Abstract
HAART has succeeded in reducing morbidity and mortality rates in patients infected with HIV. However, a small amount of replication-competent HIV can persist during HAART, allowing the virus to re-emerge if therapy is ceased. One significant source of this persistent virus is a pool of long-lived, latently infected CD4+ T cells. This article outlines what is known about how this reservoir is established and maintained, and describes the model systems that have provided insights into the molecular mechanisms governing HIV latency. The therapeutic approaches for eliminating latent cells that have been attempted are also discussed, including how improvements in understanding of these persistent HIV reservoirs are being used to develop enhanced methods for their depletion.
Keywords: AIDS, antiretroviral drug, histone deacetylase, HIV, immunotoxin, latency, macrophage, persistence, prostratin, reservoirs
The natural course of HIV disease is generally characterized by high levels of virus replication throughout infection, involving an extremely rapid turnover of both infected cells and plasma virions [1–5]. This replication occurs predominantly within CD4+ T cells and is responsible for the deterioration of the host immune system that culminates in AIDS. To inhibit HIV replication and prevent disease progression, a range of potent antiretroviral drugs that target different stages of the viral lifecycle have been developed [6–8]. Combinations including three or more of these drugs are typically administered in the context of HAART, which is often effective at suppressing viral loads to below the limit of detection of standard clinical assays (50 copies of HIV RNA per ml of plasma) [9–12]. While HAART has significantly decreased the morbidity and mortality rates associated with HIV infection, there are a number of problems associated with its long-term use, including drug toxicities, development of virologic resistance and significant financial expense. More importantly, HAART is not capable of curing infected patients. This is because a very small amount of replication-competent HIV persists during therapy, meaning that if treatment is stopped then viral loads quickly rebound, allowing disease progression to continue [13].
A substantial research effort is currently directed towards developing a more complete understanding of the sources of virus that re-emerge after cessation of HAART. It is hoped that if these reservoirs can be identified and eliminated, then virus replication could be permanently halted. While other sources of persistent virus exist, HIV latency is believed to represent a significant barrier to eradication of HIV in HAART-treated patients. Some of the current concepts about how latency may be established and maintained are outlined and the experimental models that have provided insights into the mechanisms governing this process are described in this article. Alternative potential sources of replication-competent HIV in treated patients and new experimental therapeutic approaches that may be efficacious against these elusive targets are also discussed.
Pre- & postintegration latency
Two forms of HIV latency have been described that differ fundamentally in their contribution to long-term virus persistence during therapy. The vast majority of resting (G0) CD4+ T cells exposed to HIV do not become productively infected [14,15]. Instead, the virus is inhibited at multiple steps of its replication cycle [16–18], which generally results in the formation of a non-integrated, linear, cytoplasmic DNA form of the viral genome that is labile and decays with a half-life of approximately 1 day [19]. However, if the host cell is stimulated soon after infection, then a portion of this preintegrated virus can complete its replication cycle, resulting in the production of new virions [14,15,20,21]. Since viral expression can be rescued by stimulation, this state is sometimes referred to as preintegration latency. This process may play some role in untreated HIV infection, when a large number of quiescent cells harbor these short-lived reverse transcripts. Yet, because of its short half-life, HIV DNA in an unintegrated form is unlikely to contribute to the long-term persistence of HIV during HAART and, therefore, is not considered a significant barrier to viral eradication. In some cases, quiescent cells do form complete reverse transcripts that can integrate into the host cell genome [22,23]. These integrated genomes could function as viral reservoirs similar to those discussed later.
In contrast to the labile preintegrated viral form, postintegration latency is a much more stable state where the viral genome is permanently maintained within the host cell chromosomes [24–26]. Consequently, the postintegration ‘latent reservoir’ of HIV in HAART-treated patients is a major contributor to long-term virus persistence. This reservoir consists of a pool of resting CD4+ T cells that harbor functional, integrated HIV proviruses but do not produce new virions until they are stimulated. The latent reservoir is small, comprising only around one cell per million resting CD4+ T cells, which translates into a total of approximately 1 million latently infected cells in the body as a whole [27]. This reservoir is formed soon after primary infection with HIV [28], and while initiation of HAART before seroconversion can reduce the frequency of latently infected cells, it does not prevent establishment of latency altogether [29]. These latently infected cells also decay very slowly during HAART, with a mean half-life of approximately 44 months [30]. Therefore, even under optimal treatment conditions and in the absence of other reservoirs, it could take 60 years or more for all latently infected cells to be eliminated using current therapies. For these reasons, it is believed that without new interventions, the latent reservoir is probably sufficient to ensure lifelong infection with HIV.
Establishment of latency
The precise in vivo mechanisms responsible for establishment of HIV latency have not been completely ascertained. However, the most likely scenarios for this process can be inferred based on the characteristics of latently infected cells in infected patients and the information that has been gained from various experimental models. Latent virus is found in resting CD4+ T cells, primarily within the central memory and transitional memory cell subsets [27,31]. As discussed earlier, direct infection of G0 T cells does not usually generate postintegration latency [14,15], and infection of activated CD4+ T cells generally results in a productive virus infection and death of the host cell within a few days [2,3]. Therefore, the prevailing view for how most latently infected cells are produced in vivo is that an activated T cell becomes infected but transitions into a quiescent memory cell before it can be killed by the virus or components of the immune response. This transition is associated with strong downregulation of HIV expression, which results in a latent provirus [32,33]. Because memory cells are by nature very long lived [34,35], the latently infected T cell can persist for decades before a triggering event – such as an encounter with its cognate antigen – leads to stimulation of the host cell and concomitant activation of the latent provirus. The in vivo conditions that facilitate development of latency are not known, but it may be speculated that a T lymphocyte that is already in the process of transitioning to a memory cell when it is infected would be most likely to shut down HIV expression quickly enough to survive the initial infection. It is worth noting that there is currently little evidence supporting the idea that HIV has evolved specific mechanisms that enable it to establish a latent infection. Latency may instead be an incidental by-product of the tropism of HIV for activated CD4+ T cells, which occasionally transition to long-lived memory cells that are incapable of supporting the latter portions of the virus lifecycle without further stimulation.
