Abstract
In Parkinson disease (PD) brain, a progressive loss of dopaminergic neurons leads to dopamine depletion in the striatum and reduced motor function. Lewy bodies, the characteristic neuropathological lesions found in the brain of PD patients, are composed mainly of α-synuclein protein. Three point mutations in the α-synuclein gene are associated with familial PD. In addition, genome-wide association studies indicate that α-synuclein and Tau protein synergistically increase disease susceptibility in the human population. To determine the mechanism by which α-synuclein and Tau act together, we have used PD-causing neurotoxin MPTP and pathogenic α-synuclein mutants A30P, E46K, and A53T as models. We found that exposure of human neuroblastoma M17 cells to MPTP enhances the intracellular α-synuclein protein level, stimulates Tau protein phosphorylation at Ser262, and induces apoptosis. In mouse brain, ablation of α-synuclein function significantly suppresses Tau phosphorylation at Ser262. In vitro, α-synuclein binds to phosphorylated Ser214 of Tau and stimulates PKA-catalyzed Tau phosphorylation at Ser262. PD-associated α-synuclein mutations increase α-synuclein binding to Tau and stimulate Tau phosphorylation at Ser262. In HEK-293 cells, α-synuclein and its all PD-associated mutants destabilize the microtubule cytoskeleton in a similar extent. In contrast, when co-expressed with Tau, these PD-associated mutants destabilize microtubules with significantly higher potency than WT. Our results demonstrate that α-synuclein is an in vivo regulator of Tau protein phosphorylation at Ser262 and suggest that PD-associated risk factors such as environmental toxins and α-synuclein mutations promote Tau phosphorylation at Ser262, causing microtubule instability, which leads to loss of dopaminergic neurons in PD brain.
Keywords: Neurodegeneration, Neurofilaments, Neuron, Protein Kinase A (PKA), Protein-Protein Interactions, α-Synuclein, Microtubules, Parkinson Disease, Tau Protein, Tubulin
Introduction
Parkinson disease (PD)3 is the 2nd most common neurodegenerative disorder after Alzheimer disease (AD) and is characterized as a movement disorder manifesting bradykinesia, rigidity, and tremors (1, 2). The clinical symptoms of PD are progressive loss of dopaminergic neurons in the substantia nigra pars compacta, which give rise to the nigrostriatal pathway, leading to dopamine depletion in the striatum and reduced motor function. Lewy bodies (LBs) are the characteristic neuropathological lesions found in the brain of PD patients. Biochemical studies have determined that neuronal protein α-synuclein is the major component of LBs (2–4). In vitro, recombinant α-synuclein aggregate and form LB-like structures (3, 5). Overexpression of human α-synuclein in the brain develop LB-like inclusions in mice and Drosophila (6, 7). In addition, three point mutations, A30P, E46K, and A53T, have been discovered in rare familial forms of PD (8, 9). These mutations promote α-synuclein aggregation and toxicity in vitro and in vivo (3, 10–12). Genetic and biochemical data have established α-synuclein as the central molecule involved in the development of PD (4).
Various neurotoxic paradigms have been studied in an effort to reproduce the substantia nigra neuronal loss. Of these, administration of the well characterized MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) (or related rotenone or paraquat) induces PD-like symptoms in rodents and has provided a useful model to study PD neuropathology (13–15). The biochemical and cellular changes that occur following MPTP administration are very similar to those that occur in PD brains causing selective loss of dopaminergic neurons. In the brain, MPTP is metabolized into toxic MPP+ (13). In vitro, MPP+ inhibits complex 1 of the mitochondrial respiratory chain. Inhibition of complex 1 was thought to be the major mechanism by which MPP+ causes loss of dopaminergic neurons in the brain (13, 16). However, several studies have shown that α-synuclein−/− mice are resistant to MPTP toxicity (17, 18) and MPTP exposure increases the α-synuclein protein level in neurons in culture (19). The role of α-synuclein in toxin-induced PD is not clear.
Microtubule-associated protein Tau is a neuronal protein that plays important roles in neuronal morphogenesis, brain development, and is involved in the regulation of microtubule dynamics. Tau binds to microtubules and stabilizes the microtubule structure. Tau phosphorylation reduces its affinity for microtubules causing microtubule destabilization (20). Thus Tau phosphorylation regulates microtubule-related function of Tau. In AD and related tauopathies, abnormally hyperphosphorylated Tau accumulates in the brain (see below). The abnormal Tau phosphorylation is thought to prevent Tau from binding to microtubules leading to microtubule instability and neurodegeneration. Tau in AD brain is phosphorylated on at least 21 proline and non-proline-directed sites in vivo (21). The individual contribution of many of these sites is not known. However, among all these sites, Ser262 is uniquely located within the microtubule-binding region of Tau. Phosphorylation on this site alone has a major impact on Tau microtubule binding in vitro and confers Tau neurotoxicity in vivo (22–24).
Dysfunction of the Tau gene (MAPT) is associated with a family of neurodegenerative disorders collectively called tauopathies. These disorders include AD, frontotemporal dementia, and Parkinsonism linked to chromosome 17 (FTDP-17), Picks disease, corticobasal degeneration, and progressive supranuclear palsy. In all of these disorders, hyperphosphorylated Tau filaments accumulate and form neurofibrillary tangles (NFTs) (20, 25). Mutations have been found in MAPT in the familial form of FTDP-17 (26). Similarly, a common polymorphism has been reported in MAPT to be strongly associated with progressive supranuclear palsy, corticobasal degeneration, and AD (27–30).
Several studies have reported the association of MAPT with PD. Two recent genome-wide association studies on PD found that a common variant in five genes, including α-synuclein and MAPT, which increase disease susceptibility (31, 32). Moreover, an earlier study indicated that the cognitive decline and development of PD dementia is strongly associated with a synergistic interaction between the MAPT inversion polymorphism and a single nucleotide polymorphism on the α-synuclein gene (33). It was suggested that carrying either of the genotypes marginally increases the development of PD and that the combination of risk genotypes of both loci doubles the risk of disease development (33).
In addition, several studies have indicated an overlap in the clinical symptoms and pathological findings in the tauopathies and synucleinopathies (34). For example, Tau immunoreactive pathology is detected in each of the α-synucleinopathies. The incidence of NFTs in PD is much greater than in an age-matched population (35). Tau immunoreactive LBs are detected in the medulla of 80% of individuals with sporadic PD or LB dementia (36, 37). In familial PD, Tau lesions and insoluble Tau filaments have been observed in brain tissue obtained during autopsies from patients carrying the α-synuclein A53T mutation (38, 39). In multiple systems atrophy, hyperphosphorylated Tau is associated with a subset of glia cytoplasmic inclusions, where Tau decorates the α-synuclein aggregates (34, 40). Likewise, abnormally phosphorylated Tau is present in LBs found in sporadic PD patients, and occurs in neurons near areas containing α-synuclein pathology (36, 37). Thus, the presence of Tau pathology in each of the α-synucleinopathies, and the accumulating genetic and biochemical data together, suggest that α-synuclein and Tau are involved in shared or converging pathways in the pathogenesis of PD (33).
In vitro, α-synuclein promotes Tau hyperphosphorylation (41) and Tau fibrillization (42), which are associated with NFT pathology. When compared with α-synuclein WT, the disease causing A53T mutant displays a significantly increased potency to promote Tau fibrillization in vitro (39). In transgenic mice expressing α-synuclein A30P or A53T, hyperphosphorylated Tau inclusions are formed (42, 43). Despite these studies, the molecular mechanisms by which α-synuclein and Tau act together leading to PD neuropathology are not very clear. In this study, we have used MPTP and pathogenic α-synuclein mutants A30P, E46K, and A53T as models to examine the interaction of α-synuclein with Tau in human neuroblastoma M17 cells, mouse brain, and in vitro. Here we report that α-synuclein binds to Tau and promotes Tau phosphorylation at Ser262 both in vivo and in vitro and pathogenic mutations enhance this process. We also show that MPTP, by increasing the α-synuclein protein level, promotes Tau phosphorylation at Ser262 in M17 human neuroblastoma cells. Our data suggest that both MPTP and PD-associated α-synuclein mutations act through a common pathway leading to loss of dopaminergic neurons in the brain.
MATERIALS AND METHODS
cDNA Cloning and Plasmids
The human α-synuclein gene in the Myc-pcDNA3.1 Zeo vector was amplified by platinum® TaqDNA polymerase high fidelity-catalyzed PCR using the forward primer (5-ACG CCA TAT GGA TGT ATT CAT GAA AGG ACT TTC-3) containing the NdeI (underlined) and reverse primer (5-AAC AGA TCT TTA GGC TTC AGG TTC GTA GTC TTG-3) containing the BglII (underlined) sites. The PCR product was then ligated into a pGEM®-T Easy vector (Promega). After amplification, the insert was released and ligated into the NdeI and BamHI sites of bacterial expression vector pET-3a (Novagen). The α-synuclein mutants E46K, A53T, and E83P were generated in pET-3a and Myc-pcDNA3.1 Zeo vector using forward primers containing the mutagenic sites (underlined): 5-GGC TCC AAA ACC AAG AAA GGA GTG GTG CAT GGT-3, 5-GGA GTG GTG CAT GGT GTG ACA ACA GTG GCT GAG AAG-3, 5-CAG AAG ACA GTG CCA GGA GCA GGG AGC-3, and reverse primers containing the mutagenic sites (underlined): 5-ACC ATG CAC CAC TCC TTT CTT GGT TTT GGA GCC-3, 5-CTT CTC AGC CAC TGT TGT CAC ACC ATG CAC CAC TCC-3, 5-GCT CCC TGC TCC TGG CAC TGT CTT CTG-3, respectively, by using a site-specific mutagenesis kit (Stratagene). All cloning and mutagenesis were confirmed by DNA sequencing. The pET-3a (A30P) and Myc-pcDNA3.1 (A53T) were described previously (44). FLAG-Tau(WT) and FLAG-Tau(S214A) each in pcDNA3.1 and pET-3a are also described previously (45).
