Abstract
GlcNAc-1-phosphotransferase plays a key role in the generation of mannose 6-phosphate, a recognition marker essential for efficient transport of lysosomal hydrolases to lysosomes. The enzyme complex is composed of six subunits (α2β2γ2). The α- and β-subunits are catalytically active, whereas the function of the γ-subunit is still unclear. We have investigated structural properties, localization, and intracellular transport of the human and mouse γ-subunits and the molecular requirements for the assembly of the phosphotransferase complex. The results showed that endogenous and overexpressed γ-subunits were localized in the cis-Golgi apparatus. Secreted forms of γ-subunits were detectable in media of cultured cells as well as in human serum. The γ-subunit contains two in vivo used N-glycosylation sites at positions 88 and 115, equipped with high mannose-type oligosaccharides. 35S pulse-chase experiments and size exclusion chromatography revealed that the majority of non-glycosylated γ-subunit mutants were integrated in high molecular mass complexes, failed to exit the endoplasmic reticulum (ER), and were rapidly degraded. The substitution of cysteine 245 involved in dimerization of γ-subunits impaired neither ER exit nor trafficking through the secretory pathway. Monomeric γ-subunits failed, however, to associate with other GlcNAc-1-phosphotransferase subunits. The data provide evidence that assembly of the GlcNAc-1-phosphotransferase complex takes place in the ER and requires dimerization of the γ-subunits.
Keywords: Endoplasmic Reticulum (ER), Glycoprotein Structure, Golgi Apparatus, Lysosomal Glycoproteins, Trafficking, GlcNAc-1-phosphotransferase, Mannose 6-Phosphate, Subunit Assembly
Introduction
Newly synthesized lysosomal enzymes are modified with Man-6-P residues that function as recognition markers for specific Man-6-P receptors required for lysosomal targeting. The receptor-enzyme complexes are transported to the endosomal compartment, where they dissociate because of the low pH. The lysosomal proteins are then delivered to lysosomes while the Man-6-P receptors return to the Golgi apparatus to mediate further rounds of transport (1). The Man-6-P marker is generated in the cis-Golgi apparatus by the sequential action of two enzymes. In the first step, UDP-GlcNAc lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (termed GlcNAc-1-phosphotransferase; EC 2.7.8.17) transfers GlcNAc 1-phosphate to C6 of mannose residues of lysosomal enzymes. In the second step, N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase (EC 3.1.4.45) removes N-acetylglucosamine residues, exposing the Man-6-P residues (2, 3). The bovine GlcNAc-1-phosphotransferase is composed of three subunits forming a 540-kDa heterohexameric complex (α2β2γ2) that is encoded by two genes, GNPTAB and GNPTG (4–6). Mutations in the GNPTAB and GNPTG genes have been found in patients with mucolipidosis (ML)2 types II and III, biochemically characterized by missorting of lysosomal enzymes lacking Man-6-P residues, intracellular deficiencies of acid hydrolases, and lysosomal storage of non-degraded material (reviewed in Refs. 7 and 8).
GNPTAB codes for the α/β-subunit precursor type III membrane protein of 1265 amino acids. Both the N and C termini of the precursor are located in the cytosol. The highly N-glycosylated α/β-subunit precursor is proteolytically cleaved into the individual subunits and contains the catalytic activity and binding sites for lysosomal enzymes (9–11). The γ-subunit of GlcNAc-1-phosphotransferase represents a soluble glycoprotein of 305 amino acids that forms disulfide-linked homodimers (5). It is retained in the lumen of the cis-Golgi apparatus, most likely by interactions with the integral α- and β-subunits of the GlcNAc-1-phosphotransferase complex (12, 13). The role of γ-subunits in the formation of Man-6-P residues is not yet clear. From recent studies, it has been concluded that the γ-subunit is important either to facilitate the proper folding of the subunits of GlcNAc-1-phosphotransferase and to maintain them in a conformation competent for substrate recognition and binding or to regulate the activity and expression of the α/β-subunits (9, 14, 15). Furthermore, it was shown that proteolytic fragmentation of the γ-subunit in human macrophages is associated with reduced activity of the GlcNAc-1-phosphotransferase complex (13).
In this study, we have analyzed other post-translational modifications in the γ-subunit of GlcNAc-1-phospho-transferase in detail and examined their role in the subcellular localization and assembly of GlcNAc-1-phosphotransferase. The results showed that the γ-subunit contained two in vivo used N-glycosylation sites at positions 88 and 115. Non-glycosylated γ-subunit mutants were found in high molecular mass complexes and failed to be transported from the endoplasmic reticulum (ER) to the Golgi apparatus, whereas glycosylated γ-subunits were localized mainly in the cis-Golgi apparatus and were secreted into the extracellular space. The substitution of cysteine 245 required for dimerization of γ-subunits impaired the GlcNAc-1-phosphotransferase complex assembly.
EXPERIMENTAL PROCEDURES
Reagents
The following reagents were obtained commercially as indicated: [35S]methionine, concanavalin A-Sepharose and rainbow-colored protein molecular mass marker from GE Healthcare; medium for cultivating Escherichia coli, octyl-β-d-glucopyranoside, dithiothreitol, urea, and DAPI from Roth; protein A-agarose, saponin, protamine sulfate, Geneticin, and protease inhibitor mixture from Sigma; isoelectric focusing (IEF) sample buffer, IEF marker 3–10 liquid mixture, and BSA from Serva Electrophoresis; enhanced chemiluminescence reagents from Pierce; PNGase F, endoglycosidase H, sialidase from Clostridium perfringens, Nonidet P-40, and Benzonase from Roche Diagnostics; Phusion® polymerase from Peqlab Biotechnology; DMEM, penicillin/streptomycin, and LipofectamineTM 2000 from Invitrogen; FCS and methionine-free DMEM from PAA; QuikChange® site-directed mutagenesis kit from Stratagene; restriction enzymes from Fermentas; and nickel-nitrilotriacetic acid-agarose from Qiagen. Oligonucleotides used for cloning and sequencing were synthesized by MWG Biotech.