More than one mechanism could potentially result in the formation of latency in vivo. For example, it has been reported that a low frequency of latently infected cells can be generated in vitro during acute infection of the continually proliferating Jurkat T-cell line with HIV-based reporter viruses [36]. Spontaneous generation of latency in Jurkat cells has also been described after infection of these cells with a different lentiviral vector [37]. Infection of activated and proliferating cells in vivo may therefore allow generation of some level of latently infected cells without requiring the immediate transition to a resting cell phenotype.
In vitro activation of primary CD4+ T cells for infection by HIV is generally performed by costimulation with antibodies specific for CD3 and CD28 [38], or using mitogens such as phytohemagglutinin and/or irradiated allogeneic peripheral blood mononuclear cells [39]. Yet, less powerful stimulatory signals, such as those provided by the cytokines IL-2, -4, -7 and -15, can also allow low-level, productive infection with HIV [40]. It is possible that resting cells stimulated and infected in this way may be more likely to revert to a quiescent state, resulting in the creation of a latent provirus. The role of immuno-suppressive environments in the establishment of latency has also not been fully explored. For example, extracellular milieus specifically intended to dampen immune responses, such as those produced by regulatory T cells [41], may also present an environment that either enhances formation of, or limits activation from, latency.
Another potential mechanism for the establishment of latency occurs during the process of thymopoiesis [42]. This is possible because immature CD4+CD8+ thymocytes are transcriptionally and metabolically active enough to support a productive infection by HIV. However, as these cells differentiate into naive quiescent T cells, they also become incapable of sustaining efficient HIV expression. Therefore, if a cell is infected at the CD4+CD8+ stage, it can differentiate into a naive T cell harboring a latent provirus [42]. Although the higher frequency of latent HIV in the memory T cell versus the naive T-cell compartment would perhaps argue against a large-scale establishment of latency within the thymus of infected adults, this process may nevertheless contribute at lower levels to the latent reservoir, particularly in young children where the thymus is still highly active [43].
Maintenance of latency
Latent proviruses express little mRNA, and the small amount that can be detected is generally truncated or not effectively exported from the nucleus [32,33,44,45]. A diverse collection of factors have been identified as playing a role in the maintenance of this proviral latency. Perhaps the most straightforward explanation for the lack of latent virus expression is that transcription factors such as NF-κB and nuclear factor of activated T cells (NFAT), which are required for optimal expression of HIV, cannot access the nucleus in resting T cells [46–48].
The majority of proviruses within resting T cells in HAART-treated patients are integrated in sites within actively transcribed genes [49]. This integration site preference is common during HIV infection [50], but in the context of reduced viral transcription in quiescent cells, it may lead to a phenomenon known as transcriptional interference. This is a state where transcripts initiating outside of the integration site but reading through the HIV genome could displace the transcription machinery assembled at the HIV promoter, thereby disrupting virus expression [51,52]. Yet another mechanism that can influence latency involves post-transcriptional regulation by cellular miRNAs [53]. A collection of these miRNAs that are enriched in resting versus activated CD4+ T cells are capable of inhibiting translation of viral proteins by targeting the 3′ end of HIV mRNA. Therefore, post-transcriptional mechanisms may account for a subset of the latent virus, although it is not yet clear whether these mechanisms are sufficient to maintain latency over an extended period of time.
Active inhibition of transcription via epigenetic mechanisms may also be involved in latency. An example of this is DNA methylation, which is a mechanism of transcriptional silencing that is common in adult somatic tissues [54]. Cytosine–phosphate–guanine DNA dinucleotide (CpG) methylation has long been known to be capable of silencing retroviral long terminal repeat (LTR) promoters [55,56], and there is evidence that methylation may be important for maintenance of latency in HIV infection [57]. There has also been significant interest in the possible role of histone deacetylases (HDACs) in the regulation of HIV latency. When integrated into a host cell chromosome, HIV DNA is wrapped around histone octamers that form nucleosomes [58,59]. Two of these nucleosomes are positioned at specific locations within the HIV promoter, and their acetlyation status can significantly influence HIV transcription. In general, acetlyation of lysine residues in histone tails can enhance transcriptional activity, whereas hypoacetylation is associated with the repression of gene expression [60]. Recruitment of HDACs to the HIV LTR has been documented in a process that can be mediated by several different factors, including Ying-Yang-1 (YY1) + late simian virus 40 factor (LSF), NF-κB p50 homodimers, cellular-Myc (c-Myc) + Sp1, C-promoter binding factor-1 (CBF-1) or COUP-TF interacting protein 2 (CTIP2) [61–66]. Moreover, the use of HDAC inhibitors such as valproic acid, sodium butyrate and trichostatin A can lead to transcriptional activation of latent HIV from T cells isolated from infected subjects. Thus, histone acetylation status can also influence HIV latency in some cases, and there has been substantial interest in exploiting this knowledge to develop methods for antagonizing latency.
The broad message from these and other studies is that regulation of HIV latency is complex and may be the result of several different mechanisms acting in concert to powerfully suppress HIV production in latently infected cells. It is unlikely that one therapeutic approach will be sufficient to counteract all of these different mechanisms. Hence, there is a pressing need for more research into which of these latency mechanisms are primarily responsible and, thus, merit the most attention when attempting to develop therapeutics.