Animals
Brain tissues of adult (6 weeks) female α-synuclein−/− (number 003692, Jackson Laboratories) and α-synuclein+/+ (number 00664, Jackson Laboratories) (n = 4 each) in C57BL/6 background were obtained from Jackson Laboratories. Each brain was homogenized in an equal amount (w/v) of cold extraction buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1% Triton X-100, protease inhibitor mixture (Roche Applied Science), 5 mm EDTA, 1 mm DTT, 10 mm NaF, and 20 μm okadaic acid) using a Down glass homogenizer. Homogenized samples were centrifuged at 14,000 × g for 15 min at 4 °C using a bench top centrifuge. The resulting supernatants were analyzed by Western blot analysis.
Proteins and Antibodies
Tau(WT) and Tau(S214A) were purified from their respective bacterial lysates as described previously (45). Purification of α-synuclein (WT) and its various mutants A30P, E46K, A53T, and E83P were performed following previously published procedures (46) with some modifications. The overnight bacterial culture was diluted to ×10 in fresh medium and grown for 2 h at 37 °C. Isopropyl β-d-thiogalactoside was then added to a final concentration of 0.4 mm to induce protein synthesis and shaking was continued for another 4 h. The bacterial culture was centrifuged and the pellet was suspended in ice-cold purification buffer (50 mm Tris, pH 7.4, 1 mm EDTA, 1 mm DTT, and a mixture of protease inhibitors (Roche Applied Science)). Lysozyme was added to a final concentration of 20 μg/ml and the suspension was placed on ice for 15 min. Triton X-100 (1%) was then added to the suspension on ice. The suspension was sonicated three times (10 s each) using a probe sonicator and then centrifuged at 27,000 × g for 15 min at 4 °C. The supernatant was withdrawn and heated on a boiling water bath for 20 min. Boiled sample was cooled on ice and then centrifuged at 27,000 × g for 30 min at 4 °C. The supernatant was withdrawn and loaded onto a S-Sepharose column (∼1 ml; Amersham Biosciences) previously equilibrated in purification buffer at room temperature. The flow-through fractions were collected and loaded onto a Q-Sepharose (∼1 ml; Amersham Biosciences). The column was washed with 10 ml of purification buffer and then eluted with a linear gradient of NaCl (0–0.5 m) in purification buffer. The elution fractions (0.5 ml each) were analyzed by SDS-PAGE, and the fractions containing α-synuclein were pooled and dialyzed against cold purification buffer for 4 h at 4 °C. After dialysis, samples were aliquoted and stored in −80 °C until use. Anti-α-synuclein monoclonal antibody was purchased from BD Biosciences. Monoclonal antibodies Tau 5, AT8, PHF1, 12E8, anti-Myc, and anti-FLAG are described previously (45). Polyclonal antibodies pS214 and pS262 were purchased from BIOSOURCE Inc. The PKA catalytic subunit was purchased from Sigma.
Protein Concentrations
The concentration of Tau was determined spectrophotometrically by measuring OD at 280 nm as previously described (45). Concentrations of Tau(S214A), phosphorylated Tau(WT), and phosphorylated Tau(S214A) were determined via the Bio-Rad protein assay using Tau(WT) as the standard. The concentration of α-synuclein (WT) was determined by bicinchoninic acid protein assay (BCA) (Pierce) with bovine serum albumin as the standard. Concentrations of various α-synuclein mutants were determined by BCA protein assay using α-synuclein (WT) as the standard. Concentration of PKA is based on the dry weight.
Phosphorylation Assay
Tau was phosphorylated in the presence of α-synuclein or BSA by PKA. The assay mixture contained 50 mm Tris-HCl (pH 7.4), 50 mm NaCl, 10 mm MgCl2, 1 mm DTT, 1 mm EDTA, 0.2 mg/ml of Tau(WT) or Tau(S214A), 1 mm ATP, 100 units/ml of PKA, 50 μg/ml of α-synuclein or 50 μg/ml of BSA and protease inhibitor mixture. The reaction was initiated by adding an aliquot of PKA to the vial containing the rest of the components of the assay mixture. After the indicated time at 30 °C, aliquots were withdrawn, mixed with an equal volume of SDS-PAGE sample buffer, and analyzed by Western blot analysis. Kemptide phosphorylation activity of PKA was measured by filter paper assay essentially as described previously (47).
Cell Culture, Transfection, and MPTP Treatment
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum along with penicillin-streptomycin and transfected with Lipofectamine 2000 (Invitrogen) according to the standard protocol as described previously (44). Human neuroblastoma M-17 cells were cultured in 45% Dulbecco's modified Eagle's medium, 45% Ham's F-12 (Wisent Inc), 10% fetal bovine serum and penicillin-streptomycin (44). Cells were plated in 6-well plates or 100-mm culture dishes and grown to 80% confluence. The medium was changed and the cells were treated with the indicated concentration of MPTP (Sigma) (freshly dissolved in water) for 48 h. To inhibit PKA, cells were treated with freshly prepared Rp-cAMP (Sigma), a cell permeable inhibitor of activation by cAMP of cAMP-dependent protein kinase I and II (48, 49). After 1 h of treatment, cells were then exposed to MPTP. The cells were harvested with lysis buffer (50 mm Tris (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1 mm DTT, 0.5% of Nonidet P-40, and protease inhibitor mixture (Roche Applied Science)).
In Vitro Binding
Binding mixtures containing 50 mm Tris HCl (pH 7.4), 50 mm NaCl, 10 mm MgCl2, 1 mm DTT, 1 mm EDTA, 1 mg/ml of BSA, protease inhibitor mixture, 1 mm ATP, and 0.2 mg/ml of Tau(WT) or Tau(S214A) were incubated with or without PKA overnight at 30 °C. After incubation, samples were first analyzed for phosphorylation by Western blotting and then α-synuclein or BSA (50 μg/ml) was added to each mixture and incubation was continued for another 12 h at 30 °C. To 50 μl of each binding mixture 30 μl of protein G-agarose beads (Sigma) coated with anti-Tau serum were added. Samples were then shaken end-over-end for 6 h at 4 °C. After shaking, beads from each sample were collected by centrifugation, washed three times, and dissolved in 50 μl of SDS sample buffer. Dissolved beads were boiled, centrifuged, and 15 μl of the supernatant was analyzed by Western blot analysis.
Immunoprecipitation
The cells in the each culture dish were suspended in the lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 25 mm β-glycerol phosphate, I mm EDTA, 1 mm EGTA, 10 mm NaF, 10 mm MgCl2, 1% Triton X-100, 100 nm okadaic acid and protease inhibitor mixture (Roche Applied Science)). The cell suspension was placed in ice for 30 min and then centrifuged at 4 °C for 15 min. The supernatant was used for immunoprecipitation. 200 μl of the supernatant was precleared with protein G beads equilibrated in lysis buffer. The precleared samples were incubated with 10 μl of preimmune serum or the indicated antibody, and the mixture was shaken end-over-end for 4 h at 4 °C. After shaking, 30 μl of Protein G beads were added to the mixture and shaking was continued for another 4 h. The beads were collected by centrifugation, washed three times, boiled in 50 μl of SDS-PAGE sample buffer. Boiled samples were centrifuged and 15 μl of each sample was analyzed by Western blot analysis.
Microtubule Sedimentation Assay
Microtubule sedimentation assay was performed as described previously (50). Transfected HEK-293 cells were lysed in PIPES buffer (0.1 m PIPES, 1 mm EGTA, 1 mm MgSO4, and 1 mm β-mercaptoethanol, 0.2% Nonidet P-40 containing the protease inhibitor mixture). The protein concentration of each cell lysate was adjusted to 1 mg/ml using cold PIPES buffer on ice. To samples (100 μl each) at 37 °C, pre-warmed stock GTP was added to a final concentration of 1 mm. After 1 h at 37 °C, samples were centrifuged for 30 min at 13,000 × g using a bench top centrifuge at room temperature. Supernatant was transferred to another vial and the pellet was washed 2 times with the warm PIPES buffer containing 1 mm GTP. The pellet was dissolved in 50 μl of SDS-PAGE sample buffer. Supernatant and pellet (20 μl each) were analyzed by Western blot analysis.
RESULTS
MPTP Promotes Apoptosis and Tau Phosphorylation in Human Neuroblastoma M17 Cells
When human neuroblastoma M17 cells were treated with MPTP and analyzed by Western blot analysis, there was a dose-dependent increase in the relative amount of pro-apoptotic active caspase 3 protein (supplemental Fig. S1A). Consistent with this data, the relative amount of caspase 3-cleaved poly(ADP-ribose) polymerase was 2-fold higher in extracts of cells treated with 1 μm MPTP than in vehicle-treated control cells. Likewise, in the extracts of cells treated with 5, 25, and 50 μm MPTP, the relative amounts of caspase 3-cleaved poly(ADP-ribose) polymerase increased with increasing MPTP level (supplemental Fig. S1B). This result is consistent with the idea that MPTP is cytotoxic, and demonstrated that under our experimental conditions, it induces apoptosis in M17 neuroblastoma cells.
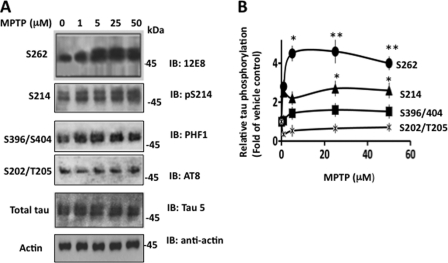
The relative amount of total Tau protein was similar in vehicle and MPTP-treated cells, indicating that this toxin does not affect Tau stability or Tau expression in these cells (Fig. 1A). However, the relative amount of 12E8 immunoreactivity, which recognizes phosphorylated Tau at Ser262 (51) was 2.8-, 4.5-, 4.6-, and 4.0-fold higher in 1, 5, 25, and 50 μm MPTP-treated cells than the basal level observed in vehicle-treated cells, respectively (Fig. 1, A and B). Similar results were obtained when polyclonal antibody pS262, specific for Ser262-phosphorylated Tau, was used (data not included). At Ser214, Tau phosphorylation of 1, 5, 25, and 50 μm MPTP-treated cells was 2.5-, 2.2-, 2.7-, and 2.8-fold more than the basal level observed in vehicle-treated cells, respectively. At Ser396/404 sites recognized by the PHF1 antibody, 1, 5, 25, and 50 μm MPTP stimulated phosphorylation 1.05-, 1.43-, 1.6-, and 1.5-fold more than the basal level, respectively. At Ser202/Thr205 sites recognized by the AT8 antibody, a relative amount of phosphorylated Tau was similar to the basal level in 1, 5, 25, and 50 μm MPTP-treated cells. Thus, MPTP did not affect Tau phosphorylation at Ser202/Thr205 but was stimulated at Ser262, Ser396/404, and Ser214. Moreover, 25 μm MPTP stimulated phosphorylation at Ser262 1.7- and 2.9-fold more than at Ser214 and Ser396/404, respectively (Fig. 1B). These results determined that MPTP significantly promotes Tau phosphorylation at Ser262 in human neuroblastoma M17 cells.