Generation of γ-Subunit Constructs
The human and mouse γ-subunit cDNAs (GenBankTM accession number NM_032520.3 and NM_172529.3, respectively) were isolated from total cDNA by PCR using Phusion® polymerase. The γ-subunit cDNA was subcloned with and without a C-terminal RGS-His6 tag into the pcDNA3.1/Hygro(+) vector (Invitrogen) or into the pEGFP-N1 vector (Promega) using restriction enzymes EcoRI and XhoI. For expression in bacteria, the mouse γ-subunit cDNA (γ-RGS-His6) was additionally cloned into the pET28a(+) vector (Novagen). For amino acid substitution in the human γ-subunit, the QuikChange® site-directed mutagenesis kit and designed mutagenic primers were used. All expression vectors were sequenced (Seqlab, Göttingen, Germany). Primers used for the generation of γ-subunit constructs are listed in supplemental Table 1.
Antibodies
Polyclonal antibodies against the human γ-subunit of GlcNAc-1-phosphotransferase were described previously (12). For generation of polyclonal antibodies against the mouse γ-subunit, the construct mouse γ-RGS-His6-pET28a(+) was transformed into E. coli. Bacteria were lysed under denaturing conditions (8 m urea, 5 mm dithiothreitol, 50 mm Tris-HCl, pH 8.0). After purification using nickel-nitrilotriacetic acid-agarose, the isolated protein was dialyzed against PBS and used for the immunization of rabbits. Monoclonal antibodies against protein-disulfide isomerase, GM130, and GFP were obtained from BIOMOL, BD Biosciences, and Roche Diagnostics, respectively. The monoclonal antibodies against human LAMP-1 were obtained from the NICHD Developmental Studies Hybridoma Bank (University of Iowa). Secondary antibodies conjugated to horseradish peroxidase and fluorochrome-conjugated antibodies were purchased from Dianova.
Cell Culture and Transfection
Baby hamster kidney (BHK) and COS-7 cells were cultured in DMEM supplemented with 10% FCS and antibiotics. To prepare mouse embryonic fibroblasts, mouse embryos were isolated at day 12.5 after terminated mating (16). Mice were housed in the animal facility of the University Medical Center Hamburg-Eppendorf. Animal care and experiments were carried out in accordance with institutional guidelines as approved by local authorities. Isolated cells were cultured in DMEM supplemented with 20% FCS and antibiotics. Cells grown on 6-cm plates were transfected with cDNAs of γ-subunit constructs using Lipofectamine 2000TM according to the manufacturer's instructions. Sixteen hours after transfection, the medium was replaced with DMEM containing 0.05% BSA and conditioned for 24 h.
Immunofluorescence Microscopy
For double immunofluorescence microscopy, BHK cells or mouse embryonic fibroblasts were grown on glass coverslips for 16 h. The cells were fixed with 3% paraformaldehyde in PBS and permeabilized with 0.5% saponin in PBS. GFP fusion proteins were detected by direct fluorescence. Cells were incubated for 16 h with primary antibodies and for 1 h with secondary antibodies conjugated to Alexa Fluor® 546. After two washes, the cells were incubated for 5 min with DAPI solution. After three washes, the cells were embedded in Mowiol. Fluorescence was detected and images were obtained using a Leica DMIRE2 digital scanning confocal microscope and Adobe Photoshop software, respectively.
Metabolic Labeling and Immunoprecipitation
Non-transfected and COS-7 cells overexpressing γ-subunit constructs were metabolically labeled with [35S]methionine (120 μCi/ml) in methionine-free DMEM for 1 h. After removing the labeling medium, cells were chased for 6 h in DMEM containing 1% BSA and 0.25 mg/ml methionine. Cell extracts and media were prepared in lysis buffer (0.4% Triton X-100, 0.2% sodium deoxycholate, 0.2% SDS, and 1% BSA in PBS). After successive removal of DNA with 50 units of Benzonase and 0.03% protamine sulfate, supernatants were preabsorbed with rabbit serum and protein A-agarose for 60 min at 4 °C. After centrifugation, rabbit anti-human γ-subunit antibody (1:200) was added to the supernatants and incubated for 12 h at 4 °C on a rotating wheel. The immunocomplexes were precipitated with protein A-agarose, washed, and processed for SDS-PAGE (10% acrylamide) under reducing conditions, followed by fluorography (17).
Other Methods
Preparation of cell extracts, measurement of protein content, and SDS-PAGE followed by Western blot analysis of the human γ-subunit were performed as described recently (14). For IEF, 20 μg of proteins were mixed with prestained IEF sample buffer and run on 5% polyacrylamide Criterion IEF precast gels (pH 3–10) (Bio-Rad) for 60 min at 100 V, for 60 min at 250 V, and for 30 min at 500 V, followed by γ-subunit Western blot analysis. To analyze serum, samples (50 μl) were diluted in 500 μl of 20 mm Tris-HCl and 0.5 m NaCl, pH 7.4 and incubated with concanavalin A-Sepharose (50 μl) overnight at 4 °C. Bound material was solubilized and processed for SDS-PAGE and Western blotting. For enzymatic deglycosylation of proteins, cell extracts were incubated in the presence or absence of PNGase F, endoglycosidase H, or sialidase for 1–3 h at 37 °C according to the manufacturer's instructions. Gel filtration chromatography of extracts of COS-7 cells overexpressing γ-subunit constructs was performed in the presence of 30 mm octyl-β-d-glucopyranoside on a SMART HPLC system as described recently (13).
