Abstract
Lantibiotics are antimicrobial peptides which can have a broad spectrum activity against many Gram positive pathogens. Many of these peptides contain charged amino acids which may be of critical importance with respect to antimicrobial activity. We have recently carried out an in-depth bioengineering based investigation of the importance of charged residues in a representative two peptide lantibiotic, lacticin 3147, and here we discuss the significance of these findings in the context of other lantibiotics and cationic antimicrobial peptides.
Key words: lantibiotics, lacticin 3147, charged residue, bioengineering, antimicrobial peptides
We have recently carried out an extensive bioengineering-based investigation of the importance of the charged amino acids within two peptide lantibiotics using lacticin 3147 as a model.1 Lantibiotics are peptide antimicrobials that are ribosomally synthesized, are distinguished by the presence of unusual post-translationally generated structures known as lanthionines and β-methylanthionines and frequently also contain other atypical residues including dehydroalanine (Dha).2–7 Although most lantibiotics are single peptide antimicrobials, an ever-increasing number of two peptide lantibiotics have been identified.8 The majority of these share a significant degree of homology (with the Enterococcus-associated cytolysin being a notable exception) and thus much of the information gleaned from the detailed investigation of a number of these two peptide lantibiotics, and lacticin 3147 and haloduracin in particular, is likely to also be relevant to the two peptide group as a whole (which also includes staphylococcin C55, plantaricin W, Smb, BHT-A, lichenicidin and a predicted pneumococcin).9–16 Two peptide lantibiotics are active as a consequence of the synergistic activity of two (β-methyl) lanthionine-containing peptides. While each individual peptide (designated a and β respectively) usually retains at least some antimicrobial activity, this solo activity is only apparent at much higher concentrations.12,17–20
It was previously noted that a number of lantibiotics resemble cationic antimicrobial peptides (cAMPS) by virtue of having long linear structures and a cationic charge as well as being able to form pores.21 cAMPS typically have a positive charge of between +2 to +9 and have broad spectrum of activity against bacteria through disruption of anionic bacterial membrane.22,23 The importance of the overall charge of cAMPS has been revealed through the creation of derivatives in which positively charged residues are manipulated. This was particularly apparent when a number of analogs were generated of the cationic peptide V13K, which has a +7 net charge. Variants with a reduced positive charge of +4 exhibited reduced activity whereas analogs made by increasing the net charge from +7 to +10 possessed increased activity against many Gram positive and Gram negative bacteria.24 Thus the importance of the net charge of this peptide is very apparent. A number of bioengineering based studies have been carried out to determine the importance of charged amino acids with respect to the antimicrobial activity of lantibiotics. However, until our recent study investigating the importance of charged residues in lacticin 3147,1 relatively few ‘charge’ mutants have been made. Nonetheless, to put the newly generated lacticin 3147-associated data into context, we will first summarise the consequences of charge residue manipulations in other lantibiotics, starting with two quite different lantibiotics, nisin and Pep5.
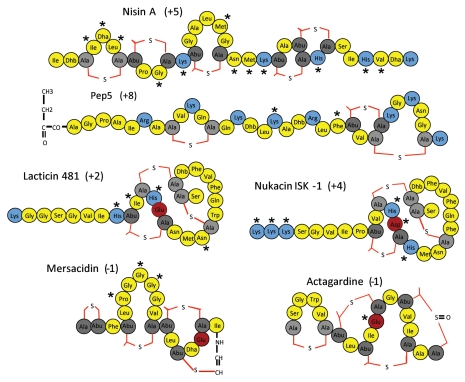
Nisin and Pep5 are subclass I lantibiotics and are distinguished from subclass II lantibiotics, such as the two peptide lantibiotics and related peptides, on the basis of the enzymes employed to generate (β-methyl) lanthionine structures. Both nisin and Pep5 are cationic and contain a number of charged residues within the central and the C-terminal regions. A number of charge-altered derivatives of nisin and, to a lesser extent, Pep5 have been generated (Fig. 1). The nisin peptides referred to are derivatives of two natural forms of nisin, i.e., nisin A and Z, which differ by one amino acid in that nisin A and Z have a histidine and an asparagine, respectively, at position 27. Within the pore-forming C-terminal region of nisin Z, changes which result in the incorporation of an additional positively charged residue [i.e., N27K (where N to K is the substitution and 27 indicates the location of the residue within the mature nisin Z peptide) and V32K] or a change from one positively charged residue to another (H31K) retained levels of activity which were similar to that of the wild type peptide but had the advantage in some instances of increased solubility.25,26 However, the importance of charge within this region of the peptide was very apparent when it was established that the introduction of a negatively charged residue (V32E) causes a 4-fold decrease in antimicrobial activity.26,27 In contrast, the consequences of manipulating charged residues at other locations within the nisin Z peptide have been much more variable. This was apparent from studies which revealed that the introduction of a lysine at position 17 (M17K) resulted in a 50% reduction in antimicrobial activity26 while the loss of a natural lysine at position 12 had no deleterious impact.25 A central three amino acid region of the nisin peptide, known as the hinge (consisting of N20-M21-K22), which links the N-terminal (receptor-binding) and C-terminal domains has been the subject of a particularly intensive engineering-based investigation and yet again a number of charge-altering changes resulted in quite variable consequences. It was noted that while variants of nisin A and nisin Z made by introducing or exchanging positively charged residues, such as N20H, N20R, M21H, M21R, K22H, K22R and N20K/M21K, generally had a slightly negative impact,28,29 exceptions were apparent as the N20K and M21K peptides showed enhanced activity against Gram negative pathogenic bacteria such as Shigella, Salmonella and Pseudomonas.28 However some variants in which a positive charge were removed, i.e., K22T and K22S, also brought about enhanced activity against targets such as Streptococcus agalactiae and Staphylococcus aureus.29 In contrast to the varying consequences of manipulating positively charged residues within the nisin hinge, the introduction of negatively charged residues (N20E, N20D, M21E, K22E and K22D) consistently had negative consequences.28 Finally, a number of charge variants have been created in which the N-terminal ring A of nisin A had been altered, i.e., KFI, KSI, TKI (letters represent the amino acids in ring A corresponding to positions 4–6 which are normally occupied by the uncharged residues IDhaL). The impact of the newly incorporated lysine residue was found to be location specific as both the KFI- and KSI-containing peptides exhibited enhanced activity whereas a TKI mutant retained no antimicrobial activity. A G10H change was also found to have a similarly negative impact.30 In contrast to the extensive charge manipulation of nisin that has occurred, only three Pep5 equivalents exist. Yet again the negative consequences of introducing an additional negative charge (F23D) was apparent while removal of one of the eight positively charged residues also impacted negatively (K18S, K18P).31 However the collection of Pep5 mutants would need to be expanded in order to establish general Pep5-related trends.
Figure 1.
Structures of the lantibiotics Nisin A, Pep5, Lacticin 481, Nukacin ISK-1 with charged amino acids highlighted (positively charged residues in blue, negatively charged residues in maroon, Ala-S-Ala [lanthionine] as light grey, Abu-S-Ala [β-methyl lanthionine] in dark grey colored, other amino acid residues in yellow color). Asterisks correspond to residues altered in the charge mutants referred to in the text.
The single peptide lantibiotics lacticin 481, nukacin ISK-1 and mersacidin are representative of subclass II lantibiotics and each possess conserved motifs which are also found among the α peptide of two peptide lantibiotics.32,33 Of the three, mersacidin is most closely related to α peptides. The antimicrobial activity of a number of derivatives of this peptide have been assessed in which positively charged residues have been incorporated (P6H, G8H and G9H) and, although the activity of G9H was found to be close to that of the wild-type peptide, the activity of both P6H and G8H was reduced.34 As a consequence of the site-saturation mutagenesis of nukacin ISK-1, a large collection of charge-altered mutants are available. The majority of these have been assessed through bioactivity-based studies alone (i.e., studies in which the antimicrobial activity of individual lantibiotic-producing strains rather than equimolar concentrations of purified