Experimental models for HIV latency
The study of latently infected cells obtained from patients has produced valuable information regarding this reservoir, but there are important limitations on the use of these primary cells that can restrict their experimental utility. Latently infected cells do not possess any distinctive cell surface or other characteristics that would permit their easy isolation from corresponding uninfected cells. These cells are also present in very low numbers, and laborious culture conditions are required to even identify them [27]. For example, to perform accurate quantification experiments many millions of highly purified resting CD4+ T cells must be obtained from HAART-treated patients with a history of consistently suppressed viral loads. These cells must then be stimulated in a limiting dilution format and cocultured with activated susceptible target cells for weeks before quantification of viral production [27].
Another confounding issue for molecular analysis of latent proviruses is that less than 1% of the total integrated HIV DNA in resting CD4+ T cells represents replication-competent virus [27]. The more abundant defective proviruses may have become inactivated prior to integration by incorporation of reverse transcriptase errors [67] or by APOBEC3G-mediated hypermutation [68]. Integration into regions of the host cell chromosomes that preclude virus expression or permanent silencing of the integrated provirus by epigenetic changes may also account for a fraction of the nonproductive genomes. Owing to their lack of cytopathicity, defective proviruses are more likely to persist for the natural lifespan of the host cell and, therefore, become enriched in the resting memory cell population.
A further limitation of patient cells is that their rarity coupled with the laborious coculture techniques required to identify them limits their use in drug-discovery procedures, such as high-throughput screening [69], which may help identify compounds capable of activating latent virus. Moreover, the HIV genome present in patient samples is predetermined by the particular strain of virus that originally infected the patient. This precludes genetic modification of the virus when investigating the contribution of specific regions of the viral genome, such as transcription factor binding sites or viral accessory genes, in the process of latency activation. Together, these limitations have provided a strong impetus for the development of relevant models of HIV latency that are more tractable than primary cells from patients. While no currently available model completely recapitulates all aspects of HIV latency in patient cells, a substantial amount of useful information has been gained from their use.
Cell line models, such as the promonocytic U1 cell line [70] and ACH2 T-cell line [71], were a focus of early research into the molecular basis for HIV latency [72]. These cells were created by infecting cultures with HIV in vitro and selecting clones that only express a high level of virus after stimulation. They have been used to investigate a number of questions including the potential role of post-transcriptional blocks in inhibition of latent virus expression [72], gene-expression profiles associated with cells harboring latent or recently reactivated HIV [73], and the relative kinetics of acute HIV infection versus activation from latency [74]. However, the main reason for the latent phenotype in these cells is a disrupted Tat/trans-activator response region (TAR) axis [75,76], which does not appear to be the prevailing mechanism for latency generation in vivo.
More recently developed cell line models utilizing reporter viruses have also provided useful insights into the processes involved in latency. The J-Lat model is based on infection of the transformed Jurkat T-cell line [36], and provides an elegant system for investigating latency that is regulated at the transcriptional level. These cells harbor a near full-length HIV genome that encodes a green fluorescent protein, which is expressed upon activation of the latent virus and greatly facilitates detection of reactivation events. Another latency model based on Jurkat cells infected with a genome consisting of only the LTRs, a Tat-coding sequence and a reporter gene was used to show that because of the powerful positive transcriptional feedback loop initiated by HIV Tat, stochastic fluctuations of Tat protein levels were sufficient to generate active or latent virus expression states in these cells [77].
Our group has utilized the severe-combined immunodeficient human (SCID-hu) thymus/liver mouse system as a model for the generation of HIV latency during thymopoiesis [78,79]. This model involves implanting human fetal liver and thymus tissue under the kidney capsule of a mouse with severe-combined immunodeficiency. The resulting conjoint organ provides an environment for maturation of human thymocytes, allowing production of phenotypically and functionally normal human T cells for longer than 1 year. Importantly, this implant is also susceptible to infection with HIV, providing an in vivo environment that is suitable for studying HIV replication and pathogenesis [80–82]. If mature CD4 single-positive thymocytes are obtained from an HIV-infected SCID-hu implant and sorted to remove productively infected cells, then the resultant cell population produces little HIV [42]. However, many of these cells harbor integrated proviruses and are capable of producing virus if stimulated. This model was used to show that latently infected cells appear phenotypically normal compared with their uninfected counterparts [83], produce very little HIV RNA [84], but can be stimulated with molecules such as the cytokine IL-7, or with the non-tumor-promoting phorbol ester prostratin, without causing the profound changes in T-cell phenotype associated with costimulation [85,86]. The model was also used to identify signaling pathways that activate HIV from latency most effectively [86,87], and to show that targeted toxins specific for the HIV envelope protein can be used in conjunction with stimulants to enhance the killing of recently activated latently infected cells [84]. An in vitro primary cell latency model based on similar thymocyte differentiation principles has also been developed [88], and was used to show that the majority of virus reactivation following costimulation was eliminated following mutation of the NF-κB binding sites in the HIV promoter. Thus, these elements are necessary for the optimal activation of HIV from latency in this model, which suggests that agents that activate via NF-κB might be useful activating agents.
Another primary cell model for HIV latency has recently been described [89]. This is a versatile system where naive T cells are first isolated from uninfected donors, and then stimulated in vitro under conditions that push them to become memory cells with either an unpolarized, T-helper 1 or 2 phenotype. After 1 week of stimulation, the cells are infected with a replication-defective (Env-deficient) HIV virus. Productively infected cells are killed by the virus, but a substantial number of the surviving memory cells harbor latent proviruses. Stimulation of these cells in the presence of signaling agonists or antagonists, and the use of LTR mutants, was used to demonstrate that Lck and NFAT, but not NF-κB, are required for optimal latency reactivation in this system. This model was also used in conjunction with J-Lat cells to show that cytosine methylation influences HIV latency and, in particular, that methyl-CpG binding domain protein 2 can participate in regulation of this process [90].
