Abstract
Mast cell secretory granules (secretory lysosomes) contain large amounts of fully active proteases bound to serglycin proteoglycan. Damage to the granule membrane will thus lead to the release of serglycin and serglycin-bound proteases into the cytosol, which potentially could lead to proteolytic activation of cytosolic pro-apoptotic compounds. We therefore hypothesized that mast cells are susceptible to apoptosis induced by permeabilization of the granule membrane and that this process is serglycin-dependent. Indeed, we show that wild-type mast cells are highly sensitive to apoptosis induced by granule permeabilization, whereas serglycin-deficient cells are largely resistant. The reduced sensitivity of serglycin−/− cells to apoptosis was accompanied by reduced granule damage, reduced release of proteases into the cytosol, and defective caspase-3 activation. Mechanistically, the apoptosis-promoting effect of serglycin involved serglycin-dependent proteases, as indicated by reduced sensitivity to apoptosis and reduced caspase-3 activation in cells lacking individual mast cell-specific proteases. Together, these findings implicate serglycin proteoglycan as a novel player in mast cell apoptosis.
Keywords: Apoptosis, Cysteine Protease, Mast Cell, Protease, Proteoglycan Structure, Proteolytic Enzymes, Chymase, Secretory Granule, Serglycin, Tryptase
Introduction
Serglycin (SG)2 is a proteoglycan expressed by numerous hematopoietic cell types, including mast cells (MCs), cytotoxic T lymphocytes, platelets, neutrophils, and macrophages (1, 2). Like all proteoglycans, SG contains a protein “core” to which highly sulfated glycosaminoglycan chains are attached, with the type of glycosaminoglycan (heparin or chondroitin/heparan sulfate) and extent of glycosaminoglycan sulfation being cell type-specific (1–3). The genetic targeting of SG has revealed a major function for this proteoglycan in regulating the storage of a variety of compounds present in the secretory granules of hematopoietic cells. For example, SG-deficient MCs showed an essentially complete inability to store a number of MC-specific granule proteases of chymase (mouse mast cell protease 4 (mMCP-4) and mMCP-5), tryptase (mMCP-6), and MC carboxypeptidase A-type (MC-CPA) (4, 5), of which mMCP-4–6 are serine proteases and MC-CPA is a metalloprotease (reviewed in Refs. 6, 7). Furthermore, SG has been shown to have a key role in promoting the storage of granzyme B in cytotoxic T lymphocytes (8), N-elastase in neutrophils (9), and platelet factor 4/CXCL4 in platelets (10).
It has recently been demonstrated that animals lacking SG spontaneously develop an enlargement of multiple lymphoid tissues/organs (11) and a markedly delayed contraction of the CD8+ T cell response following virus infection (12). Although multiple explanations for these findings may apply, one possibility would be that the absence of SG affects the ability of certain leukocyte populations to undergo apoptosis.
Apoptosis can be achieved by a multitude of pathways, including activation through death receptors, the perforin/granzyme pathway, and through mitochondrial damage (13, 14), but it is also known that apoptosis can be initiated via a lysosomal pathway triggered by permeabilization of the lysosomal membrane (15–18). Notably, the lysosomal pathway of apoptosis has been shown to contribute to cell death in several immunological contexts, such as CD95-mediated apoptosis of germinal center B cells (19), models of peripheral T cell deletion (20), macrophage cell death induced by NF-κB inhibition (21), CD2 activation of NK cells (22), and activation-induced cell death of CD8+ T cells (23). Apoptosis via the lysosomal pathway has been shown to involve the release into the cytosol of various lysosomal proteases, such as cysteine cathepsins, and the translocation of these into the cytosol leads to downstream proteolytic activation of numerous pro-apoptotic compounds along with degradation of anti-apoptotic components (15–18).