FIGURE 1.
MPTP promotes Tau protein phosphorylation in human neuroblastoma M17 cells. M17 cells treated with the indicated amounts of MPTP for 48 h were analyzed by Western blot analysis using the indicated antibodies. Based on blot band intensities, relative Tau phosphorylation was determined. A, Western blots. B, relative Tau phosphorylation. The relative Tau phosphorylation at each point was determined by normalizing the band intensity of phosphorylated Tau at the indicated site at that point with the respective total Tau band intensity. Data shown represent mean ± S.E. from three independent experiments. *, p < 0.001, and **, p < 0.005 relative to vehicle-treated control cells (t test).
Identification of the Kinase That Phosphorylates Tau at Ser262 in MPTP-treated Cells
Previous studies have shown that Ser262, a non-proline-directed site, is phosphorylated by cAMP-dependent protein kinase (PKA), protein kinase C (PKC), calmodulin-dependent protein kinase 2, and phosphorylase kinase in vitro (25, 47). To identify the kinase that phosphorylates Tau at Ser262 in response to MPTP treatment, we treated M17 cells with MPTP in the presence of the Ca2+ chelator EGTA (1 mm). MPTP increased Tau Ser262 phosphorylation 2.2-fold compared with the basal level in MPTP-treated cells as expected. In cells treated with MPTP and EGTA, Tau phosphorylation at Ser262 was 2.3-fold more than the basal level observed in vehicle-treated cells (data not shown). This data precluded Ca2+-dependent kinases: PKC, calmodulin-dependent protein kinase 2, and phosphorylase kinase as possible candidates for phosphorylating Tau at Ser262 in MPTP-treated cells.
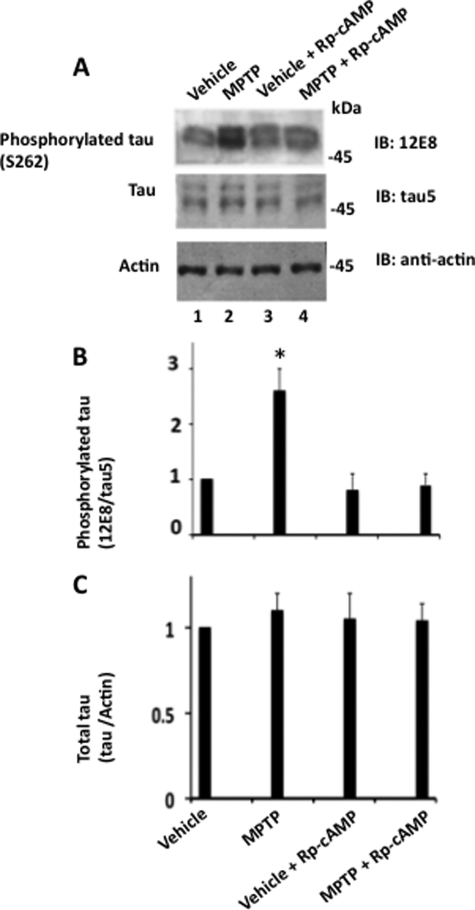
We then treated M17 cells with MPTP in the presence of Rp-cAMP, an inhibitor of PKA activation (48). Treated cells were analyzed by Western blot analysis. As shown in Fig. 2, in MPTP-treated cells Tau phosphorylation at Ser262 was 2.5-fold more than the basal level, as expected (compare lanes 1 and 2). When Rp-cAMP was included with MPTP, phosphorylation was reduced to an almost basal level (compare lanes 2 and 4). Thus, inhibition of PKA activation almost completely blocked MPTP-induced Tau phosphorylation at Ser262 without affecting the Tau protein level (Fig. 2, B and C). Based on this data, we concluded that PKA phosphorylates Tau at Ser262 in MPTP-treated cells.
FIGURE 2.
Inhibition of PKA activation inhibits Tau phosphorylation in MPTP-treated human neuroblastoma M17 cells. Human neuroblastoma M17 cells were treated with vehicle, MPTP (50 μm), vehicle + Rp-cAMP (25 μm), or MPTP (50 μm) + Rp-cAMP (25 μm) as described under “Materials and Methods.” Treated cells were analyzed by Western blot analysis using the indicated antibodies. Based on blot band intensities, relative Tau phosphorylation was determined as in the legend to Fig. 1. A, Western blots. B, relative Tau phosphorylation. C, total Tau. Values are mean ± S.E. from three determinations. *, p < 0.001 with respect to vehicle treated cells (t test).
α-Synuclein Regulates Tau Ser262 Phosphorylation in Vivo
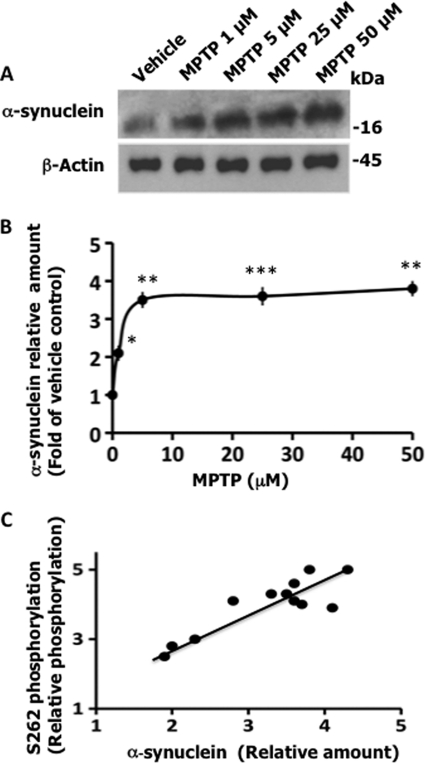
MPTP induces LB formation in the rodent brain by enhancing the α-synuclein level in neurons (52, 53). Consistent with these results, MPTP caused a dose-dependent increase in the intracellular α-synuclein level in our M17 human neuroblastoma cells (Fig. 3, A and B). More importantly, there was a positive correlation between the increase in α-synuclein protein and Tau Ser262 phosphorylation in MPTP-treated cells (Fig. 3C). This observation suggested that MPTP may promote Tau Ser262 phosphorylation by enhancing the α-synuclein protein level in M17 cells. This in turn suggested an interesting possibility that α-synuclein may be an in vivo regulator of Tau Ser262 phosphorylation.
FIGURE 3.
MPTP up-regulates intracellular α-synuclein protein in neuroblastoma M17 cells. MPTP or vehicle-treated cells from Fig. 1 were analyzed by Western blot analysis using the indicated antibodies. Based on blot band intensities, the relative levels of α-synuclein were determined by normalizing the α-synuclein band intensity with the respective actin band intensity. A, Western blots. B, relative amounts. The relative amount of α-synuclein at each point is presented as the fold of vehicle-treated cells. Values are mean ± S.E. from three independent experiments (*, p < 0.05; **, p < 0.005; ***, p < 0.001 with respect to vehicle treated controls (t test)). C, the correlation between Ser262 phosphorylation and α-synuclein level. Relative amounts of α-synuclein are from panel B, whereas relative Tau Ser262 phosphorylation values are from Fig. 1B.
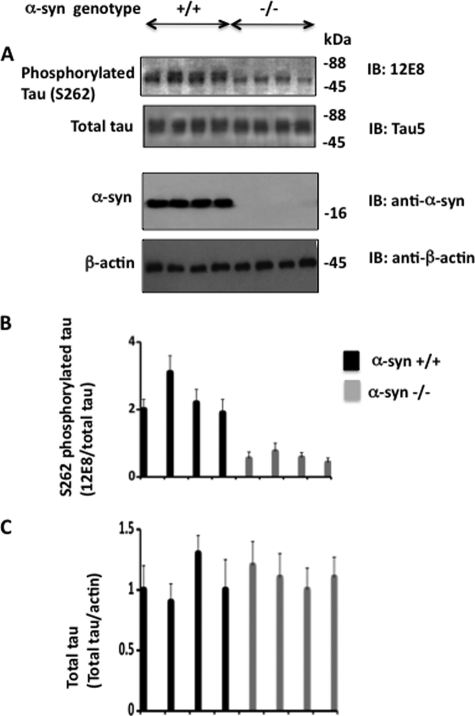
To test the above possibility, we examined Tau phosphorylation in α-synuclein+/+ and α-synuclein−/− mouse (4 per group) brain extracts (Fig. 4). Total Tau levels were similar in both α-synuclein+/+ and α-synuclein−/− brains. 12E8 immunoreactivity on the other hand was ∼4-fold less in α-synuclein−/− than in α-synuclein+/+ brains (Fig. 4, A and B). This data indicated that Ser262 phosphorylation in α-synuclein−/− brains is significantly less than in α-synuclein+/+ brains. To substantiate this data, Western blotted brain samples using pS262 polyclonal antibody specific for Ser262-phosphorylated Tau were used. Compared with the α-synuclein+/+ brains, the average pS262 band intensity value was 3.2-fold less in α-synuclein−/− brains (data not shown). Thus, ablation of α-synuclein function significantly reduced Tau phosphorylation at Ser262 in the mouse brain. This result demonstrated that α-synuclein is a positive regulator of Tau Ser262 phosphorylation in vivo.
FIGURE 4.
Tau phosphorylation is suppressed in α-synuclein−/− mouse brain. Mouse brains (4 in each group) of the indicated genotypes were homogenized and subjected to Western blotting using the indicated antibodies. Relative amounts of the indicated protein were calculated based on blot band intensities. Values are mean ± S.E. from three determinations.