These and other models have provided a wealth of information on how HIV latency may be regulated and important clues as to how latently infected cells could be eliminated [91,92]. However none of the model systems is perfect and, wherever possible, it is important to verify information gained from these experiments with latently infected cells obtained from HAART-treated patients.
Depleting the latent reservoir
Previous attempts to reduce the frequency of latently infected cells in patients undergoing HAART have often involved immune-activation therapy approaches. The rationale for this is that if resting latently infected T cells can be activated in vivo, then this will result in HIV expression and death of the host cell from viral cytopathic effects or immune effector mechanisms. Meanwhile, the continued presence of antiretroviral drugs would prevent spread of the newly produced virus to uninfected cells. One such approach involved the use of IL-2, either alone [93] or in conjunction with an agonistic anti-CD3 monoclonal antibody (OKT3) [94]. While high doses of OKT3 induced quite serious side effects [94], the IL-2 therapy significantly reduced latently infected cell numbers but failed to prevent viral rebound when HAART was withdrawn [13,93,95].
Induction of generalized immune activation is undesirable in purging strategies because it leads to toxicity in patients (sometimes referred to as a ‘cytokine storm’) and produces an abundance of activated target cells for the newly induced virus to spread to, which may not be adequately contained by regular HAART. The former problem may be avoided by identifying molecules that are capable of activating the latent provirus without strongly stimulating either the host cell or uninfected bystander cells. The latter potential issue of virus spread from newly activated cells may be partially offset by intensification of HAART during the treatment period. With these ideas in mind, the HDAC inhibitor valproic acid (VPA) has also been tested for its capacity to deplete latently infected cells. VPA was administered either alone in the context of HAART [96], or in combination with HAART intensification using the fusion inhibitor enfuvirtide [97]. Again, some reduction in the frequency of latently infected cells was observed in these studies, particularly when combined with intensification of antiviral therapy, but detectable levels of latently infected cells remained. VPA treatment alone also did not appear to affect decay of the latent reservoir in another study of HAART-treated patients who were taking VPA therapy for neurological or psychiatric conditions [98], suggesting that the utility of VPA when used as a lone activating agent in purging strategies may be limited. High-dose intravenous immunoglobulin treatment has also been tested in a proof-of-concept study attempting depletion of latently infected cells in HAART-treated patients [99]. This resulted in a median 68% decrease in the latent pool of cells. The precise mechanisms for latency activation with this therapy are not clear, but may involve ligation of Fc receptors and subsequent cytokine production by multiple immune cell types.
Further attempts at purging persistent reservoirs in the context of various HAART regimens have been made where latently infected cells were not directly measured, but other indicators of viral replication, such as plasma viral loads and proviral DNA quantification, were used to assess the impact of treatment. Examples of these include studies using IL-2 [100], IL-2 + OKT3 [94,101,102], IL-2 + IFN-γ [103] and cyclophosphamide [104]. These treatments had varying impacts on levels of HIV DNA or RNA, but none led to eradication of the infection.
The pioneering clinical studies outlined earlier have demonstrated that latent reservoirs of HIV can be reduced by therapeutic means. However, previously tested approaches are not capable of eliminating the infection completely or preventing viral rebound if therapy is stopped. Therefore, more basic research is required in order to develop effective strategies for eliminating the in vivo reservoirs of HIV in HAART-treated patients.
Of the molecules that have proven effective at activating latent HIV in vitro, a few stand out as strong candidates for further development with the potential for use as a therapeutic. One such molecule is the non-tumor-promoting phorbol ester prostratin [105]. This is a molecule originally isolated from the Samoan plant Homalanthus nutans, extracts of which are traditionally used to treat viral diseases. Prostratin shows promise for a number of reasons. First, it is capable of activating latent HIV via a PKC/NF-κB pathway [106,107]. Second, it can do so without inducing strong stimulation and proliferation in resting T cells [86]. Third, it also downregulates the HIV receptors CD4, CXCR4 and CCR5 and, therefore, might help prevent virus spread from newly activated latent virus [106]. A positive development in this area is the report that prostratin and its derivatives can be chemically synthesized and potentially ‘tuned for performance’ [108]. This opens the possibility of designing new compounds based on prostratin that are more effective than the parent molecule at specifically and efficiently activating latent virus.
IL-7 has also received interest for its ability to activate latent HIV without undue stimulation of the host cell [85], and may potentially be useful in the context of a broader purging strategy. Similarly, further work on HDAC inhibitors will improve our understanding of that particular mechanism for latency, and may help with development of targeted, more potent drugs that only inhibit those HDACs that play a role in HIV latency. Work on alternative HDAC inhibitors for use in this context is ongoing. For example, suberoylanilide hydroxamic acid has also been identified as being capable of activating latent virus in a model system [109]. Other mechanisms of latency activation should also be explored. One example of this is hexamethylbisacetamide, which is capable of activating latent HIV by interacting with Sp1 and inducing CDK9 recruitment to the HIV promoter, leading to phosphorylation of the C-terminal domain of RNA polymerase II in a Tat-independent manner [110,111].