RESULTS
The γ-Subunit of GlcNAc-1-phosphotransferase Is N-Glycosylated at Asn-88 and Asn-115
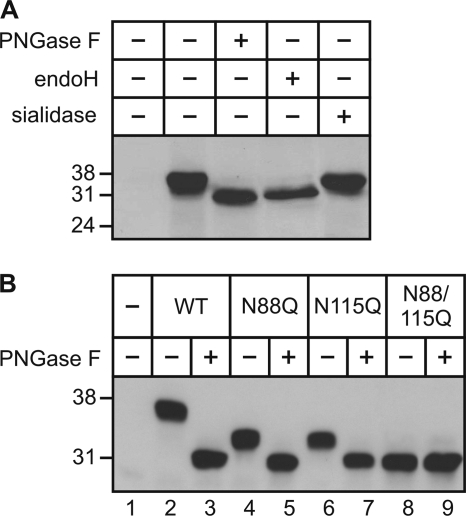
The human γ-subunit of the GlcNAc-1-phosphotransferase represents a soluble glycoprotein that contains two potential N-glycosylation sites at Asn-88 and Asn-115 (5). Western blot analysis showed that treatment of COS-7 cells overexpressing the human γ-subunit with glycosidases hydrolyzing either all N-linked oligosaccharides (PNGase F) or high mannose-type oligosaccharides (endoglycosidase H) resulted in a shift of the electrophoretic mobility of the γ-subunit from 36 to 31 kDa (Fig. 1A). The γ-subunit was not sensitive to sialidase treatment. The data indicate the presence of high mannose-type oligosaccharides on the human γ-subunit of GlcNAc-1-phosphotransferase.
FIGURE 1.
The γ-subunit of GlcNAc-1-phosphotransferase is glycosylated at Asn-88 and Asn-115. A, extracts (75 μg) from γ-subunit-overexpressing COS-7 cells were incubated for 1 h in the presence (+) or absence (−) of PNGase F, endoglycosidase H (endoH), or sialidase. SDS-PAGE (12.5% acrylamide) was performed under reducing conditions, and Western blot analysis was against the human γ-subunit (1:250 dilution). B, extracts (75 μg) from COS-7 cells overexpressing the wild-type (WT; lanes 2 and 3) and mutant (N88Q, N115Q, and N88Q/N115Q (N88/115Q); lanes 4-9) γ-subunits were incubated for 1 h in the presence (+) or absence (−) of PNGase F. The proteins were analyzed by SDS-PAGE (10% acrylamide) under reducing conditions, and human γ-subunit Western blot analysis was performed. Non-transfected cell extracts were used as a control (lane 1). The positions of the molecular mass marker proteins in kilodaltons are indicated. Representative blots of three to four experiments are shown.
Similar results were obtained when cell extracts of BHK cells overexpressing the mouse γ-subunit were incubated in the presence or absence of PNGase F, followed by Western blot analyses using a polyclonal antibody against the murine γ-subunit (supplemental Fig. S1A). The antibody recognizes a 36/34-kDa doublet and a 31-kDa polypeptide upon deglycosylation. Under nonreducing conditions, dimeric forms of the γ-subunit were detected (supplemental Fig. S1B). No immunoreactive polypeptides were detectable in non-transfected cells or cell extracts overexpressing the human γ-subunit, demonstrating the specificity of the antibody. Short-term incubation of cell extracts expressing mouse γ-subunits with PNGase F showed the sequential deglycosylation and indicated the presence of two N-linked oligosaccharides (supplemental Fig. S1C).
Next, we substituted Asn-88 and/or Asn-115 with glutamine residues by site-directed mutagenesis. COS-7 cells were transiently transfected with wild-type and mutant γ-subunit (N88Q, N115Q, and N88Q/N115Q) cDNAs. Cell extracts were incubated in the presence or absence of PNGase F and subjected to Western blot analysis. In cell extracts, the mutant N88Q and N115Q γ-subunits exhibited the same electrophoretic migration pattern with a molecular mass of 34 kDa. Both N88Q and N115Q were sensitive to PNGase F treatment (Fig. 1B, lanes 5 and 7). These data were confirmed by Western blot analysis of cells expressing the double mutant (N88Q/N115Q), which represents a PNGase F-insensitive non-glycosylated polypeptide band of 31 kDa (lanes 8 and 9). Similar results were obtained in BHK cells (data not shown), demonstrating that Asn-88 and Asn-115 are N-glycosylated in a cell type-independent manner. To analyze whether the N-glycosylation affects the dimerization of the γ-subunits, cell extracts were analyzed under nonreducing conditions by SDS-PAGE and Western blotting (supplemental Fig. S2). In extracts of wild-type γ-subunit-overexpressing cells, a 72-kDa dimeric form was detected (lane 2), which was absent in non-transfected cells (lane 1). Treatment with PNGase F resulted in a molecular mass shift of ∼10 kDa to the 62-kDa non-glycosylated dimer (lane 3). The three mutants lacking one (N88Q or N115Q) or both N-glycosylation sites (N88Q/N115Q) exhibited 66-kDa monoglycosylated and 62-kDa non-glycosylated dimeric forms (lanes 4–9). These data showed that the loss of one or both N-glycosylation sites does not affect the dimerization of the γ-subunit.