α-Synuclein Stimulates Tau Phosphorylation by PKA in Vitro
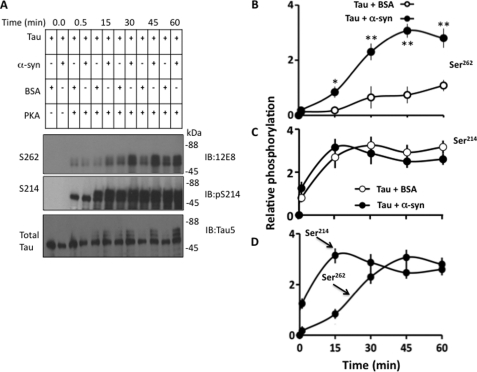
To test if α-synuclein activates PKA, we measured Kemptide (a synthetic peptide substrate of PKA) phosphorylation by filter paper assay in vitro (47). PKA phosphorylated Kemptide both in the presence and absence of α-synuclein. However, α-synuclein showed no effect on Kemptide phosphorylation by PKA (data not shown). This result indicated that α-synuclein does not affect PKA activity directly. This in turn suggested that α-synuclein in the brain may promote Tau phosphorylation by activating kinase(s) other than PKA. Alternatively, α-synuclein may activate PKA indirectly by making Tau more accessible to PKA. To evaluate the 2nd possibility, we phosphorylated recombinant human Tau with recombinant PKA in the presence of α-synuclein or BSA control. After various time points, aliquots were withdrawn and analyzed for phosphorylation at Ser262 and Ser214, the major PKA phosphorylation sites.
Tau was progressively phosphorylated at both Ser214 and Ser262 with increasing time points (Fig. 5A). Phosphorylation at Ser214 was similar in the presence and absence of α-synuclein at all time points (Fig. 5, A and C). However, at 15-, 30-, 45-, and 60-min time points, phosphorylation at Ser262 was 4.6-, 3.5-, 4.1-, and 2.6-fold greater in the presence than the absence of α-synuclein, respectively (Fig. 5, A and B). This data indicates that α-synuclein stimulates Tau phosphorylation by PKA at Ser262 but not at Ser214. Moreover, Ser262 phosphorylation progressed relatively slowly throughout in the absence of α-synuclein. In the presence of α-synuclein, Ser262 phosphorylation progressed relatively slowly until 15 min and then increased rapidly plateauing at 45 min (Fig. 5B). Thus, in the presence of α-synuclein, Ser262 phosphorylation displayed a lag that disappeared after 15 min. Phosphorylation at Ser214, on the other hand, increased rapidly from the beginning and plateaued at the 15-min time point in both the presence and absence of α-synuclein (Fig. 5, A and C).
FIGURE 5.
α-Synuclein stimulates Tau phosphorylation by PKA in vitro. Tau was phosphorylated by PKA in the presence of α-synuclein or BSA. At the indicated time points, aliquots were removed and analyzed by Western blot analysis. Based on blot band intensities, the relative amounts of phosphorylated Tau at each site at each time point were determined. A, Western blots. B and C, relative Tau phosphorylation at the indicated sites in the presence of the indicated proteins. Values represent mean ± S.E. of three independent experiments. *, p < 0.04; **, p < 0.001 against respective Tau phosphorylated in the presence of BSA control (t test). D, the comparison of Tau phosphorylation at the indicated sites in the presence of α-synuclein. Values are from panels B and C.
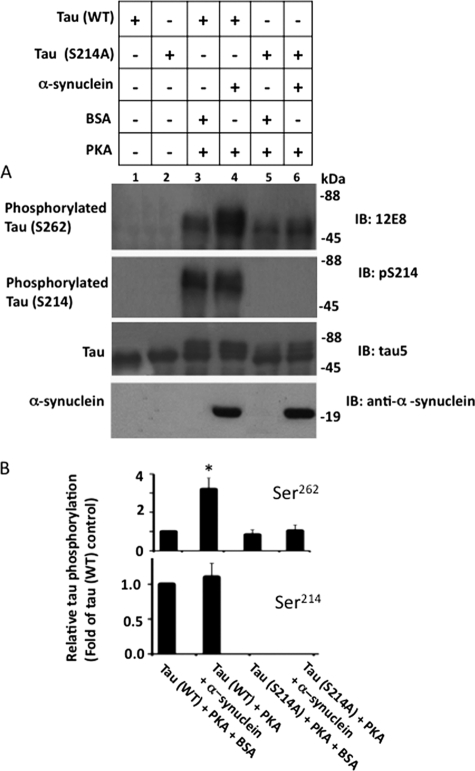
In the presence of α-synuclein, Tau phosphorylation at Ser214 occurred prior to phosphorylation at Ser262 (Fig. 5D). In addition, completion of Ser214 phosphorylation and disappearance of the lag in Ser262 phosphorylation occurred sequentially. To determine whether Ser214 phosphorylation has any role on Ser262 phosphorylation, we blocked Ser214 phosphorylation by replacing Ser214 to alanine. We phosphorylated Tau(WT) and Tau(S214A) with PKA in the presence and absence of α-synuclein. The relative amount of Ser214 phosphorylation of Tau(WT) was similar in the presence of α-synuclein or BSA control (Fig. 6, A and B). Phosphorylation of Tau(WT) Ser262 was 3.2-fold higher in the sample containing α-synuclein than the control BSA (compare lanes 3 and 4 in Fig. 6, A, and see B). This data are as expected, and confirmed the observation made in Fig. 5, demonstrating that α-synuclein stimulates phosphorylation of Tau(WT) by PKA at Ser262, but not at Ser214. As shown in Fig. 6A, Tau(S214A) was phosphorylated at Ser262 in the absence of α-synuclein (lane 5). More importantly, the relative amount of Ser262 phosphorylation of Tau(S214A) was not significantly different between samples with and without α-synuclein (Fig. 6B). Thus, in contrast to Tau(WT), Ser262 phosphorylation of Tau(S214A) was insensitive to α-synuclein. This data indicated that blocking Ser214 phosphorylation almost completely blocks the ability of α-synuclein to promote Tau phosphorylation at Ser262 by PKA.
FIGURE 6.
Blocking Ser214 phosphorylation inhibits α-synuclein-mediated Ser262 phosphorylation of Tau by PKA in vitro. Tau(WT) and Tau(S214A) were phosphorylated in the presence of α-synuclein or BSA by PKA as indicated for 45 min. After phosphorylation, samples were analyzed via Western blot analysis using the indicated antibodies. Based on blot band intensities, relative Tau phosphorylation at the indicated sites was determined. A, Western blots, B, relative Tau phosphorylation. Relative Tau phosphorylation was determined by normalizing phosphorylated Tau band intensity at the indicated site with the respective total Tau band intensity. Values are mean ± S.E. of three independent determinations. *, p < 0.01 against corresponding BSA control (t test).
α-Synuclein Binds to Ser214-phosphorylated Tau
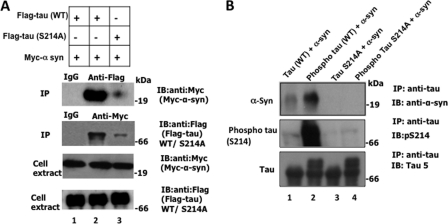
To determine the molecular basis of α-synuclein-mediated Ser262 phosphorylation, we asked if α-synuclein binds to Tau and if Ser214 phosphorylation plays any role in the binding. We co-transfected FLAG-Tau(WT) or FLAG-Tau(S214A) with Myc-α-synuclein in HEK-293 cells. Transfected cells were lysed, and Tau and α-synuclein were immunoprecipitated using anti-FLAG and anti-Myc antibodies, respectively. Indeed, α-synuclein was present in an anti-FLAG immune complex, and Tau was present in the anti-Myc immune complex (Fig. 7A, lane 2). Furthermore, the relative amount of α-synuclein that co-immunoprecipitated from cells co-transfected with FLAG-Tau(S214A) and Myc-α-synuclein was significantly less than the amount from cells co-transfected with FLAG-Tau(WT) and Myc-α-synuclein (Fig. 7A, compare lanes 2 and 3 in the top blot). Likewise, compared with Tau(WT) the amount of Tau(S214A) that co-immunoprecipitated with Myc-α-synuclein was significantly less (compare lanes 2 and 3 in the anti-Myc IP blot). This data indicated that Tau binds to α-synuclein, and that mutating Ser214 to Ala significantly inhibits this binding.
FIGURE 7.
α-Synuclein binds to Ser214-phosphorylated Tau. A, mutation of Ser214 to Ala inhibits α-synuclein-Tau binding in HEK-293 cells. HEK-293 cells transfected with the indicated genes were lysed and first Western blotted to examine the expressions of the transfected constructs. Cell lysates were then subjected to immunoprecipitation using antibodies, anti-FLAG, anti-Myc, or a nonspecific IgG control. Each resulting immune complex was analyzed by Western blot analysis using the indicated antibody. The lower panels corresponding to the cell extract shows expression of the indicated genes. The top panels corresponding to immunoprecipitation show α-synuclein or Tau co-immunoprecipitation as indicated. B, Ser214 phosphorylation of Tau promotes binding of α-synuclein with Tau in vitro. Tau(WT), PKA-phosphorylated Tau(WT), Tau(S214A), or PKA-phosphorylated Tau(S214A) were mixed with α-synuclein in a binding buffer containing BSA and then immunoprecipitated with anti-Tau antibody. Each immune complex was immunoblotted with indicated antibody.
To determine whether α-synuclein binds to Tau directly and to exclude the possibility that a conformational change caused by mutation of Ser214 to Ala may inhibit the binding, we performed an in vitro binding experiment. We phosphorylated Tau(WT) and Tau(S214A) with PKA under identical conditions. PKA-phosphorylated and non-phosphorylated species were mixed with α-synuclein in the presence of BSA (1 mg/ml). Each Tau species was immunoprecipitated from the mixture using anti-Tau antibody and analyzed by Western blot analysis.