peptides) and these have revealed that mutagenesis of the N-terminal lysine's, K1, K2 and K3, is generally well tolerated except when aspartate or glutamate residues are incorporated. Although mutagenesis of H15 is also generally well tolerated, changes to either H12 or D13 (with the exception of D13E) have very detrimental consequences indicating that these natural residues have an important role. It was also noted that, in the majority of cases, the introduction of charged residues (either positive or negative) at positions not normally occupied by similarly charged residues also had a negative impact. As noted above the D13E change did not have a detrimental impact. Indeed this alteration was beneficial in that it resulted in a derivative with enhanced activity against a number of targets.35 Notably this alteration results in the introduction of a glutamate at a location occupied by glutamate residues in very many related peptides including the a component of two peptide lantibiotics, mersacidin and lacticin 481. With respect to lacticin 481, while a variety of charge variants of lacticin 481 have been generated (N41D, H32D, H36R and I34D-H36R [NB. the numbering system employed for describing lacticin 481 mutants starts at the N-terminal end of a leader peptide which is cleaved during export rather than at the beginning of the propeptide]) and exhibit antimicrobial activity, it is not apparent if this activity is enhanced or reduced relative to the wild type peptide.36
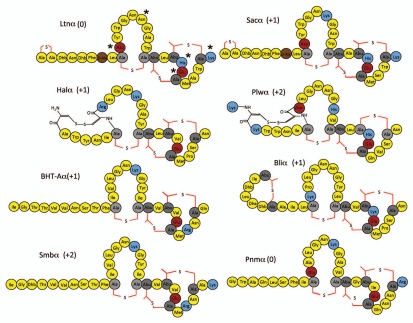
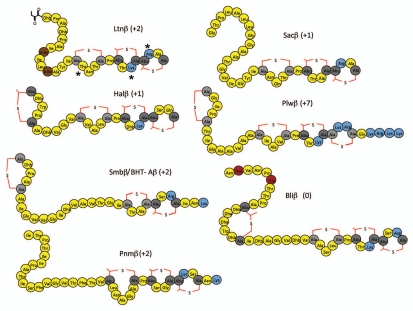
Comparison of the individual components of the two peptide lantibiotics (designated α and β) reveals that in most cases both peptides have an overall positive charge (Figs. 2 and 3). Although the α peptides are usually cationic, they all possess at least one negatively charged residue and as a consequence of being globular do not resemble classical cationic antimicrobial peptides. Indeed two of these peptides, Ltnα of Lacticin 3147,33,37 and putative Pnmα of Pneumococcin SP23-BS72,14 have a net neutral charge. These peptides also resemble a number of one peptide lantibiotics such as the aforementioned mersacidin and actagardine which are anionic,7,10,17,38,39 thereby establishing that an overall net positive charge is not an essential pre-requisite for this extended group of peptides. These peptides do however share one common charge-related feature in that all possess a conserved glutamate residue (referred to in the context of nukacin ISK-1 above) which is thought to play a key role in receptor binding, and mutagenesis of which resulted in the abolition of antimicrobial activity.10,33,40,41 In contrast, β peptides are linear, appear to represent the pore-forming component and thus more closely resemble classical cationic antimicrobial peptides. Bliβ of licheniciden is the only β component which contains negatively charged residues and as a result has a neutral rather than a cationic charge.14,42 It is notable however that negatively charged residues of Bliβ are located at the extreme N-terminus and thus are relatively distant from the positively charged amino acids which are exclusively located within the C-terminal region of this and indeed all β peptides. It was thus anticipated that manipulation of the positively charged residues in the β component of these antimicrobials would have the more significant impact. To investigate this and other related theories the most extensively studied two peptide lantibiotic, lacticin 3147 (consisting of the Ltnα and Ltnβ peptides) was employed to ascertain the consequences of charge manipulation among this group of lantibiotics.
Figure 2.
Structures of Ltnα like peptides with charged amino acids highlighted (positively charged residues in blue, negatively charged residues in maroon, Ala-S-Ala [lanthionine] as light grey, Abu-S-Ala [β-methyl lanthionine] in dark grey colored, D-alanine in brown, other amino acid residues in yellow color). Asterisks correspond to residues altered in the charge mutants referred to in the text.
Figure 3.
Ltnβ like peptides with charged amino acids highlighted (positively charged residues in blue, negatively charged residues in maroon, Ala-S-Ala [lanthionine] as light grey, Abu-S-Ala [β-methyl lanthionine] in dark grey colored, D-alanine in brown, other amino acid residues in yellow color). Asterisks correspond to residues altered in the charge mutants referred to in the text.