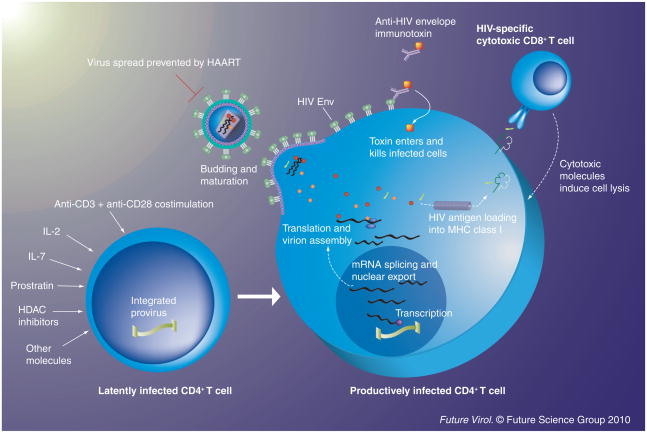
While developing effective activators of latent virus is a central research focus, there is also some interest in methods that could improve the killing of latently infected cells after the virus is activated. Anti-HIV envelope immunotoxins have the potential to do precisely this. Immunotoxins are hybrid molecules consisting of a targeting domain (often derived from a monoclonal antibody) linked to a toxic moiety [112,113]. Several immunotoxins specific for the Env protein of HIV have been developed [114–116], which are capable of killing cells productively infected with HIV. An early anti-HIV targeted toxin was tested in Phase 1 clinical trials in the pre-HAART era, but did not show any in vivo antiviral effects [117,118]. However, enhanced anti-HIV immunotoxins have now been developed, and if these were to be used in the context of HAART in conjunction with agents that activate latent virus, then they may prove to be more effective. In support of this idea, one such immunotoxin successfully enhanced killing of latently infected cells ex vivo when used in combination with latency activators [84]. An illustration outlining the activation–elimination approach to depleting the latent HIV reservoir is provided in Figure 1.
Figure 1. Potential methods for the activation of latent virus and subsequent killing of infected cells.
A wide range of molecules are capable of inducing expression of HIV from a latent provirus. Some of these (such as anti-CD3+ anti-CD28 costimulation) lead to powerful activation of both the host cell and the latent provirus, whereas others (e.g., treatment with prostratin or IL-7) can activate latent HIV expression without inducing significant stimulation and proliferation of the host cell. After stimulation, the latent provirus continues with the latter part of the virus lifecycle, resulting in expression of viral proteins and production of new virions. Once it has begun expressing viral proteins, the host cell is susceptible to viral cytopathic effects and immune effector mechanisms (such as killing by cytotoxic T cells). Anti-HIV Env immunotoxins can specifically bind to and kill cells expressing HIV Env proteins; therefore, they may prove useful in purging strategies in conjunction with latency activators. Throughout this process, virus spread is prevented by the presence of HAART. HDAC: Histone deacetylase.
Alternative sources of persistent virus
In addition to viral latency, other sources of HIV can also persist during HAART. Even in patients who respond well to therapy and have successfully suppressed viral loads for years, low levels of HIV virions can often be detected in the plasma using highly sensitive methods [119–121]. This virus is apparently not entirely derived from activation of latently infected CD4+ T cells, and a portion of it may instead be released from rare productively infected cells [122,123]. If some low-level replication does occur during HAART, then intensification of current HAART regimens or their complementation with newly available antiretroviral drugs may be necessary to fully suppress virus spread.
In some patients, a significant proportion of the residual viremia appears to originate in another, less well-defined reservoir with the capacity to produce a genetically homogeneous population of virus over the course of years [124]. This has been speculated to be derived from the rare infection of a monocyte–macrophage lineage stem cell. Hence, complete eradication of HIV from an infected individual may require identification and elimination of virus in several distinct locations with very different characteristics.
The potential role of other infected cell types, such as macrophages, in long-term HIV persistence is unclear. Macrophages are more resistant to the cytopathic effects of HIV than activated T cells, and can survive for weeks or months after their infection [125,126]. Macrophages and microglial cells are also the principal infected cell types in the CNS [127], which can be an anatomical reservoir with limited access to some antiretroviral drugs. Combinations of molecules suggested for use in targeting the latency reservoir, such as prostratin and anti-Env immunotoxins, can also be effective against HIV-infected macrophages [128]. Hence, there is the potential for overlap between purging strategies directed towards the latent reservoir and treatment intended to target other HIV-infected cell types.
Conclusion
HIV persistence during HAART is an exceptionally challenging problem compounded by the presence of multiple reservoirs of replication-competent virus. Postintegration latency is perhaps the most clearly understood barrier to HIV eradication, and this latent reservoir is probably sufficient to ensure lifelong infection unless new therapeutics are developed that are capable of depleting it. Numerous mechanisms acting in concert may be responsible for preventing virus expression in latently infected cells. These can be relieved by overt stimulation of the host cell [27], or by using molecules that activate the virus more specifically, without necessarily inducing host cell stimulation and proliferation [85,106,129]. It is encouraging that in spite of the multiple mechanisms governing latency, a variety of factors acting through different pathways can activate a significant proportion of latent virus. This indicates that relieving one block may be sufficient to overcome other potential barriers to virus expression that are also in place in resting CD4+ T cells. Ultimately, a combinatorial approach involving several different therapeutic agents may prove most effective in activation and elimination of latently infected cells.
Future perspective
In any area of scientific endeavor, it is difficult to predict what advances will be made in the future. This is particularly true of HIV persistence and latency, in part because it is unclear how many distinct underlying sources of replication-competent virus are present in HAART-treated patients. If the larger repositories of virus can be eliminated, such as the latent reservoir in resting CD4+ T cells, then other sources may become easier to identify and investigate. Most researchers in the field believe that more than one approach will probably be necessary if HIV is to be eradicated from infected patients. This may end up taking the form of antiretroviral drugs that prevent virus spread in conjunction with several different compounds that activate latent virus. Potentially, HIV-specific immunotoxins or other cytotoxic molecules could be utilized alongside these purging regimens to target cells harboring the newly produced virus and also clear any remaining chronically infected cells.