All Tau species, Tau(WT), phosphorylated Tau(WT), Tau(S214A), and phosphorylated Tau(S214A), were immunoprecipitated with anti-Tau antibody from their respective mixtures (Fig. 7B, lower blot). α-Synuclein did not co-immunoprecipitate with either phosphorylated or nonphosphorylated Tau(S214A) (Fig. 7B, lanes 3 and 4). More importantly, α-synuclein co-immunoprecipitated significantly more with phosphorylated compared with non-phosphorylated Tau(WT) (Fig. 7B, compare lanes 1 and 2 of top blot). This data showed that Ser214-phosphorylated Tau binds to α-synuclein significantly more that the non-phosphorylated counterpart. Based on this result, we concluded that Tau phosphorylation at Ser214 is the major determinant for the α-synuclein Tau interaction.
Effect of PD-specific α-Synuclein Mutations
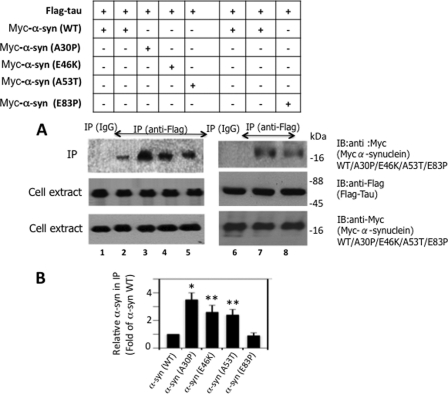
To evaluate the pathological implication of our findings, we examined the effects of PD-specific α-synuclein mutations, A30P, E46K, and A53T, on the Tau α-synuclein interaction. We co-transfected FLAG-Tau with Myc-α-synuclein (WT), Myc-α-synuclein (A30P), Myc-α-synuclein (E46K), and Myc-α-synuclein (A53T) in HEK-293 cells. We included a nonspecific mutant Myc-α-synuclein (E83P) as a control in our experiment. Transfected cells were lysed and Tau was immunoprecipitated using anti-FLAG antibody. As shown in Fig. 8, A and B, Myc-α-synuclein co-immunoprecipitated with FLAG-Tau as expected (lane 2). Under identical conditions, the relative amount of Myc-α-synuclein (A30P) that co-immunoprecipitated with FLAG-Tau was 3.5-fold more than the Myc-α-synuclein (WT) (compare lanes 2 and 3). Likewise, the relative amount of co-immunoprecipitated Myc-α-synuclein (E46K) and Myc-α-synuclein (A53T) was 2.6- and 2.4-fold more than the Myc-α-synuclein (WT), respectively (lanes 4 and 5). The relative amount of the Myc-α-synuclein (E83P) control that co-immunoprecipitated with FLAG-Tau, on the other hand, was similar to that of Myc-α-synuclein (WT) (compare lanes 7 and 8). Thus, the nonspecific mutation E83P did not affect the interaction of α-synuclein with Tau. All PD-specific mutations, A30P, E46K, and A53T, on the other hand, significantly enhanced binding of α-synuclein with Tau.
FIGURE 8.
Pathogenic α-synuclein mutations promote the binding of α-synuclein with Tau in HEK-293 cells. HEK-293 cells co-transfected with the indicated constructs were analyzed by Western blot analysis to confirm expression of the respective genes and then immunoprecipitated with the indicated antibodies. The resulting immune complexes were Western blotted as indicated. Based on blot band intensities, the relative amount of α-synuclein co-immunoprecipitated with Tau was determined. A, Western blots, B, relative amount of α-synuclein in the immunoprecipitate. To calculate the relative amount of α-synuclein, the blot band intensity of α-synuclein in the immunoprecipitation blot was normalized against the respective α-synuclein band intensity in cell extract blot. Values are mean ± S.E. of three determinations and are expressed as the -fold of α-synuclein (WT). *, p < 0.001; **, p < 0.04 with respect to α-synuclein (syn) (WT) (t test).
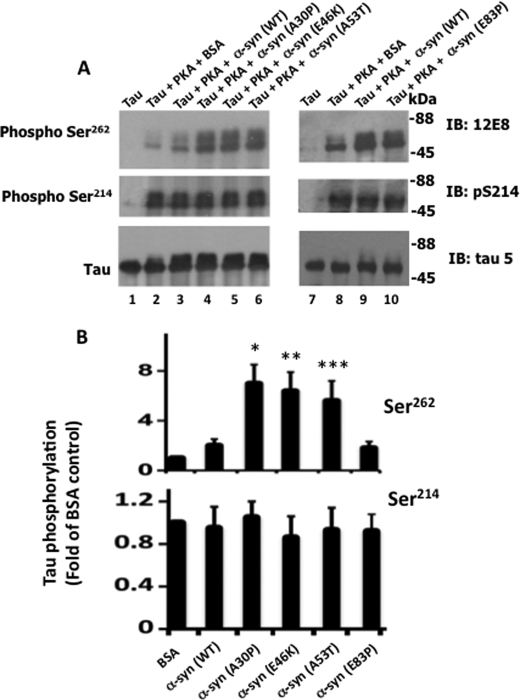
PD-specific Mutations Promote Tau Phosphorylation at Ser262 in Vitro
To further evaluate pathogenic α-synuclein mutations, we performed an in vitro Tau phosphorylation assay. We incubated Tau with PKA in the presence of α-synuclein (WT), α-synuclein (A30P), α-synuclein (E46K), α-synuclein (A53T), or the nonspecific mutant α-synuclein (E83P) and the rest of the phosphorylation mixture components. After incubation, Tau phosphorylation was monitored. As shown in Fig. 9, Tau was phosphorylated at Ser262 by PKA in the absence of α-synuclein (lane 2). In the presence of α-synuclein (WT), Tau was phosphorylated at Ser262 2-fold more than in the presence of control BSA (compare lanes 2 and 3). In the presence of α-synuclein A30P, E46K, and A53T, Tau was phosphorylated 7.1-, 6.4-, and 5.6-fold more than in the presence of BSA control, respectively. In the presence of the nonspecific mutant E83P, on the other hand, Tau remained phosphorylated at the level observed in the mixture containing α-synuclein (WT) (compare lanes 9 and 10). Thus, pathogenic mutations A30P, E46K, and A53T promoted Tau phosphorylation at Ser262 at levels 3.5-, 3.2-, and 2.8-fold greater than α-synuclein (WT), respectively (Fig. 9B). Furthermore, Ser214 phosphorylation was similar in samples containing BSA control, α-synuclein (WT), α-synuclein (A30P), α-synuclein (E46K), α-synuclein (A53T), and α-synuclein (E83P) (Fig. 9B). Thus, α-synuclein pathogenic mutations specifically enhanced phosphorylation by PKA at Ser262 but not at Ser214.
FIGURE 9.
Pathogenic α-synuclein mutations promote Tau phosphorylation at Ser262in vitro. Tau was phosphorylated by PKA in the presence of the indicated α-synuclein species for 45 min. After phosphorylation, an aliquot from each sample was analyzed by Western blot analysis using the indicated antibodies. Based on blot band intensities, relative Tau phosphorylation was determined. A, Western blots. B, relative Tau phosphorylation. Relative Tau phosphorylation at the indicated sites were determined as described in the legend to Fig. 1. Values represent mean ± S.E. of three determinations and are expressed as -fold of Tau phosphorylated in the presence of α-synuclein (WT). *, p < 0.002; **, p < 0.005; ***, p < 0.01 with respect to Tau phosphorylated in the presence of BSA control (t test).
Effects of Pathogenic α-Synuclein Mutations on the Microtubule Cytoskeleton
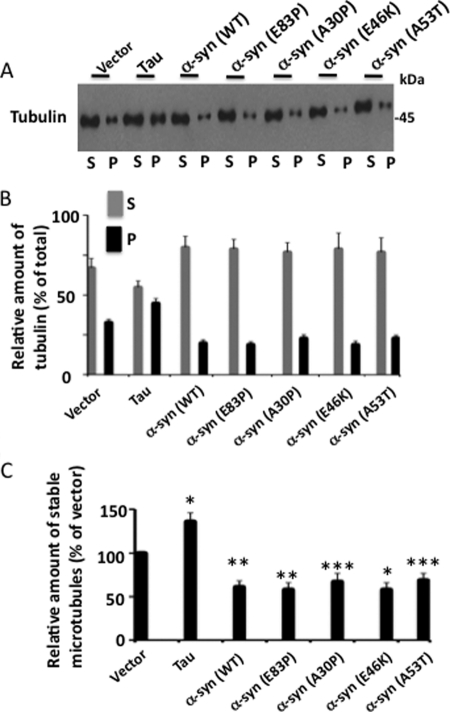
Studies have suggested that α-synuclein may cause loss of dopaminergic neurons in PD brain by destabilizing the microtubule cytoskeleton (54–56). To examine how α-synuclein pathogenic mutations affect the microtubules, we first examined the impact of α-synuclein in the absence of Tau. We transfected α-synuclein and its various mutants individually in HEK-293 cells and analyzed them by Western blot analysis. The relative amount of total tubulin was similar in α-synuclein (WT) and all of its mutants used compared with vector-transfected cells (supplemental Fig. S2). This observation determined that overexpression of α-synuclein and all of the mutants does not alter the intracellular tubulin level.
We then mixed each lysate with buffer containing GTP/Mg2+ and incubated them at 37 °C to induce microtubule polymerization. After incubation, each sample was centrifuged. Tubulins that formed stable microtubules were recovered in the pellet (P) and those that were unstable and did not polymerize remained in the supernatant (S). Pellet and supernatants were analyzed by Western blot analysis.
As shown in Fig. 10, A and B, in vector-transfected cells 33% of the total amount of microtubules were in the pellet and 67% in the supernatant. This data indicated that in vector-transfected control cells, 33% of the total amount of microtubules were stable. In Tau-transfected cells, 45% of the total amount of microtubules were stable and were recovered in the pellet, indicating that Tau overexpression increased microtubule stability by 1.36-fold (Fig. 10C). In α-synuclein (WT) overexpressing cells, 20% of the total microtubules were stable and present in the pellet (Fig. 10B). This data showed that in cells overexpressing α-synuclein (WT), the relative amount of stable microtubules is 60.6% of those expressing vector control. This, in turn, determined that overexpression of α-synuclein (WT) destabilized microtubules by 39.4% compared with vector expressing cells (Fig. 10C). Likewise, microtubules were 32, 33, 32, and 31% less stable than in vector-expressing cells in α-synuclein: A30P, E46K, A53T, and E83P expressing cells, respectively (Fig. 10C). Thus, whereas Tau increased microtubule stability, α-synuclein and each of its mutants decreased microtubule stability in HEK-293 cells. Surprisingly, as shown in Fig. 10C, the relative amount of stable microtubules in cells overexpressing A30P, E46K, or A53T is similar to that of α-synuclein (WT) or the nonspecific mutant E83P. This data showed that pathogenic mutations did not affect the ability of α-synuclein to destabilize microtubules in our experiment.