In the associated study 16 new charge variants were created which, when combined with 6 charge variants previously generated during an alanine scanning study33 and a Ltnα N15K mutant,13 made a total of 23 charge variants which were investigated by assessing their activity against the indicator strain Lactococcus lactis HP.1 It was noted that five of the six variants in which charged residues were replaced with alanine (i.e., Ltnα D10A, Ltnα H23A, LtnαK30A, Ltnβ K24A and Ltnβ R27A) retained at least some antimicrobial activity. Ltnβ R27A was particularly notable as, although its activity when combined with Ltnα was reduced relative to that of the wild-type combination, it possessed enhanced ‘solo’ activity compared to Ltnβ. The exceptional alanine ‘charge’ mutant (LtnαE24A) which lacked activity involved the glutamate mentioned earlier which is conserved across several lantibiotics. It was noted, however, that replacement of glutamate with another negatively charged residue resulted in the retention of some activity, albeit greatly reduced. Indeed, in general, replacement of a charged residue with another, such as Ltnβ K24R and Ltnβ R27K, was well tolerated. This contrasted with the consequences when the charge at some specific locations were inverted, e.g., in Ltnα D10K, Ltnβ K24D, Ltnβ R27D, all of which resulted in the complete loss of activity. It should be noted however that Ltnα H23D and Ltnα K30D retained a significant level of activity, suggesting that the charged residues naturally present at these locations are of lesser importance. The remaining peptides were designed such that an additional positively charged residue was incorporated or multiple alanines were introduced. Within the former category, the activity of Ltnα N15K was slightly reduced whereas Ltnβ T17R was more dramatically affected, while in the latter category the activity of Ltnα H23A/K30A, Ltnα D10A/H23A and Ltnβ K24A/R27A was slightly reduced but Ltnα D10A/K30A lacked activity.
Assays were also carried out using Staphylococcus aureus sa113 and associated mutants, ΔdltA and ΔmprF, in which positively charged D-alanines and lysines which decorate cell wall teichoic acids and membrane phospholipids are removed, respectively.1,43,44 It was apparent that both the wild-type and mutant peptides were more active against the ΔdltA and ΔmprF mutants. It was particularly notable that, although the absence of positively charged residues in Ltnβ did not dramatically impact on its activity against L. lactis HP, the relative decrease in activity relative to the wild-type peptide was much more dramatic against the ΔdltA and ΔmprF targets, thereby establishing that the importance of charge can vary depending on the target, and more specifically, the cell envelope charge of that target.
Here the outcome of a number of additional bioactivity-based assays (i.e., assays with producers of these charge mutants) against Streptococcus pyogenes NCDO2381, Listeria monocytogenes H7858 and Enterococcus faecium s.dc3 is presented (Table 1). While these targets are less sensitive than L. lactis HP to lacticin 3147 and its derivatives in general, the relative activity of the various mutants is quite consistent. One notable observation was that the strain producing Ltnα N15K and its wild type Ltnβ partner produced larger zones than the Ltnα-Ltnβ producer against the three additional indicators. Specific activity assays, in the form of broth-based minimum inhibitory concentration studies, were carried out with the corresponding purified peptides to further investigate this phenomenon using Lactococcus lactis HP, Streptococcus pyogenes NCDO2381, Listeria monocytogenes H7858, Listeria monocytogenes 33013, Enterococcus faecium s.dc3 and Enterococcus faecalis 4B as indicators (Table 2). However these specific activity studies revealed that Ltnα N15K activity is not enhanced in these conditions, thereby suggesting that enhanced bioactivity in agar based studies may be reflective of an enhanced diffusion rate in agar (NB. production of Ltnα N15K is not enhanced relative to Ltnα).
Table 1.
Bioactivity* of lacticin 3147 charged mutant against different indicator strains
| Producer | Total charge | Lactococcus lactis HP | Enterococcus faecium s.dc3 | Listeria monocytogenes H7858 | Streptococcus pyogenes NCDO2381 |
| MGpMRC01 (wild type) | 2 | 16 | 9.5 | 10 | 9.5 |
| Ltnα D10A | 3 | 9 | no zone | no zone | no zone |
| Ltnα H23A | 1 | 15 | 6.5 | 7 | 7 |
| Ltnα E24A | 3 | no zone | no zone | no zone | no zone |
| Ltnα K30A | 1 | 15 | 6.5 | 8 | 9 |
| Ltnα H23A/K30A | 0 | 12 | no zone | no zone | no zone |
| Ltnα H23D | 0 | 11 | no zone | no zone | no zone |
| Ltnα K30D | 0 | 11 | no zone | no zone | no zone |
| Ltnα D10A/K30A | 2 | no zone | no zone | no zone | no zone |
| Ltnα D10K | 4 | no zone | no zone | no zone | no zone |
| Ltnα E24D | 2 | 8 | no zone | no zone | no zone |
| Ltnα N15K | 3 | 15 | 10 | 10.5 | 10 |
| Ltnα D10A, H23A | 2 | 10 | no zone | no zone | no zone |
| Ltnβ K24A | 1 | 11 | no zone | 6.5 | no zone |
| Ltnβ R27A | 1 | 15 | no zone | 6.5 | 6.5 |
| Ltnβ K24E | 0 | 8 | no zone | no zone | no zone |
| Ltnβ K24R | 2 | 14 | no zone | 6.5 | 7 |
| Ltnβ K24D | 2 | no zone | no zone | no zone | no zone |
| Ltnβ K24A/R27A | 0 | 11 | no zone | no zone | 6.5 |
| Ltnβ R27K | 2 | 13 | 6.5 | 7 | 8 |
| Ltnβ R27D | 0 | no zone | no zone | no zone | no zone |
| Ltnβ T17R | 3 | 9 | 6.5 | 6.5 | 8 |
Zone size = diameter in mm; Bioactivity assay was carried out as agar well diffusion assay with cell free supernatant of overnight culture of each individual strain against the specified indicator organisms, well size = 5.6 mm in diameter, 50 µl of overnight cell free supernatant added to the wells and incubated at 30°C. Further details on bioactivity see the original article.1
Table 2.