N-Glycosylation Is Required for the Intracellular Trafficking of the γ-Subunit
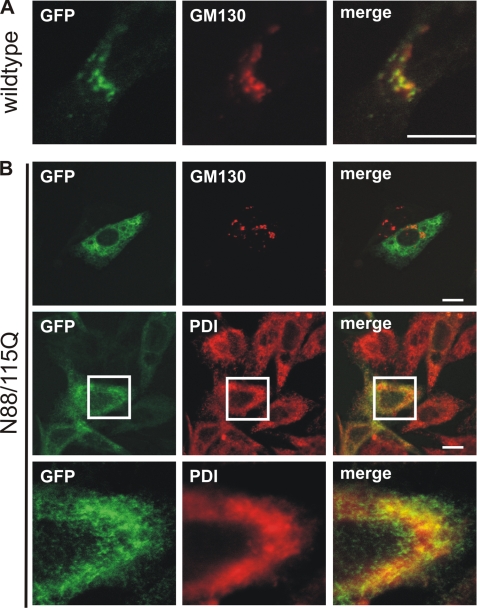
The endogenous human γ-subunit of GlcNAc-1-phosphotransferase is localized in the cis-Golgi apparatus in human fibroblasts and cultured macrophages (12, 13, 18). Double immunofluorescence microscopy of mouse embryonic fibroblasts demonstrated the presence of the endogenous γ-subunit in the cis-Golgi apparatus by complete colocalization with the Golgi apparatus marker GM130 (supplemental Fig. S1D) but not with the ER marker protein-disulfide isomerase or the lysosomal marker LAMP-1 (data not shown). The same results were obtained when the human γ-subunit C-terminally fused to GFP was overexpressed in BHK cells. Immunoreactive polypeptides were observed in the Golgi apparatus (Fig. 2A) but not in the ER (data not shown). The endoglycosidase H sensitivity and the colocalization with GM130 confirmed that the γ-subunit of GlcNAc-1-phosphotransferase is localized in the cis-Golgi apparatus cisternae in different cell types.
FIGURE 2.
Intracellular localization of the human γ-subunit. BHK cells were transfected with cDNA encoding the human wild-type γ-subunit C-terminally fused to GFP (A) or the non-glycosylated γ-subunit (N88Q/N115Q (N88/115Q)) C-terminally fused to GFP (B). Cells were costained for either the cis-Golgi apparatus marker protein GM130 (red; 1:200 dilution) or the ER marker protein-disulfide isomerase (red; 1:400 dilution). Colocalization in merged images appears yellow. Scale bars = 10 μm. Magnified views of the indicated white squares are shown at the bottom.
To examine whether N-glycosylation is required for the intracellular transport of the γ-subunit of GlcNAc-1-phosphotransferase, we analyzed BHK cells overexpressing the mutant N88Q/N115Q C-terminally fused to GFP by double immunofluorescence microscopy. Non-glycosylated γ-subunits did almost not colocalize with the Golgi apparatus marker GM130, whereas the majority costained with the ER marker protein-disulfide isomerase (Fig. 2B), indicating that N-glycosylation of the γ-subunit is essential for ER exit.
Interestingly, the γ-subunit was also detectable in conditioned medium of overexpressing COS-7 cells as a broad polypeptide band with a higher molecular mass than intracellular γ-subunit forms (Fig. 3A, lanes 2 and 4). The increase in molecular mass was most likely due to complex oligosaccharide formation during passage of distal stacks of the Golgi apparatus. The treatment of media with PNGase F resulted in a molecular mass shift to the 31-kDa non-glycosylated γ-subunit form (lane 5). Under nonreducing conditions, the secreted γ-subunit was detectable as a dimer (lane 6). These data indicate that parts of the dimeric γ-subunit not only were retained in the cis-Golgi apparatus but were secreted into the extracellular space. Similar data were obtained by analyzing conditioned medium of COS-7 cells expressing the mouse γ-subunit (supplemental Fig. S1E).
FIGURE 3.