FIGURE 10.
α-Synuclein and its pathogenic mutations destabilize microtubules in vitro. HEK-293 cells transfected with the indicated genes were lysed in microtubule assembly buffer containing GTP/Mg2+. An aliquot was analyzed for expression of the transfected gene (supplemental Fig. S2) and the remaining aliquot was incubated at 37 °C to induce microtubule polymerization and subjected to a microtubule sedimentation assay. The resulting supernatant (S) and pellet (P) were analyzed via Western blot. Based on the blot band intensity, the relative amount of tubulin and stable microtubules in each fraction were calculated. A, Western blot. B, relative amount of tubulin. To calculate the relative amount of tubulin, the band intensity value of tubulin in each fraction of the indicated sample was divided by the sum of the band intensity values of tubulin in P and S fractions of that sample. C, relative amount of stable microtubules. The relative amount of stable microtubules of a sample is the relative amount of tubulin in the P fraction of that sample from panel B and is expressed as the % of vector-expressing cells. Values are mean ± S.E. from three independent experiments. *, p < 0.01; **, p < 0.001; p < 0.05 with respect to vector-transfected control cells (t test).
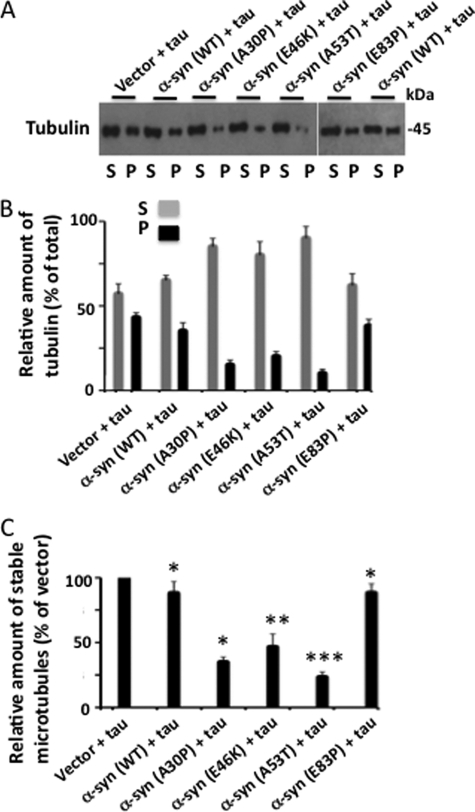
We next examined the impact of α-synuclein and its mutants on microtubules in Tau-expressing cells. We co-transfected Tau with α-synuclein (WT) or its various mutants into HEK-293 cells. Transfected cells were lysed and first analyzed by Western blot analysis (supplemental Fig. S3). As expected, the level of tubulin was similar in cells co-expressing Tau with vector, α-synuclein (WT), A30P, E46K, E53T, or E83P. In contrast, Tau phosphorylation at Ser262 was 1.5-, 2.1-, 2.2-, and 2.0-fold higher in cells co-expressing Tau with α-synuclein (WT), A30P, E46K, or A53T, respectively, than those co-expressing Tau with vector. Moreover, compared with cells co-expressing Tau with α-synuclein (WT), those co-expressing Tau with A30P0, E46K, or A53T displayed Ser262-phosphorylated Tau 1.4-, 1.4-, and 1.3-fold higher, respectively. Thus as observed in Fig. 9, in intact cells, α-synuclein promoted Tau phosphorylation at Ser262 and its pathogenic mutations enhanced its ability.
We then subjected the rest of the lysate to a microtubule sedimentation assay as described above. In control cells that were co-transfected with Tau and vector, 43% of the total microtubules were stable and present in the pellet (Fig. 11, A and B). In Tau and α-synuclein (WT) co-transfected cells, this number decreased to 34.8%. Thus, compared with Tau and vector co-transfected cells, Tau and α-synuclein (WT) co-transfected cells, microtubule stability decreased by 11.9% (Fig. 11C). Likewise, compared with Tau and vector co-transfected cells, in Tau and A30P, E46K, A53T, or E83P co-transfected cells, microtubule stability decreased by 65.2, 53.5, 76.2, and 11.7%, respectively (Fig. 11C). Thus, all α-synuclein species destabilized microtubules to different extents in Tau-expressing cells.
FIGURE 11.
Pathogenic mutations promote microtubule destabilizing activity of α-synuclein in the presence of Tau. The indicated α-synuclein species or vector were co-transfected with Tau in HEK-293 cells. Transfected cells were lysed and an aliquot was analyzed for Tau phosphorylation and expression of the transfected gene (supplemental Fig. S3). The remaining sample was then analyzed by a microtubule sedimentation assay as described in the legend to Fig. 10. Based on tubulin band intensities, the relative amount of tubulin in S and P fractions as well as the relative amount of stable microtubules were determined. A, Western blot. B, relative amount of tubulin. C, the relative amount of stable microtubules of a sample is the relative amount of tubulin in the P fraction of that sample from panel B and is expressed as % of vector-expressing cells. Values are mean ± S.E. of three independent experiments. *, p < 0.001; **, p < 0.05; ***, p < 0.01 with respect to α-synuclein (WT) and Tau-transfected cells (t test).
As shown in 11C, in Tau and α-synuclein (WT) co-expressing cells, the microtubule stability is 88.1% of that observed in Tau and vector co-transfected cells. In contrast, in Tau and A30P co-expressing cells, the amount of stable microtubules is 34.8% of the Tau and vector co-expressing cells. Thus, compared with α-synuclein (WT), A30P-expressing cells, the relative amount of stable microtubules is 2.5-fold less (Fig. 11C). This indicated that the A30P mutant destabilizes microtubules 2.5-fold more than α-synuclein (WT) in these cells. Likewise, the relative amount of stable microtubules in cells co-expressing Tau and α-synuclein (E46K) or α-synuclein (A53T) was 1.9- and 3.8-fold less than those co-expressing Tau and α-synuclein (WT), respectively (Fig. 11C). Cells co-expressing the Tau and nonspecific α-synuclein (E83P) mutant, on the other hand, the relative amount of stable microtubules was similar to that observed in Tau and α-synuclein (WT) co-expressing cells. This data indicated that when co-expressed with Tau, pathogenic A30P, E46K, and A53T but not nonspecific E83P α-synuclein mutants destabilize microtubules more than the α-synuclein (WT).
DISCUSSION
Tau is the major component of paired helical filaments (PHFs), which are the major fibrous components of NFTs, the characteristic neuropathological lesions found in the brains of patients suffering from AD and related tauopathies (20, 25). Tau isolated from PHFs is abnormally hyperphosphorylated and is incapable of binding to microtubules and promoting microtubule assembly in vitro. Upon dephosphorylation, Tau isolated from PHFs (PHF Tau) regains the ability to bind to and promote microtubule assembly (57, 58). Abnormal Tau phosphorylation is thought to prevent Tau from binding to microtubules, causing an accumulation of hyperphosphorylated Tau that then leads to PHF formation, microtubule instability, and neurodegeneration (25). PHF-Tau is phosphorylated on 21 sites at least (21). Among these sites, Ser262 is uniquely located within the first microtubule-binding region of Tau. Phosphorylation at this site alone was found to detach Tau from microtubules, cause microtubule instability, and make Tau neurotoxic in Drosophila and primary neurons in culture (22–24).
PKA phosphorylates Tau at both Ser214 and Ser262 (see Ref. 59 and Fig. 5). In the presence of α-synuclein, PKA phosphorylates Ser262 significantly more than in the absence. Phosphorylation of Tau at Ser214, on the other hand, is similar in the presence and absence of α-synuclein (Fig. 5). This data indicates that α-synuclein stimulates Tau phosphorylation by PKA at Ser262 but not at Ser214. Interestingly, when Tau is phosphorylated by PKA in the presence of α-synuclein, Ser214 phosphorylation precedes Ser262 phosphorylation (Fig. 5). When Ser214 phosphorylation is blocked, phosphorylation at Ser262 becomes similar in the presence and absence of α-synuclein (Fig. 6). In vitro, α-synuclein binds to phosphorylated Ser214 of Tau (Fig. 7). This step occurs prior to and is required for α-synuclein to stimulate phosphorylated at Ser262. Our data indicate that PKA phosphorylation at Ser262 occurs in two steps. In the first step, PKA phosphorylates Tau at Ser214 and generates an α-synuclein binding site to which α-synuclein subsequently binds. α-Synuclein binding changes the Tau conformation exposing Ser262. PKA then phosphorylates the exposed Ser262 site of Tau. More importantly, as shown in Fig. 9B, compared with α-synuclein (WT), in vitro Ser262 phosphorylation-promoting activities of α-synuclein mutants, A30P, E46K, and A53T, are 3.5-, 3.1-, and 2.9-fold, respectively, higher. Our results indicate that α-synuclein is a regulator of phosphorylation of Tau at Ser262 and that pathogenic α-synuclein mutations interfere with this regulation.
We found that pathogenic α-synuclein mutations promote Tau phosphorylation at Ser262 by enhancing the binding of α-synuclein with Tau (Figs. 8 and 9). As discussed above, α-synuclein binds to Tau phosphorylated at Ser214 (Fig. 7). Surprisingly, none of the pathological mutations enhanced Ser214 phosphorylation in vitro (Fig. 9). This means that pathogenic mutations do not promote Tau phosphorylation at Ser262 by promoting Ser214 phosphorylation. It is possible that pathogenic mutations change the α-synuclein conformation that has higher affinity for Tau phosphorylated at Ser214. Alternatively, as shown in Fig. 7B, lane 1, nonphosphorylated Tau bind to α-synuclein. Likewise, Tau(S214A) co-immunoprecipitates with α-synuclein (Fig. 7A, lane 3). This data shows that α-synuclein binds Tau independent of Ser214 phosphorylation and that Ser214 phosphorylation enhances the binding. Consistent with this suggestion, a previous study reported that α-synuclein binds to the microtubule-binding region of Tau in vitro (41). Pathogenic mutations may enhance the affinity of α-synuclein for the microtubule-binding region of Tau.