MIC activity* of lacticin 3147 and N15K variant against selected targets
| Indicator | Ltnα + Ltnβ | N15KLtnα + Ltnβ |
| Lactococcus lactis HP | 9.8 nM | 20 nM |
| Streptococcus pyogenes NCDO2381 | 312.5 nM | 625 nM |
| Listeria monocytogenes H7858 | 625 nM | 1.25 µM |
| Listeria monocytogenes 33013 | 625 nM | 2.5 µM |
| Enterococcus faecium s.dc3 | 625 nM | 2.5 µM |
| Enterococcus faecalis 4B | 625 nM | 2.5 µM |
| Indicator | Ltnα | N15KLtnα |
| Lactococcus lactis HP | 1.25 µM | >2.5 µM |
| Streptococcus pyogenes NCDO2381 | 2.5 µM | >2.5 µM |
MIC assay was carried out same as in the original paper, further details please refer original article.1
In conclusion, it is apparent from these studies that the consequences of manipulating charged residues in lantibiotics differ from corresponding manipulations in cAMPs in that they are variable and difficult to predict. There are, however, some general conclusions that can be made in that it would seem that positively charged residues consistently play an important role within specific domains of some lantibiotics which resemble classical cationic antimicrobials. These include the C-terminus of nisin Z (and therefore presumably related peptides) as well as the C-terminus of the β peptide of two peptide lantibiotics (but in this case only against certain targets such as ΔdltA and ΔmprF strains). It is also apparent that there can be benefits associated with manipulating charged residues in lantibiotics (Ltnβ R27A of Lacticin 3147; N20K, M21K of Nisin Z; K22T, K22S of Nisin A; D13E of Nukacin ISK-1). Further bioengineering based studies with an emphasis on manipulating the charged amino acids of the peptide with a view to improving peptide structure, solubility and/or orientation in the membrane of target bacteria could yield rich rewards.
Acknowledgements
This work was supported by the Irish Government under the National Development Plan, through a Science Foundation Ireland Investigator award to C.H., R.P.R. and P.D.C. (06/IN.1/B98).