If effective means of depleting these reservoirs are not found, then the continued use of antiretroviral drugs may allow the virus to be indefinitely contained. Ideally, future improvements in antiretroviral drug development would provide cheaper, more effective, less toxic agents that can be taken on an infrequent basis. Gene-therapy approaches also offer exciting opportunities for preventing virus spread. For example stem cells can be engineered to express genes that inhibit HIV infection or viral gene expression, with the goal of repopulating a patient’s immune system with CD4+ cells that are not susceptible to HIV infection [130–134]. This type of approach may ultimately be effective against all of the sources of replication-competent HIV in HAART-treated patients because it could deprive the virus of susceptible host cells, thereby preventing virus expansion from these cryptic sources. While this may not be a complete cure for HIV infection, it may represent an acceptable outcome until one can be found.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors and some of the studies outlined in this manuscript were supported by NIH grant # AI70010 to Jerome A Zack. The authors’ laboratory has received funding at various times from Amgen, Johnson and Johnson Research and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Matthew D Marsden, Email: mmarsden@ucla.edu, David Geffen School of Medicine at UCLA, 615 Charles E Young Drive South, BSRB 188-10, Los Angeles, CA 90095, USA, Tel.: +1 310 206 2152, Fax: +1 310 267 1875.
Jerome A Zack, Email: jzack@ucla.edu, David Geffen School of Medicine at UCLA, 615 Charles E Young Drive South, BSRB 173, Los Angeles, CA 90095, USA, Tel.: +1 310 825 0876, Fax: +1 310 267 1875.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Piatak M, Jr, Saag MS, Yang LC, et al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 2.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 3.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 4.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 5.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276(5314):960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 6.Fatkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11(11):1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 7.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a Phase II randomised controlled trial. Lancet. 2007;369(9569):1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 8.Pomerantz Rj, Horn Dl. Twenty years of therapy for HIV-1 infection. Nat Med. 2003;9(7):867–873. doi: 10.1038/nm0703-867. [DOI] [PubMed] [Google Scholar]
- 9.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 10.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 11.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less AIDS clinical trials group 320 study team. N Engl J Med. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch M, Steigbigel R, Staszewski S, et al. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced human immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis. 1999;180(3):659–665. doi: 10.1086/314948. [DOI] [PubMed] [Google Scholar]
- 13.Chun T-W, Davey RT, Engel D, Lane HC, Fauci AS. AIDS: re-emergence of HIV after stopping therapy. Nature. 1999;401(6756):874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 14.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 15.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korin YD, Zack JA. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J Virol. 1999;73(8):6526–6532. doi: 10.1128/jvi.73.8.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganesh L, Burstein E, Guha-Niyogi A, et al. The gene product murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426(6968):853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 18.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435(7038):108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J Virol. 2005;79(4):2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zack JA, Haislip AM, Krogstad P, Chen IS. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korin YD, Zack JA. Progression to the g1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72(4):3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatakis DN, Kim S, Kim N, Chow SA, Zack JA. Human immunodeficiency virus integration efficiency and site selection in quiescent CD4+ T cells. J Virol. 2009;83(12):6222–6233. doi: 10.1128/JVI.00356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai J, Agosto LM, Baytop C, et al. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J Virol. 2009;83(9):4528–4537. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells quantitative analysis of the transition to stable latency. Nat Med. 1995;1(12):1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 25.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 26.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 27▪▪.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387(6629):183–188. doi: 10.1038/387183a0. Provides the first accurate quantification of the latent reservoir. [DOI] [PubMed] [Google Scholar]
- 28.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95(15):8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191(9):1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. Provides evidence for the slow in vivo decay rate of latently infected cells in treated patients, indicating that HAART alone is unlikely to clear this reservoir. [DOI] [PubMed] [Google Scholar]
- 31.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermankova M, Siliciano JD, Zhou Y, et al. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77(13):7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassen KG, Bailey JR, Siliciano RF. Analysis of human immunodeficiency virus type 1 transcriptional elongation in resting CD4+ T cells in vivo. J Virol. 2004;78(17):9105–9114. doi: 10.1128/JVI.78.17.9105-9114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 35.Combadiere B, Boissonnas A, Carcelain G, et al. Distinct time effects of vaccination on long-term proliferative and IFN-γ-producing T cell memory to smallpox in humans. J Exp Med. 2004;199(11):1585–1593. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪▪.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22(8):1868–1877. doi: 10.1093/emboj/cdg188. Original description of the widely used J-Lat model for HIV latency in Jurkat T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YK, Bourgeois CF, Pearson R, et al. Recruitment of TFIIH to the HIV LTR is a rate-limiting step in the emergence of HIV from latency. EMBO J. 2006;25(15):3596–3604. doi: 10.1038/sj.emboj.7601248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsden MD, Zack JA. Human immunodeficiency virus bearing a disrupted central DNA flap is pathogenic in vivo. J Virol. 2007;81(11):6146–6150. doi: 10.1128/JVI.00203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koup RA, Ho DD, Poli G, Fauci AS. Isolation and quantitation of HIV in peripheral blood. Curr Protoc Immunol. 1993;12(Suppl 5):2.1–2.11. doi: 10.1002/0471142735.im1202s05. [DOI] [PubMed] [Google Scholar]