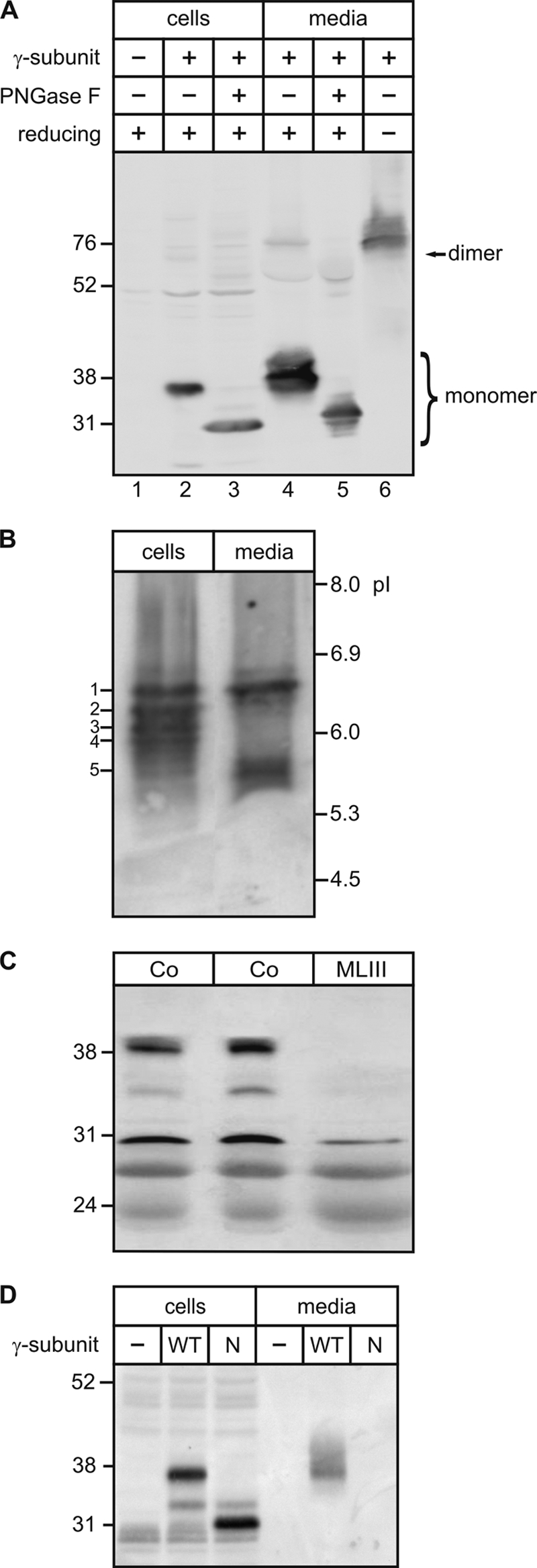
N-Glycosylation is required for the intracellular trafficking of the γ-subunit. A, cell extracts (50 μg) and media conditioned for 24 h (20%) from COS-7 cells overexpressing the human γ-subunits (lanes 2 and 3) were incubated for 1 h in the presence (+) or absence (−) of PNGase F. Non-transfected COS-7 cells were used as a control (lane 1). The proteins were analyzed by SDS-PAGE under reducing (+; lanes 1-5) and nonreducing (−; lane 6) conditions. The positions of monomeric and dimeric forms are indicated. B, for separation of γ-subunit isoforms, cell extracts and conditioned media from COS-7 cells overexpressing the human γ-subunits were mixed with IEF sample buffer and analyzed by IEF. The pH gradient and the γ-subunit forms (1–5) are indicated. C, sera (50 μl) of two healthy individuals (Co) and an MLIII patient lacking the γ-subunit protein (15) were incubated with concanavalin A-Sepharose. Bound glycoproteins were analyzed by SDS-PAGE under reducing conditions (12.5% acrylamide), followed by γ-subunit Western blotting. D, cell extracts (75 μg) and media conditioned for 24 h (15%) from COS-7 cells overexpressing the human wild-type (WT) and non-glycosylated mutant N88Q/N115Q (N) γ-subunits were analyzed by SDS-PAGE under reducing conditions and by γ-subunit Western blotting.
To investigate the N-glycosylation of cellular and secreted γ-subunits in more detail, we used IEF. The cellular forms of the γ-subunit showed a complex pattern of at least four immunoreactive bands between pI 6.6 and 5.9 (Fig. 3B). In the medium, both a pI 6.6 form of the human γ-subunit and a pI 5.6 form of higher negative charge were visible (Fig. 3B). To exclude that secretion of the γ-subunit of GlcNAc-1-phosphotransferase was caused by overexpression in COS-7 cells, we analyzed whether secreted γ-subunit polypeptides were detectable at the endogenous level. Therefore, serum samples of two healthy individuals and an MLIII patient lacking the γ-subunit due to an intronic mutation in the coding GNPTG gene (15) were analyzed by Western blotting. In control serum, two γ-subunit-specific immunoreactive bands of 39 and 35 kDa were detectable, which were absent in the serum of the MLIII patient (Fig. 3C), confirming that γ-subunits were partially secreted and present in the serum. The mutant non-glycosylated human γ-subunit (N88Q/N115Q) could not be observed in the medium of overexpressing COS-7 cells (Fig. 3D), confirming the immunofluorescence microscopic data that N-glycosylation was required for exit from the ER and trafficking of the γ-subunit along the secretory route.
Cysteine 245 Is Responsible for Dimerization of the γ-Subunits
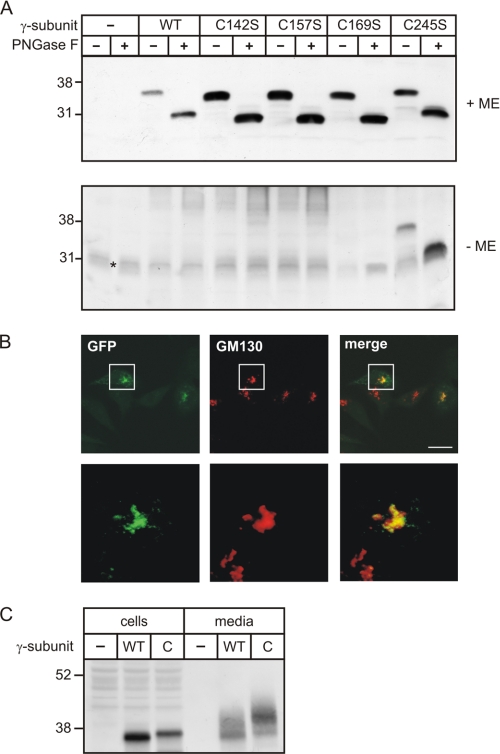
There are seven cysteine residues in the amino acid sequence of the γ-subunit, Cys-71, Cys-84, Cys-129, Cys-142, Cys-157, Cys-169, and Cys-245, which might form intra- and intermolecular disulfide linkages (prediction programs DISULFIND and DIANNA (19, 20)). We generated a series of γ-subunit mutants substituting single, double, or triple cysteine residues with serine by site-directed mutagenesis. COS-7 or BHK cells were transiently transfected with wild-type or mutant γ-subunit cDNA. Subsequently, cell extracts were analyzed by γ-subunit Western blotting. When SDS-PAGE was performed under nonreducing conditions, only mutants comprising C245S substitutions (C245S, C157S/C245S, and C84S/C157S/C245S) could be detected as 36-kDa monomeric forms (Fig. 4A and supplemental Fig. S3). All other cysteine mutants (C84S, C142S, C157S, C169S, and C84S/C157S) were expressed under these conditions as dimers. Upon reducing SDS-PAGE, immunoreactive glycosylated 36-kDa monomeric bands were observed in extracts of wild-type and C245S-expressing cells, whereas the C142S, C157S, and C169S mutants exhibited a slightly increased electrophoretic mobility (Fig. 4A). These differences persisted upon PNGase F-mediated deglycosylation, supporting the observation that the availability of one or more free cysteine residues impairs the compact structure compared with proteins containing intact disulfide bonds (21, 22). The data indicated that Cys-142, Cys-157, and Cys-169 are involved in intramolecular disulfide bonds and that Cys-245 is responsible for the formation of an intermolecular disulfide linkage required for homodimerization of γ-subunits. Additionally, the data showed that all cysteine mutants tested are stable.