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/12353
References
- 1.Deegan LH, Suda S, Lawton EM, Draper LA, Hugenholtz F, Peschel A, et al. Manipulation of charged residues within the two-peptide lantibiotic lacticin 3147. Microbiol Biotechnol. 2010;3:222–234. doi: 10.1111/j.1751-7915.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter PD, O'Connor PM, Draper LA, Lawton EM, Deegan LH, Hill C, et al. Posttranslational conversion of L-serines to D-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc Natl Acad Sci USA. 2005;102:18584–18589. doi: 10.1073/pnas.0509371102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee C, Paul M, Xie L, van der Donk WA. Biosynthesis and mode of action of lantibiotics. Chem Rev. 2005;105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 4.Pag U, Sahl HG. Multiple activities in lantibiotics—models for the design of novel antibiotics? Curr Pharm Des. 2002;8:815–833. doi: 10.2174/1381612023395439. [DOI] [PubMed] [Google Scholar]
- 5.Xie L, van der Donk WA. Post-translational modifications during lantibiotic biosynthesis. Curr Opin Chem Biol. 2004;8:498–507. doi: 10.1016/j.cbpa.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Bierbaum G, Sahl HG. Lantibiotics—unusually modified bacteriocin-like peptides from gram-positive bacteria. Zentralbl Bakteriol. 1993;278:1–22. doi: 10.1016/s0934-8840(11)80275-6. [DOI] [PubMed] [Google Scholar]
- 7.Ryan MP, Jack RW, Josten M, Sahl HG, Jung G, Ross RP, et al. Extensive post-translational modification, including serine to D-alanine conversion, in the two-component lantibiotic, lacticin 3147. J Biol Chem. 1999;274:37544–37550. doi: 10.1074/jbc.274.53.37544. [DOI] [PubMed] [Google Scholar]
- 8.Lawton EM, Ross RP, Hill C, Cotter PD. Two-peptide lantibiotics: a medical perspective. Mini Rev Med Chem. 2007;7:1236–1247. doi: 10.2174/138955707782795638. [DOI] [PubMed] [Google Scholar]
- 9.Lawton EM, Cotter PD, Hill C, Ross RP. Identification of a novel two-peptide lantibiotic, haloduracin, produced by the alkaliphile Bacillus halodurans C-125. FEMS Microbiol Lett. 2007;267:64–71. doi: 10.1111/j.1574-6968.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper LE, McClerren AL, Chary A, van der Donk WA. Structure-activity relationship studies of the two-component lantibiotic haloduracin. Chem Biol. 2008;15:1035–1045. doi: 10.1016/j.chembiol.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navaratna MA, Sahl HG, Tagg JR. Identification of genes encoding two-component lantibiotic production in Staphylococcus aureus C55 and other phage group II S. aureus strains and demonstration of an association with the exfoliative toxin B gene. Infect Immun. 1999;67:4268–4271. doi: 10.1128/iai.67.8.4268-4271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holo H, Jeknic Z, Daeschel M, Stevanovic S, Nes IF. Plantaricin W from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology. 2001;147:643–651. doi: 10.1099/00221287-147-3-643. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor EB, Cotter PD, O'Connor P, O'Sullivan O, Tagg JR, Ross RP, et al. Relatedness between the two-component lantibiotics lacticin 3147 and staphylococcin C55 based on structure, genetics and biological activity. BMC Microbiol. 2007;7:24. doi: 10.1186/1471-2180-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begley M, Cotter PD, Hill C, Ross RP. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl Environ Microbiol. 2009;75:5451–5460. doi: 10.1128/AEM.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonezawa H, Kuramitsu HK. Genetic analysis of a unique bacteriocin, Smb, produced by Streptococcus mutans GS5. Antimicrob Agents Chemother. 2005;49:541–548. doi: 10.1128/AAC.49.2.541-548.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyink O, Balakrishnan M, Tagg JR. Streptococcus rattus strain BHT produces both a class I two-component lantibiotic and a class II bacteriocin. FEMS Microbiol Lett. 2005;252:235–241. doi: 10.1016/j.femsle.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Wiedemann I, Bottiger T, Bonelli RR, Wiese A, Hagge SO, Gutsmann T, et al. The mode of action of the lantibiotic lacticin 3147—a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol Microbiol. 2006;61:285–296. doi: 10.1111/j.1365-2958.2006.05223.x. [DOI] [PubMed] [Google Scholar]
- 18.Morgan SM, O'Connor PM, Cotter PD, Ross RP, Hill C. Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob Agents Chemother. 2005;49:2606–2611. doi: 10.1128/AAC.49.7.2606-2611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navaratna MA, Sahl HG, Tagg JR. Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl Environ Microbiol. 1998;64:4803–4808. doi: 10.1128/aem.64.12.4803-4808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oman TJ, van der Donk WA. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem Biol. 2009;4:865–874. doi: 10.1021/cb900194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung G. Lantibiotics-Ribosomally synthesized biologically active polypeptides containing sulfide bridges and alpha,beta-didehydroamino acids. Angewandte Chemie (Int Ed Engl) 1991;30:1051–1192. [Google Scholar]