Previous studies have shown that tubulin co-localizes with α-synuclein in Lewy bodies and α-synuclein positive pathological structures (55). In vitro, tubulin promotes α-synuclein fibrillization, and α-synuclein was reported to directly interact with microtubules in vitro (55). When neurons were exposed to fibrillar α-synuclein, it caused microtubule depolymerization (54). Consistent with these reports, we found that when overexpressed in HEK-293 cells, α-synuclein reduced the amount of microtubules formed when compared with vector-transfected cells (Fig. 10C). Surprisingly, in cells expressing any of the pathogenic mutations, A30P, E46K, and A53T, the amount of microtubules formed was similar to that observed in cells expressing α-synuclein (WT) (Fig. 10C). This data indicates that under our experimental condition, pathogenic mutations do not affect the interaction of α-synuclein with microtubules and appears to contradict the idea that α-synuclein may cause microtubule instability in a PD brain. However, when α-synuclein (WT) is co-expressed with Tau in HEK-293 cells, the relative amount of microtubules formed is 88% of that observed in control cells co-expressing vector and Tau (Fig. 11C). In cells co-expressing α-synuclein A30P and Tau, on the other hand, the relative amount of microtubules formed was 34.8% of the control. Likewise, cells co-expressing α-synuclein E46K and A53T and Tau were 46.5 and 23.3% of control, respectively. Thus when co-expressed with Tau, pathogenic α-synuclein mutants A30P, E46K, and A53T caused microtubule instability 2.5-, 1.9-, and 3.8-fold more that the α-synuclein (WT) (Fig. 11C). These finding indicate that pathogenic mutations enhance the ability of α-synuclein to destabilize microtubules via Tau in neurons.
We find that in cells deficient in Tau, α-synuclein reduces microtubule stability by 39.4% (Fig. 10C). In Tau-expressing cells, this value decreases to 11.9% (Fig. 11C), a drop in more than 25%. This data suggests that in neurons, Tau protects microtubules from α-synuclein. More importantly, as discussed above, pathogenic α-synuclein mutants are more potent to destabilize microtubules than α-synuclein WT in only Tau-expressing cells. Furthermore, as shown in Fig. 9B, and supplemental Fig. S3, A30P, E46K, and A53T mutants stimulate Tau phosphorylation at Ser262 significantly more than the WT did. As Tau stabilizes microtubules and Tau phosphorylation at Ser262 has a profoundly negative effect on Tau microtubule binding (22, 23), it is very likely that pathogenic α-synuclein mutations promote microtubule instability by promoting Tau phosphorylation at Ser262 in neurons.
MPTP, in addition to Ser262 and Ser214, also promotes Tau phosphorylation at Ser396/404 in human neuroblastoma M17 cells (Fig. 1). A previous study has shown that MPTP activates GSK3β in neurons in culture and mouse brain, and that inhibition of GSK3β suppresses MPTP-induced Tau Ser396/404 phosphorylation in SH-SY5Y cells (60). PKA does not phosphorylate Tau at Ser396/404 (59) and GSK3β does not phosphorylate Tau at Ser262 (61). Thus, MPTP appears to promote Tau phosphorylation in the brain by activating both PKA and GSK3β.
Environmental toxins, MPTP, rotenone, and paraquat, induce many features of PD, including motor deficit and the accumulation of α-synuclein inclusions in rodents (13). Several studies have indicated that these toxins exert their effects by inhibiting the mitochondrial complex 1 of dopaminergic neurons of substantia nigra (13). However, recently a study showed that primary midbrain mesencephalic neurons in culture without a functional complex 1 are viable and displayed sensitivity to MPTP, paraquat, or rotenone similar to those containing fully functional complex 1. Thus, the absence of complex 1 activity did not protect dopaminergic neurons from MPTP, parquat, or rotenone (62). This study suggested that MPTP, rotenone, or paraquat cause a loss of dopaminergic neurons in a complex 1-independent mechanism. In the current study, we showed that in human neuroblastoma M17 cells, MPTP increases the intracellular α-synuclein level, promotes PKA-catalyzed Tau phosphorylation at Ser262, and induces apoptosis. Previous studies have shown that phosphorylation at Ser262 confers Tau toxicity in Drosophila and primary neurons in culture and destabilizes microtubules in vitro (22–24). Our data along with these studies suggest that MPTP by enhancing the intracellular α-synuclein level, promotes Tau phosphorylation at Ser262 by PKA, which may lead to loss of dopaminergic neurons in the brain. Consistent with this idea, α-synuclein−/− mice were reported to display resistance to MPTP (17).
Specific loss of dopaminergic neurons of substantia nigra is one of the hallmarks of PD. One study has reported that overexpression of α-synuclein (WT) in dopaminergic neurons in culture induces dopamine oxidation and promotes apoptosis, and pathogenic α-synuclein mutations A30P and A53T promote this process (63). Another study demonstrated that the exposure of toxin rotenone to dopaminergic neurons in culture induces dopamine oxidation, apoptosis, and microtubule depolymerization (56). This study also showed that microtubule depolymerization disrupts vesicular transport, and leads to an accumulation of dopamine in soma, which then leads to oxidation of cytosolic dopamine.
Using transfected HEK-293 cells, microtubule sedimentation, and Tau phosphorylation assays, we demonstrated that α-synuclein promotes Tau phosphorylation at Ser262 and destabilizes microtubules, and that α-synuclein pathogenic mutations accelerate this process (Figs. 9–11). Our data and previous reports together suggest that in normal neurons, α-synuclein regulates microtubule dynamics by regulating Tau phosphorylation at Ser262. PD-associated risk factors such as α-synuclein mutations, increased α-synuclein level, or exposure to environmental toxins may modify this pathway and promote Tau phosphorylation at Ser262. Increased Ser262 phosphorylation may confer Tau toxicity and induce microtubule depolymerization, causing oxidation of cytosolic dopamine. It is to be noted that abundant filamentous Tau has been reported in the brains of PD patients (38, 39). In vitro, α-synuclein promotes Tau fibrillization, and disease causing mutations accelerate this process (39, 42). In the current study, we showed that α-synuclein binds to Tau and PD-specific mutations enhance this binding. It is possible that in the PD brain, the mutant form or excess level of α-synuclein may interact with Tau and induce Tau fibrillization.
This study emphasizes the mechanism by which pathogenic α-synuclein mutations promote neurotoxicity. However, our data, for the first time, demonstrate that α-synuclein binds to Tau phosphorylated at Ser214. α-Synuclein shares physical and functional homology with phosphoserine-binding protein 14-3-3 and forms heterodimers with 14-3-3 isoforms (64). Interestingly, 14-3-3 was reported to bind to Tau phosphorylated at Ser214 in vitro (65). It is possible that α-synuclein also binds to Tau in a manner that is similar to 14-3-3. More studies will be needed to elucidate the biochemical basis of the binding of α-synuclein to phosphorylated Ser214 of Tau.
Supplementary Material
Acknowledgment
We thank Ryen MacDonald for reading the manuscript.
This work was supported in part by operating grants from Canadian Institute for Health Research, The Alzheimer's Society of Canada, and National Scientific and Engineering Research Council of Canada (NSERC).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- PD
- Parkinson disease
- AD
- Alzheimer disease
- FTDP-17
- frontotemporal dementia and Parkinsonism linked to chromosome 17
- LB
- Lewy body
- MAPT
- microtubule-associated protein Tau gene
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NFT
- neurofibrillary tangle
- PHF
- paired helical filament.