FIGURE 4.
Cys-245 is involved in dimerization of the γ-subunits. A, cell extracts from COS-7 cells overexpressing the human wild-type (WT) and mutant (C142S, C157S, C169S, and C245S) γ-subunits were incubated for 1 h in the presence (+) or absence (−) of PNGase F. The proteins were separated by SDS-PAGE under reducing (+ ME) and nonreducing (− ME) conditions and analyzed by γ-subunit Western blotting. The asterisk indicates an unspecific band. B, the mutant C245S γ-subunit C-terminally fused to GFP shows colocalization with the cis-Golgi apparatus marker protein GM130 in BHK cells. Scale bar = 10 μm. Magnified views of the indicated white squares are shown below. C, cell extracts (75 μg) and media conditioned for 24 h (15%) from COS-7 cells overexpressing the human wild-type (WT) and mutant C245S (C) γ-subunits were analyzed by γ-subunit Western blotting.
Double immunofluorescence microscopy showed that the mutant C245S γ-subunit colocalized with the Golgi apparatus marker protein GM130 (Fig. 4B). This indicated that dimerization was not essential for ER exit of the γ-subunit. Western blot analysis of cell extracts and media of COS-7 cells overexpressing the wild-type or mutant C245S γ-subunit demonstrated that comparable amounts of secreted forms were found in the media (Fig. 4C), indicating that the formation of homodimers is not required for the passage of the γ-subunits through the Golgi apparatus.
Dimerization of the γ-Subunits Is Important for the Assembly of the GlcNAc-1-phosphotransferase Complex
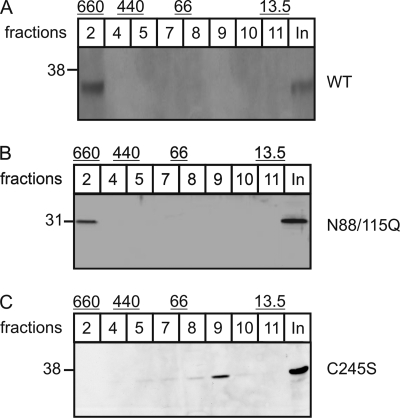
It has been reported that the subunits of bovine GlcNAc-1-phospho-transferase form a 540-kDa hexameric α2β2γ2 complex (4). Size exclusion chromatography represents a suitable method to follow the assembly of the GlcNAc-1-phosphotransferase subunits into higher molecular mass complexes (13). Here, we analyzed the importance of N-linked oligosaccharides and the capability of the γ-subunit for dimerization to assemble in high molecular mass GlcNAc-1-phosphotransferase complexes. First, octyl-β-d-glucopyranoside extracts of BHK cells expressing wild-type and the non-glycosylated N88Q/N115Q γ-subunit were analyzed by size exclusion chromatography. Both wild-type and non-glycosylated mutant forms were eluted in fraction 2, corresponding to elution profiles of protein complexes with estimated molecular masses of 440–660 kDa (Fig. 5, A and B). The data indicated that N-linked oligosaccharides were not required for GlcNAc-1-phosphotransferase complex formation. Second, when extracts of COS-7 cells overexpressing the mutant C245S γ-subunit were analyzed by size exclusion chromatography, immunoreactive γ-subunits were recovered in fractions 8 and 9, coeluting with proteins smaller than 60 kDa (Fig. 5C). No mutant C245S γ-subunit was found in fraction 2 containing high molecular mass protein complexes. The data demonstrate that monomeric γ-subunits fail to assemble with endogenous GlcNAc-1-phosphotransferase subunits.
FIGURE 5.
Dimerization but not N-glycosylation of the γ-subunits is important for the complex formation of GlcNAc-1-phosphotransferase. Size exclusion chromatography was performed using a Sephadex 200 column. Lysates of BHK cells overexpressing wild-type (WT; A), mutant N88Q/N115Q (N88/115Q; B), and mutant C245S (C) γ-subunits were run under identical conditions. Eluted fractions (100 μl) were analyzed by Western blotting for the γ-subunit. The elution of the standard proteins thyroglobulin (660 kDa), ferritin (440 kDa), albumin (66 kDa), and ribonuclease A (13.5 kDa) are indicated at the top of the blots (see also Ref. 13). For comparison, 50 μl of the loaded extract (input (In)) was included.