- 22.Wu Z, Li X, de Leeuw E, Ericksen B, Lu W. Why is the Arg5-Glu13 salt bridge conserved in mammalian alpha-defensins? J Biol Chem. 2005;280:43039–43047. doi: 10.1074/jbc.M510562200. [DOI] [PubMed] [Google Scholar]
- 23.Hancock RE, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z, Vasil AI, Hale JD, Hancock RE, Vasil ML, Hodges RS. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers. 2008;90:369–383. doi: 10.1002/bip.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuipers OP, Bierbaum G, Ottenwalder B, Dodd HM, Horn N, Metzger J, et al. Protein engineering of lantibiotics. Antonie Van Leeuwenhoek. 1996;69:161–169. doi: 10.1007/BF00399421. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann I, Breukink E, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, et al. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem. 2001;276:1772–1779. doi: 10.1074/jbc.M006770200. [DOI] [PubMed] [Google Scholar]
- 27.Van Kraaij C, Breukink E, Rollema HS, Siezen RJ, Demel RA, De Kruijff B, et al. Influence of charge differences in the C-terminal part of nisin on antimicrobial activity and signaling capacity. Eur J Biochem. 1997;247:114–120. doi: 10.1111/j.1432-1033.1997.00114.x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Zhang ZZ, Chen XZ, Yang W, Huan LD. Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl Microbiol Biotechnol. 2004;64:806–815. doi: 10.1007/s00253-004-1599-1. [DOI] [PubMed] [Google Scholar]
- 29.Field D, Connor PM, Cotter PD, Hill C, Ross RP. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol Microbiol. 2008;69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 30.Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJ, Kuipers OP, et al. Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl Environ Microbiol. 2007;73:5809–5816. doi: 10.1128/AEM.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierbaum G, Reis M, Szekat C, Sahl HG. Construction of an expression system for engineering of the lantibiotic Pep5. Appl Environ Microbiol. 1994;60:4332–4338. doi: 10.1128/aem.60.12.4332-4338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter PD, Hill C, Ross RP. Bacterial lantibiotics: strategies to improve therapeutic potential. Curr Protein Pept Sci. 2005;6:61–75. doi: 10.2174/1389203053027584. [DOI] [PubMed] [Google Scholar]
- 33.Cotter PD, Deegan LH, Lawton EM, Draper LA, O'Connor PM, Hill C, et al. Complete alanine scanning of the two-component lantibiotic lacticin 3147: generating a blueprint for rational drug design. Mol Microbiol. 2006;62:735–747. doi: 10.1111/j.1365-2958.2006.05398.x. [DOI] [PubMed] [Google Scholar]
- 34.Appleyard AN, Choi S, Read DM, Lightfoot A, Boakes S, Hoffmann A, et al. Dissecting structural and functional diversity of the lantibiotic mersacidin. Chem Biol. 2009;16:490–498. doi: 10.1016/j.chembiol.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam MR, Shioya K, Nagao J, Nishie M, Jikuya H, Zendo T, et al. Evaluation of essential and variable residues of nukacin ISK-1 by NNK scanning. Mol Microbiol. 2009;72:1438–1447. doi: 10.1111/j.1365-2958.2009.06733.x. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee C, Patton GC, Cooper L, Paul M, van der Donk WA. Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem Biol. 2006;13:1109–1117. doi: 10.1016/j.chembiol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 37.McAuliffe O, Ryan MP, Ross RP, Hill C, Breeuwer P, Abee T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol. 1998;64:439–445. doi: 10.1128/aem.64.2.439-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee S, Lad SJ, Phansalkar MS, Rupp RH, Ganguli BN, Fehlhaber HW, et al. Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization. J Antibiot (Tokyo) 1992;45:832–838. doi: 10.7164/antibiotics.45.832. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann N, Metzger JW, Jung G. The tetracyclic lantibiotic actagardine. 1H-NMR and 13C-NMR assignments and revised primary structure. Eur J Biochem. 1995;228:786–797. [PubMed] [Google Scholar]
- 40.Szekat C, Jack RW, Skutlarek D, Farber H, Bierbaum G. Construction of an expression system for site-directed mutagenesis of the lantibiotic mersacidin. Appl Environ Microbiol. 2003;69:3777–3783. doi: 10.1128/AEM.69.7.3777-3783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boakes S, Cortes J, Appleyard AN, Rudd BA, Dawson MJ. Organization of the genes encoding the biosynthesis of actagardine and engineering of a variant generation system. Mol Microbiol. 2009;72:1126–1136. doi: 10.1111/j.1365-2958.2009.06708.x. [DOI] [PubMed] [Google Scholar]
- 42.Dischinger J, Josten M, Szekat C, Sahl HG, Bierbaum G. Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS One. 2009;4:6788. doi: 10.1371/journal.pone.0006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 44.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]