REFERENCES
- 1. Lee V. M., Giasson B. I., Trojanowski J. Q. (2004) Trends Neurosci. 27, 129–134 [DOI] [PubMed] [Google Scholar]
- 2. Kim C., Lee S. J. (2008) J. Neurochem. 107, 303–316 [DOI] [PubMed] [Google Scholar]
- 3. Giasson B. I., Uryu K., Trojanowski J. Q., Lee V. M. (1999) J. Biol. Chem. 274, 7619–7622 [DOI] [PubMed] [Google Scholar]
- 4. Waxman E. A., Giasson B. I. (2009) Biochim. Biophys. Acta 1792, 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wood S. J., Wypych J., Steavenson S., Louis J. C., Citron M., Biere A. L. (1999) J. Biol. Chem. 274, 19509–19512 [DOI] [PubMed] [Google Scholar]
- 6. Feany M. B., Bender W. W. (2000) Nature 404, 394–398 [DOI] [PubMed] [Google Scholar]
- 7. Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. (2000) Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 8. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 9. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 10. Giasson B. I., Duda J. E., Quinn S. M., Zhang B., Trojanowski J. Q., Lee V. M. (2002) Neuron 34, 521–533 [DOI] [PubMed] [Google Scholar]
- 11. Lee M. K., Stirling W., Xu Y., Xu X., Qui D., Mandir A. S., Dawson T. M., Copeland N. G., Jenkins N. A., Price D. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8968–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins N. A., Price D. L., Lee M. K. (2006) J. Neurosci. 26, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smeyne R. J., Jackson-Lewis V. (2005) Brain Res. Mol. Brain Res. 134, 57–66 [DOI] [PubMed] [Google Scholar]
- 14. Kim R. H., Smith P. D., Aleyasin H., Hayley S., Mount M. P., Pownall S., Wakeham A., You-Ten A. J., Kalia S. K., Horne P., Westaway D., Lozano A. M., Anisman H., Park D. S., Mak T. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cappelletti G., Surrey T., Maci R. (2005) FEBS Lett. 579, 4781–4786 [DOI] [PubMed] [Google Scholar]
- 16. Abou-Sleiman P. M., Muqit M. M., Wood N. W. (2006) Nat. Rev. Neurosci. 7, 207–219 [DOI] [PubMed] [Google Scholar]
- 17. Dauer W., Kholodilov N., Vila M., Trillat A. C., Goodchild R., Larsen K. E., Staal R., Tieu K., Schmitz Y., Yuan C. A., Rocha M., Jackson-Lewis V., Hersch S., Sulzer D., Przedborski S., Burke R., Hen R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14524–14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drolet R. E., Behrouz B., Lookingland K. J., Goudreau J. L. (2004) Neurotoxicology 25, 761–769 [DOI] [PubMed] [Google Scholar]
- 19. Duka T., Rusnak M., Drolet R. E., Duka V., Wersinger C., Goudreau J. L., Sidhu A. (2006) FASEB J. 20, 2302–2312 [DOI] [PubMed] [Google Scholar]
- 20. Brunden K. R., Trojanowski J. Q., Lee V. M. (2009) Nat. Rev. Drug Discov. 8, 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Titani K., Ihara Y. (1995) J. Biol. Chem. 270, 823–829 [DOI] [PubMed] [Google Scholar]
- 22. Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. (1993) Neuron 11, 153–163 [DOI] [PubMed] [Google Scholar]
- 23. Nishimura I., Yang Y., Lu B. (2004) Cell 116, 671–682 [DOI] [PubMed] [Google Scholar]
- 24. Whiteman I. T., Gervasio O. L., Cullen K. M., Guillemin G. J., Jeong E. V., Witting P. K., Antao S. T., Minamide L. S., Bamburg J. R., Goldsbury C. (2009) J. Neurosci. 29, 12994–13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanger D. P., Anderton B. H., Noble W. (2009) Trends Mol. Med. 15, 112–119 [DOI] [PubMed] [Google Scholar]
- 26. Lee V. M., Goedert M., Trojanowski J. Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 27. Baker M., Litvan I., Houlden H., Adamson J., Dickson D., Perez-Tur J., Hardy J., Lynch T., Bigio E., Hutton M. (1999) Hum. Mol. Genet. 8, 711–715 [DOI] [PubMed] [Google Scholar]
- 28. Conrad C., Andreadis A., Trojanowski J. Q., Dickson D. W., Kang D., Chen X., Wiederholt W., Hansen L., Masliah E., Thal L. J., Katzman R., Xia Y., Saitoh T. (1997) Ann. Neurol. 41, 277–281 [DOI] [PubMed] [Google Scholar]
- 29. Myers A. J., Kaleem M., Marlowe L., Pittman A. M., Lees A. J., Fung H. C., Duckworth J., Leung D., Gibson A., Morris C. M., de Silva R., Hardy J. (2005) Hum. Mol. Genet. 14, 2399–2404 [DOI] [PubMed] [Google Scholar]
- 30. Pittman A. M., Fung H. C., de Silva R. (2006) Hum. Mol. Genet. 15, Spec. 2, R188–95 [DOI] [PubMed] [Google Scholar]
- 31. Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., Toda T. (2009) Nat. Genet. 41, 1303–1307 [DOI] [PubMed] [Google Scholar]
- 32. Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M. A., Illig T., Gieger C., Houlden H., Steffens M., Okun M. S., Racette B. A., Cookson M. R., Foote K. D., Fernandez H. H., Traynor B. J., Schreiber S., Arepalli S., Zonozi R., Gwinn K., van der Brug M., Lopez G., Chanock S. J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N. W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J. A., Singleton A. B., Gasser T. (2009) Nat. Genet. 41, 1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goris A., Williams-Gray C. H., Clark G. R., Foltynie T., Lewis S. J., Brown J., Ban M., Spillantini M. G., Compston A., Burn D. J., Chinnery P. F., Barker R. A., Sawcer S. J. (2007) Ann. Neurol. 62, 145–153 [DOI] [PubMed] [Google Scholar]
- 34. Geddes J. W. (2005) Exp. Neurol. 192, 244–250 [DOI] [PubMed] [Google Scholar]
- 35. Boller F., Mizutani T., Roessmann U., Gambetti P. (1980) Ann. Neurol. 7, 329–335 [DOI] [PubMed] [Google Scholar]
- 36. Arima K., Hirai S., Sunohara N., Aoto K., Izumiyama Y., Uéda K., Ikeda K., Kawai M. (1999) Brain Res. 843, 53–61 [DOI] [PubMed] [Google Scholar]
- 37. Ishizawa T., Mattila P., Davies P., Wang D., Dickson D. W. (2003) J. Neuropathol. Exp. Neurol. 62, 389–397 [DOI] [PubMed] [Google Scholar]
- 38. Duda J. E., Giasson B. I., Mabon M. E., Miller D. C., Golbe L. I., Lee V. M., Trojanowski J. Q. (2002) Acta Neuropathol. 104, 7–11 [DOI] [PubMed] [Google Scholar]
- 39. Kotzbauer P. T., Giasson B. I., Kravitz A. V., Golbe L. I., Mark M. H., Trojanowski J. Q., Lee V. M. (2004) Exp. Neurol. 187, 279–288 [DOI] [PubMed] [Google Scholar]
- 40. Giasson B. I., Mabon M. E., Duda J. E., Montine T. J., Robertson D., Hurtig H. I., Lee V. M., Trojanowski J. Q. (2003) Acta Neuropathol. 106, 243–250 [DOI] [PubMed] [Google Scholar]
- 41. Jensen P. H., Hager H., Nielsen M. S., Hojrup P., Gliemann J., Jakes R. (1999) J. Biol. Chem. 274, 25481–25489 [DOI] [PubMed] [Google Scholar]
- 42. Giasson B. I., Forman M. S., Higuchi M., Golbe L. I., Graves C. L., Kotzbauer P. T., Trojanowski J. Q., Lee V. M. (2003) Science 300, 636–640 [DOI] [PubMed] [Google Scholar]
- 43. Frasier M., Walzer M., McCarthy L., Magnuson D., Lee J. M., Haas C., Kahle P., Wolozin B. (2005) Exp. Neurol. 192, 274–287 [DOI] [PubMed] [Google Scholar]
- 44. Song W., Patel A., Qureshi H. Y., Han D., Schipper H. M., Paudel H. K. (2009) J. Neurochem. 110, 719–733 [DOI] [PubMed] [Google Scholar]
- 45. Han D., Qureshi H. Y., Lu Y., Paudel H. K. (2009) J. Biol. Chem. 284, 13422–13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murray I. V., Giasson B. I., Quinn S. M., Koppaka V., Axelsen P. H., Ischiropoulos H., Trojanowski J. Q., Lee V. M. (2003) Biochemistry 42, 8530–8540 [DOI] [PubMed] [Google Scholar]
- 47. Paudel H. K. (1997) J. Biol. Chem. 272, 1777–1785 [PubMed] [Google Scholar]
- 48. Botelho L. H., Rothermel J. D., Coombs R. V., Jastorff B. (1988) Methods Enzymol. 159, 159–172 [DOI] [PubMed] [Google Scholar]
- 49. Dostmann W. R., Taylor S. S., Genieser H. G., Jastorff B., Døskeland S. O., Ogreid D. (1990) J. Biol. Chem. 265, 10484–10491 [PubMed] [Google Scholar]
- 50. Sun W., Qureshi H. Y., Cafferty P. W., Sobue K., Agarwal-Mawal A., Neufield K. D., Paudel H. K. (2002) J. Biol. Chem. 277, 11933–11940 [DOI] [PubMed] [Google Scholar]
- 51. Seubert P., Mawal-Dewan M., Barbour R., Jakes R., Goedert M., Johnson G. V., Litersky J. M., Schenk D., Lieberburg I., Trojanowski J. Q. (1995) J. Biol. Chem. 270, 18917–18922 [DOI] [PubMed] [Google Scholar]
- 52. Vila M., Vukosavic S., Jackson-Lewis V., Neystat M., Jakowec M., Przedborski S. (2000) J. Neurochem. 74, 721–729 [DOI] [PubMed] [Google Scholar]
- 53. Kühn K., Wellen J., Link N., Maskri L., Lübbert H., Stichel C. C. (2003) Eur. J. Neurosci. 17, 1–12 [DOI] [PubMed] [Google Scholar]
- 54. Zhou R. M., Huang Y. X., Li X. L., Chen C., Shi Q., Wang G. R., Tian C., Wang Z. Y., Jian Y. Y., Gao C., Dong X. P. (2010) Mol. Biol. Rep. 37, 3183–3192 [DOI] [PubMed] [Google Scholar]
- 55. Alim M. A., Hossain M. S., Arima K., Takeda K., Izumiyama Y., Nakamura M., Kaji H., Shinoda T., Hisanaga S., Ueda K. (2002) J. Biol. Chem. 277, 2112–2117 [DOI] [PubMed] [Google Scholar]
- 56. Ren Y., Liu W., Jiang H., Jiang Q., Feng J. (2005) J. Biol. Chem. 280, 34105–34112 [DOI] [PubMed] [Google Scholar]
- 57. Alonso A. C., Zaidi T., Grundke-Iqbal I., Iqbal K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang J. Z., Gong C. X., Zaidi T., Grundke-Iqbal I., Iqbal K. (1995) J. Biol. Chem. 270, 4854–4860 [DOI] [PubMed] [Google Scholar]
- 59. Scott C. W., Spreen R. C., Herman J. L., Chow F. P., Davison M. D., Young J., Caputo C. B. (1993) J. Biol. Chem. 268, 1166–1173 [PubMed] [Google Scholar]
- 60. Duka T., Duka V., Joyce J. N., Sidhu A. (2009) FASEB J. 23, 2820–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reynolds C. H., Betts J. C., Blackstock W. P., Nebreda A. R., Anderton B. H. (2000) J. Neurochem. 74, 1587–1595 [DOI] [PubMed] [Google Scholar]
- 62. Choi W. S., Kruse S. E., Palmiter R. D., Xia Z. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15136–15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu J., Kao S. Y., Lee F. J., Song W., Jin L. W., Yankner B. A. (2002) Nat. Med. 8, 600–606 [DOI] [PubMed] [Google Scholar]
- 64. Ostrerova N., Petrucelli L., Farrer M., Mehta N., Choi P., Hardy J., Wolozin B. (1999) J. Neurosci. 19, 5782–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sadik G., Tanaka T., Kato K., Yamamori H., Nessa B. N., Morihara T., Takeda M. (2009) J. Neurochem. 108, 33–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.