Increased Secretion of the Mutant C245S Monomeric γ-Subunit
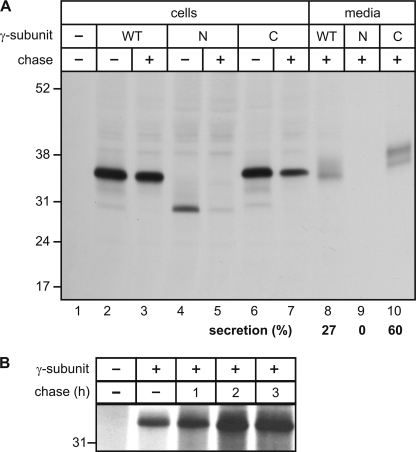
BHK cells overexpressing the wild-type or mutant (N88Q/N115Q and C245S) γ-subunits were metabolically labeled with [35S]methionine for 1 h and either harvested or chased in nonradioactive medium for 6 h. 35S-Labeled wild-type γ-subunits were detectable as 36-kDa polypeptides after a 1-h pulse (Fig. 6, lane 2). Radioactive quantification of 35S-containing bands cut from the gel revealed that after a chase period of 6 h, approximately 73 and 27% of wild-type γ-subunits were found in cell extracts and media, respectively (lanes 3 and 8). The non-glycosylated mutant N88Q/N115Q γ-subunit could be immunoprecipitated as a 31-kDa 35S-labeled protein (lane 4). After a 6-h chase, no polypeptides were detectable in cell extracts and media (lanes 5 and 9), suggesting that the loss of N-glycosylation sites affects the folding and transport to the Golgi apparatus and results in degradation of the γ-subunits. The mutant C245S γ-subunit was synthesized as a 36-kDa protein (lane 6). After the 6-h chase period, 40% of the newly synthesized protein was found in cell extracts and 60% in the media (lanes 7 and 10). The data suggest that the inability of monomeric γ-subunits to form high molecular mass complexes with α/β-subunits of GlcNAc-1-phosphotransferase leads to an increased secretion into the medium.
FIGURE 6.
Biosynthesis and sorting of human wild-type and mutant γ-subunits. Non-transfected and BHK cells overexpressing the wild-type (WT), N88Q/N115Q (N), and C245S (C) γ-subunit constructs were metabolically labeled with [35S]methionine. Human γ-subunits were immunoprecipitated from cells that were either harvested (−) or chased (+) for 6 h (A) or for 1, 2, and 3 h (B) in media as described under “Experimental Procedures.” The amounts of secreted γ-subunits were determined by excision of 35S-labeled gel pieces and are expressed as a percentage of totally synthesized γ-subunits in cells and media (A).
Of note, when COS-7 cells overexpressing the human γ-subunit were metabolically labeled for 1 h and either harvested or chased for 1, 2, and 3 h, followed by immunoprecipitation of the γ-subunit, the intensity of 35S-labeled precipitated polypeptide increased at least until 2 h of chase. The data suggest that the folding of the γ-subunits is a slow process accompanied by an increased number of antibody-accessible epitopes.
DISCUSSION
The human γ-subunit has two potential N-glycosylation sites (Asn-88 and Asn-115) that are both used in vivo and are conserved in human, monkey, mouse, rat, and chicken ortholog proteins (supplemental Fig. S4). Here, we have shown that mouse γ-subunits are also glycosylated at Asn-88 and Asn-115. When expressed in COS-7 cells, the presence of high mannose-type oligosaccharides and the colocalization with the Golgi apparatus marker GM130 suggested that the γ-subunits were localized in the cis-Golgi apparatus. In addition, Western blot analysis and pulse-chase experiments of metabolically labeled cells followed by immunoprecipitation demonstrated that ∼25% of newly synthesized γ-subunits are secreted as dimers. During their passage through distal parts of the Golgi apparatus, the glycans on γ-subunits are processed to complex-type oligosaccharides, which contribute to the more acidic pI of the secreted γ-subunit in comparison with the cellular γ-subunit forms. Secretory forms of γ-subunits are not caused by the overexpression but can also be observed in the serum of healthy individuals, whereas γ-subunits were not detectable in the circulation of an MLIII patient who lacks γ-subunits because of an intronic mutation in the GNPTG gene (15). At present, the physiological significance of the secretory γ-subunit and the mechanism by which soluble γ-subunits are retained in the Golgi apparatus are unknown. It is likely that the formation of the hexameric GlcNAc-1-phosphotransferase complex prevents the secretion of γ-subunits for which N-linked oligosaccharides of γ-subunits might be important. Our data demonstrate that the absence of oligosaccharides, however, reduces the stability and impairs the transport of γ-subunits from the ER to the Golgi apparatus. These data may explain how the deletion of one N-glycosylation site (Asn-115) in the γ-subunit found in two MLIII siblings resulted in reduced activity of GlcNAc-1-phosphotransferase and sorting efficiency of several lysosomal enzymes (12). One N-linked oligosaccharide on the γ-subunit monomers appears to be not sufficient to assist the proper interaction with molecular chaperones or with quality control sorting receptors (23).
Replacement of Cys-245 prevented the formation of disulfide bonds between γ-subunit monomers. Pulse-chase experiments and size exclusion chromatography provided evidence that mutant C245S γ-subunits were stable and were found in the cis-Golgi apparatus but lost their capability to assemble with the α/β-subunit precursor protein. The data were supported by the higher percentage of secreted mutant C245S γ-subunits. How the non-assembled γ-subunit mutant is transported and retained in the Golgi apparatus remains to be examined.
