin R. S., Jones W. J., Hayslett J. P. Animal model to study the effect of adrenal hormones on epithelial function. Kidney Int. 1983 Sep;24(3):386–391. doi: 10.1038/ki.1983.171. [DOI] [PubMed] [Google Scholar]
- McCabe R. D., Smith P. L., Sullivan L. P. Ion transport by rabbit descending colon: mechanisms of transepithelial potassium transport. Am J Physiol. 1984 May;246(5 Pt 1):G594–G602. doi: 10.1152/ajpgi.1984.246.5.G594. [DOI] [PubMed] [Google Scholar]
- McCabe R., Cooke H. J., Sullivan L. P. Potassium transport by rabbit descending colon. Am J Physiol. 1982 Jan;242(1):C81–C86. doi: 10.1152/ajpcell.1982.242.1.C81. [DOI] [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am J Physiol. 1987 Aug;253(2 Pt 1):C177–C192. doi: 10.1152/ajpcell.1987.253.2.C177. [DOI] [PubMed] [Google Scholar]
- O'Grady S. M., Palfrey H. C., Field M. Na-K-2Cl cotransport in winter flounder intestine and bovine kidney outer medulla: [3H] bumetanide binding and effects of furosemide analogues. J Membr Biol. 1987;96(1):11–18. doi: 10.1007/BF01869330. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Sansom S. C. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82(3):281–295. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- Plass H., Gridl A., Turnheim K. Absorption and secretion of potassium by rabbit descending colon. Pflugers Arch. 1986 May;406(5):509–519. doi: 10.1007/BF00583375. [DOI] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A., Grady T. P. Intracellular K+ activity and its relation to basolateral membrane ion transport in Necturus gallbladder epithelium. J Gen Physiol. 1980 Jul;76(1):33–52. doi: 10.1085/jgp.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Kinetics of the inhibition of the Na-K pump by external sodium. J Physiol. 1977 Jan;264(2):449–470. doi: 10.1113/jphysiol.1977.sp011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle G. I., Foster E. S., Lewis S. A., Binder H. J., Hayslett J. P. The electrical basis for enhanced potassium secretion in rat distal colon during dietary potassium loading. Pflugers Arch. 1985 Apr;403(4):433–439. doi: 10.1007/BF00589258. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Biagi B. Active potassium transport by rabbit descending colon epithelium. J Membr Biol. 1982;64(3):195–203. doi: 10.1007/BF01870886. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A., Eaton D. C. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol. 1979 Mar 28;45(1-2):81–108. doi: 10.1007/BF01869296. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Zeiske W., Van Driessche W. Noise analysis reveals K+ channel conductance fluctuations in the apical membrane of rabbit colon. J Membr Biol. 1982;69(3):187–197. doi: 10.1007/BF01870398. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Zweifach A. Recent advances in the characterization of epithelial ionic channels. Biochim Biophys Acta. 1987 Apr 27;906(1):1–31. doi: 10.1016/0304-4157(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Wingo C. S. Cortical collecting tubule potassium secretion: effect of amiloride, ouabain, and luminal sodium concentration. Kidney Int. 1985 Jun;27(6):886–891. doi: 10.1038/ki.1985.96. [DOI] [PubMed] [Google Scholar]