- 40.Unutmaz D, Kewalramani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189(11):1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 42.Brooks DG, Kitchen SG, Kitchen CM, Scripture-Adams DD, Zack JA. Generation of HIV latency during thymopoiesis. Nat Med. 2001;7(4):459–464. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 43.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 44.Adams M, Sharmeen L, Kimpton J, et al. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc Natl Acad Sci USA. 1994;91(9):3862–3866. doi: 10.1073/pnas.91.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2(7):e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6(3):235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 48.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 49.Han Y, Lassen K, Monie D, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78(12):6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 51.Lenasi T, Contreras X, Peterlin BM. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell Host Microbe. 2008;4(2):123–133. doi: 10.1016/j.chom.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duverger A, Jones J, May J, et al. Determinants of the establishment of human immunodeficiency virus type 1 latency. J Virol. 2009;83(7):3078–3093. doi: 10.1128/JVI.02058-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13(10):1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 54.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 55.Harbers K, Schnieke A, Stuhlmann H, Jahner D, Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci USA. 1981;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2) J Virol. 2005;79(2):876–883. doi: 10.1128/JVI.79.2.876-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blazkova J, Trejbalova K, Gondois-Rey F, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5(8):e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verdin E. Dnase I-hypersensitive sites are associated with both long terminal repeats and with the intragenic enhancer of integrated human immunodeficiency virus type 1. J Virol. 1991;65(12):6790–6799. doi: 10.1128/jvi.65.12.6790-6799.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verdin E, Paras P, JR, van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12(8):3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370(Pt 3):737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imai K, Okamoto T. Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J Biol Chem. 2006;281(18):12495–12505. doi: 10.1074/jbc.M511773200. [DOI] [PubMed] [Google Scholar]
- 62.Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26(24):4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coull JJ, Romerio F, Sun JM, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74(15):6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-κb p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25(1):139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marban C, Suzanne S, Dequiedt F, et al. Recruitment of chromatin-modifying enzymes by ctip2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26(2):412–423. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang G, Espeseth A, Hazuda DJ, Margolis DM. c-Myc and sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81(20):10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roberts JD, Bebenek K, Kunkel TA. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 68.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral vif protein. Nature. 2002;418(6898):646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 69.Sundberg SA. High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. Curr Opin Biotechnol. 2000;11(1):47–53. doi: 10.1016/s0958-1669(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 70.Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 71.Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor α induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pomerantz RJ, Trono D, Feinberg MB, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 73.Krishnan V, Zeichner Sl. Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. J Virol. 2004;78(17):9458–9473. doi: 10.1128/JVI.78.17.9458-9473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arlen PA, Brooks DG, Gao LY, Vatakis D, Brown HJ, Zack JA. Rapid expression of human immunodeficiency virus following activation of latently infected cells. J Virol. 2006;80(3):1599–1603. doi: 10.1128/JVI.80.3.1599-1603.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emiliani S, Fischle W, Ott M, van Lint C, Amella CA, Verdin E. Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the u1 cell line. J Virol. 1998;72(2):1666–1670. doi: 10.1128/jvi.72.2.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emiliani S, van Lint C, Fischle W, et al. A point mutation in the HIV-1 tat responsive element is associated with postintegration latency. Proc Natl Acad Sci USA. 1996;93(13):6377–6381. doi: 10.1073/pnas.93.13.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 tat fluctuations drive phenotypic diversity. Cell. 2005;122(2):169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Mccune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 79.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, Mccune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172(4):1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aldrovandi GM, Feuer G, Gao L, et al. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363(6431):732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 81.Bonyhadi ML, Rabin L, Salimi S, et al. HIV induces thymus depletion in vivo. Nature. 1993;363(6431):728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 82.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, Mccune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 83.Brooks DG, Zack JA. Effect of latent human immunodeficiency virus infection on cell surface phenotype. J Virol. 2002;76(4):1673–1681. doi: 10.1128/JVI.76.4.1673-1681.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84▪.Brooks DG, Hamer DH, Arlen PA, et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19(3):413–423. doi: 10.1016/s1074-7613(03)00236-x. Utilization of the SCID-hu (Thy/Liv) mouse model for HIV latency to show that latently infected cells express little HIV mRNA, and can be induced with IL-7 or prostratin then killed with an anti-HIV immunotoxin. [DOI] [PubMed] [Google Scholar]
- 85.Scripture-Adams DD, Brooks DG, Korin YD, Zack JA. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J Virol. 2002;76(24):13077–13082. doi: 10.1128/JVI.76.24.13077-13082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brooks DG, Arlen PA, Gao L, Kitchen CM, Zack JA. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. Proc Natl Acad Sci USA. 2003;100(22):12955–12960. doi: 10.1073/pnas.2233345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burke B, Brown HJ, Marsden MD, Bristol G, Vatakis DN, Zack JA. Primary cell model for activation-inducible human immunodeficiency virus. J Virol. 2007;81(14):7424–7434. doi: 10.1128/JVI.02838-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113(1):58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kauder Se, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5(6):e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marini A, Harper JM, Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol. 2008;181(11):7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 92.Sahu GK, Lee K, Ji J, Braciale V, Baron S, Cloyd MW. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355(2):127–137. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 93.Chun TW, Engel D, Mizell SB, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5(6):651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 94.Prins JM, Jurriaans S, van Praag RM, et al. Immuno-activation with anti-CD3 and recombinant human Il-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13(17):2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 95.Davey RT, Jr, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96(26):15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Archin NM, Eron JJ, Palmer S, et al. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22(10):1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siliciano JD, Lai J, Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195(6):833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 99.Lindkvist A, Eden A, Norstrom MM, et al. Reduction of the HIV-1 reservoir in resting CD4+ T-lymphocytes by high dosage intravenous immunoglobulin treatment: a proof-of-concept study. AIDS Res Ther. 2009;6:15. doi: 10.1186/1742-6405-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stellbrink HJ, van Lunzen J, Westby M, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (cosmic trial) AIDS. 2002;16(11):1479–1487. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- 101.van Praag RM, Prins JM, Roos MT, et al. Okt3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21(3):218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- 102.Kulkosky J, Nunnari G, Otero M, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186(10):1403–1411. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 103.Lafeuillade A, Poggi C, Chadapaud S, et al. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. J Acquir Immune Defic Syndr (1999) 2001;26(1):44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- 104.Bartlett JA, Miralles GD, Sevin AD, et al. Addition of cyclophosphamide to antiretroviral therapy does not diminish the cellular reservoir in HIV-infected persons. AIDS Res Hum Retroviruses. 2002;18(8):535–543. doi: 10.1089/088922202753747888. [DOI] [PubMed] [Google Scholar]
- 105.Gustafson KR, Cardellina JH, 2nd, McMahon JB, et al. A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J Med Chem. 1992;35(11):1978–1986. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 106▪.Kulkosky J, Culnan D, MmRoman J, et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98(10):3006–3015. doi: 10.1182/blood.v98.10.3006. Describes the potential merits of prostratin as an activator of latent HIV. [DOI] [PubMed] [Google Scholar]
- 107.Williams SA, Chen LF, Kwon H, et al. Prostratin antagonizes HIV latency by activating NF-κb. J Biol Chem. 2004;279(40):42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- 108▪.Wender PA, Kee JM, Warrington JM. Practical synthesis of prostratin, DPP, and their analogs, adjuvant leads against latent HIV. Science. 2008;320(5876):649–652. doi: 10.1126/science.1154690. Description of the successful chemical synthesis of prostratin and analogs, which could allow the design of more specific and effective latency activators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: Activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses. 2009;25(9):883–887. doi: 10.1089/aid.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choudhary SK, Archin NM, Margolis DM. Hexamethylbisacetamide and disruption of human immunodeficiency virus type 1 latency in CD4+ T cells. J Infect Dis. 2008;197(8):1162–1170. doi: 10.1086/529525. [DOI] [PubMed] [Google Scholar]
- 111.Vlach J, Pitha PM. Hexamethylene bisacetamide activates the human immunodeficiency virus type 1 provirus by an NF-κb-independent mechanism. J Gen Virol. 1993;74(Pt 11):2401–2408. doi: 10.1099/0022-1317-74-11-2401. [DOI] [PubMed] [Google Scholar]
- 112.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med. 2007;58:221–237. doi: 10.1146/annurev.med.58.070605.115320. [DOI] [PubMed] [Google Scholar]
- 113.Thrush GR, Lark LR, Clinchy BC, Vitetta ES. Immunotoxins: an update. Annu Rev Immunol. 1996;14:49–71. doi: 10.1146/annurev.immunol.14.1.49. [DOI] [PubMed] [Google Scholar]
- 114.Pincus SH, Fang H, Wilkinson RA, Marcotte TK, Robinson JE, Olson WC. In vivo efficacy of anti-glycoprotein 41, but not anti-glycoprotein 120, immunotoxins in a mouse model of HIV infection. J Immunol. 2003;170(4):2236–2241. doi: 10.4049/jimmunol.170.4.2236. [DOI] [PubMed] [Google Scholar]
- 115.Lueders KK, De Rosa SC, Valentin A, Pavlakis GN, Roederer M, Hamer DH. A potent anti-HIV immunotoxin blocks spreading infection by primary HIV type 1 isolates in multiple cell types. AIDS Res Hum Retroviruses. 2004;20(2):145–150. doi: 10.1089/088922204773004851. [DOI] [PubMed] [Google Scholar]
- 116.Kennedy PE, Bera TK, Wang QC, et al. Anti-HIV-1 immunotoxin 3b3(FV)-pe38: Enhanced potency against clinical isolates in human PBMCS and macrophages, and negligible hepatotoxicity in macaques. J Leukoc Biol. 2006;80(5):1175–1182. doi: 10.1189/jlb.0306139. [DOI] [PubMed] [Google Scholar]
- 117.Ramachandran RV, Katzenstein DA, Wood R, Batts DH, Merigan TC. Failure of short-term CD4-pe40 infusions to reduce virus load in human immunodeficiency virus-infected persons. J Infect Dis. 1994;170(4):1009–1013. doi: 10.1093/infdis/170.4.1009. [DOI] [PubMed] [Google Scholar]
- 118.Davey RT, Jr, Boenning CM, Herpin BR, et al. Use of recombinant soluble CD4 pseudomonas exotoxin, a novel immunotoxin, for treatment of persons infected with human immunodeficiency virus. J Infect Dis. 1994;170(5):1180–1188. doi: 10.1093/infdis/170.5.1180. [DOI] [PubMed] [Google Scholar]
- 119.Dornadula G, Zhang H, Vanuitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282(17):1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 120.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharkey ME, Teo I, Greenough T, et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med. 2000;6(1):76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J Virol. 2005;79(8):5203–5210. doi: 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80(13):6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Orenstein JM, Meltzer MS, Phipps T, Gendelman HE. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gartner S, Markovits P, Markovitz DM, Betts RF, Popovic M. Virus isolation from and identification of HTLV-III/LAV-producing cells in brain tissue from a patient with AIDS. JAMA. 1986;256(17):2365–2371. [PubMed] [Google Scholar]
- 128.Marsden MD, Xu J, Hamer D, Zack JA. Activating stimuli enhance immunotoxin-mediated killing of HIV-infected macrophages. AIDS Res Hum Retroviruses. 2008;24(11):1399–1404. doi: 10.1089/aid.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18(8):1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 130.Amado RG, Mitsuyasu RT, Rosenblatt JD, et al. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a Phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum Gene Ther. 2004;15(3):251–262. doi: 10.1089/104303404322886101. [DOI] [PubMed] [Google Scholar]
- 131.Li MJ, Kim J, Li S, et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing tar decoy. Mol Ther. 2005;12(5):900–909. doi: 10.1016/j.ymthe.2005.07.524. [DOI] [PubMed] [Google Scholar]
- 132.An DS, Donahue RE, Kamata M, et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci USA. 2007;104(32):13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitsuyasu RT, Merigan TC, Carr A, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15(3):285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100(1):183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]