The analysis of brain extracts of γ-subunit targeted mice (11) and of γ-subunit-deficient fibroblasts of MLIII patients (15) revealed that the formation of the Man-6-P recognition marker and the sorting efficiency of lysosomal enzymes were reduced in a hydrolase-depending manner. The affected GlcNAc-1-phosphotransferase activity in these cells might have been partially compensated by an increase in α/β-subunit precursor mRNA expression (14). In addition, the proteolytic cleavage of the γ-subunit in human macrophages also inhibits the formation of high molecular mass complexes with other GlcNAc-1-phosphotransferase subunits (13) and is associated with a strongly reduced capability to form Man-6-P residues on lysosomal enzymes.
In summary, this study provides insight into the structural requirements of γ-subunits to assemble with other subunits of the GlcNAc-1-phosphotransferase complex. The data demonstrate the importance of γ-subunit dimerization for subunit assembly and the requirement of N-linked high mannose-type oligosaccharides for stability and exit from the ER. The transport of the heterohexameric GlcNAc-1-phosphotransferase complex from the ER to the Golgi apparatus is prerequisite for Man-6-P formation on lysosomal proteins and lysosomal functions.
Supplementary Material
Acknowledgments
We thank Johannes Brand, Inke Stange, Christina Gottschild, and Hendrik Müller for expert technical assistance and Erin C. Boyle for English editing of the manuscript.
This study was supported by Deutsche Forschungsgemeinschaft Grants GRK1459, FOR885, and SFB877.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Table S1.
- ML
- mucolipidosis
- ER
- endoplasmic reticulum
- IEF
- isoelectric focusing
- BHK
- baby hamster kidney
- PNGase F
- peptide N-glycosidase F.
REFERENCES
- 1. Braulke T., Bonifacino J. S. (2009) Biochim. Biophys. Acta 1793, 605–614 [DOI] [PubMed] [Google Scholar]
- 2. Reitman M. L., Kornfeld S. (1981) J. Biol. Chem. 256, 4275–4281 [PubMed] [Google Scholar]
- 3. Waheed A., Pohlmann R., Hasilik A., von Figura K. (1981) J. Biol. Chem. 256, 4150–4152 [PubMed] [Google Scholar]
- 4. Bao M., Booth J. L., Elmendorf B. J., Canfield W. M. (1996) J. Biol. Chem. 271, 31437–31445 [DOI] [PubMed] [Google Scholar]
- 5. Raas-Rothschild A., Cormier-Daire V., Bao M., Genin E., Salomon R., Brewer K., Zeigler M., Mandel H., Toth S., Roe B., Munnich A., Canfield W. M. (2000) J. Clin. Invest. 105, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tiede S., Storch S., Lübke T., Henrissat B., Bargal R., Raas-Rothschild A., Braulke T. (2005) Nat. Med. 11, 1109–1112 [DOI] [PubMed] [Google Scholar]
- 7. Kornfeld S., Sly W. S. (2001) in The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K. W., Vogelstein B. eds) pp. 3421–3452, McGraw-Hill Inc., New York [Google Scholar]
- 8. Braulke T., Pohl S., Storch S. (2008) J. Inherit. Metab. Dis. 31, 253–257 [DOI] [PubMed] [Google Scholar]
- 9. Lee W. S., Payne B. J., Gelfman C. M., Vogel P., Kornfeld S. (2007) J. Biol. Chem. 282, 27198–27203 [DOI] [PubMed] [Google Scholar]
- 10. Kudo M., Canfield W. M. (2006) J. Biol. Chem. 281, 11761–11768 [DOI] [PubMed] [Google Scholar]
- 11. Qian Y., Lee I., Lee W. S., Qian M., Kudo M., Canfield W. M., Lobel P., Kornfeld S. (2010) J. Biol. Chem. 285, 3360–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiede S., Cantz M., Raas-Rothschild A., Muschol N., Bürger F., Ullrich K., Braulke T. (2004) Hum. Mutat. 24, 535. [DOI] [PubMed] [Google Scholar]
- 13. Pohl S., Tiede S., Marschner K., Encarnação M., Castrichini M., Kollmann K., Muschol N., Ullrich K., Müller-Loennies S., Braulke T. (2010) J. Biol. Chem. 285, 23936–23944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pohl S., Tiede S., Castrichini M., Cantz M., Gieselmann V., Braulke T. (2009) Biochim. Biophys. Acta 1792, 221–225 [DOI] [PubMed] [Google Scholar]
- 15. Pohl S., Encarnacão M., Castrichini M., Müller-Loennies S., Muschol N., Braulke T. (2010) Am. J. Med. Genet. 152A, 124–132 [DOI] [PubMed] [Google Scholar]
- 16. Hogan B., Constantini F., Lacy E. (1986) in A Laboratory Manual: Manipulating the Mouse Embryo (Bradley A. ed) pp. 89–197, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 17. Hasilik A., Neufeld E. F. (1980) J. Biol. Chem. 255, 4937–4945 [PubMed] [Google Scholar]
- 18. Tiede S., Cantz M., Spranger J., Braulke T. (2006) Hum. Mutat. 27, 830–831 [DOI] [PubMed] [Google Scholar]
- 19. Lippi M., Passerini A., Punta M., Rost B., Frasconi P. (2008) Bioinformatics 24, 2094–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrè F., Clote P. (2005) Nucleic Acids Res. 33, W230–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. (1991) J. Cell Biol. 114, 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang N., Daniels R., Hebert D. N. (2005) Mol. Biol. Cell 16, 3740–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.