MC secretory granules share many features with lysosomes, such as acidic pH and similar membrane components. Moreover, MC secretory granules contain a number of lysosomal proteases such as cysteine and aspartic acid cathepsins (24–27) as well as other lysosomal hydrolases, including β-hexosaminidase. Hence, the distinction between lysosomes and secretory granules is not clearly defined in many cell types, and secretory granules, e.g. in MCs, are therefore often referred to as “secretory lysosomes” (28). Considering that MC secretory granules, in addition to containing vast amounts of MC-specific proteases of chymase, tryptase, and MC-CPA-type, also contain various lysosomal proteases, MC granules are thus equipped with an impressive arsenal of proteolytic activity. Accordingly, damage to the MC secretory granules will lead to a massive release of active proteases into the cytosol. Potentially, these may proteolytically activate various pro-apoptotic compounds, and we therefore hypothesized that MCs may be prone to apoptosis via agents that induce secretory granule/lysosome permeabilization. Moreover, because many of the proteases within MC granules are dependent on SG for proper storage (4, 5), we also hypothesized that apoptosis initiated by a granule/lysosome pathway may be SG-dependent. Indeed, we show here that MCs are highly sensitive to apoptosis induced by permeabilization of the secretory granules and that this pathway of MC apoptosis is strongly dependent on SG. We have thus identified SG as a novel player in apoptosis.
EXPERIMENTAL PROCEDURES
Reagents
H-Leu-Leu-OMe (LLME), HBr, and the chromogenic peptide substrate Z-Phe-Arg-AMC were from Bachem (Bubendorf, Switzerland). (S)-(+)-Camptothecin, acridine orange, E64d (membrane-permeable), and Z-DEVD-FMK were from Sigma. Pefabloc SC was from Roche Applied Science. Donkey anti-rabbit and anti-goat Ig, both conjugated to horseradish peroxidase, were purchased from GE Healthcare and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Polyclonal antibodies were as follows: anti-procaspase-3/CPP32 was from Invitrogen; anti-active caspase-3 was from Abcam (catalog no. ab2302, Cambridge, UK); anti-Bid was from Abcam; anti-β-actin was from Santa Cruz Biotechnology; and anti-murine cathepsin C was from R&D Systems (Minneapolis, MN).
Mice
Mice (8–18 weeks old) deficient in SG (SG−/−)(5), mMCP-4−/− (29), mMCP-6−/− (30), and MC-CPA−/− (31) as well as wild-type (WT) controls were all on C57BL/6J genetic background. Mice with an inactivating mutation in the active site of MC-CPA (MC-CPAY356L,E378A) were as described previously (32). All animal experiments were approved by the local ethical committee.
Bone Marrow-derived MCs (BMMCs)
Mice were sacrificed by CO2 asphyxiation. Femurs and tibiae were removed, and marrow was flushed from the bones with phosphate-buffered saline (PBS). BMMCs were obtained by culturing bone marrow cells in Dulbecco's modified Eagle's medium (SVA, Uppsala, Sweden), supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 50 μg/ml streptomycin sulfate, 60 μg/ml penicillin G, 2 mm l-glutamine (SVA), and 30% WEHI-3B conditioned medium (which contains IL-3). The cells were kept at a concentration of 0.5 × 106 cells/ml, at 37 °C in 5% CO2, and the medium was changed every 3rd day.
LLME Assays
Triplicates of 1 ml of BMMCs (0.5 × 106 cells/ml) were transferred into individual wells of a 24-well flat-bottomed plate and were either left untreated or induced to undergo apoptosis with different concentrations of LLME in complete culture medium, followed by incubation for different time points (as specified in the figure legends). Inhibitory assays were performed using the same methodology. However, before LLME treatment, cells were previously incubated with a broad spectrum cysteine cathepsin inhibitor E64d (10 μm), the caspase-3-type inhibitor Z-Asp(O-Me)-Glu(O-Me)-Val-Asp(O-Me) fluoromethyl ketone (Z-DEVD-FMK) (40 μm), or pepstatin A (1 μm).
Staining for Acidic Compartments
BMMCs (0.5 × 106 cells/ml) were either left untreated or previously induced to undergo apoptosis with LLME (as described above) and incubated with 5 μg/ml of acridine orange for 15 min. Next, the medium was removed by centrifugation (1,200 rpm; 8 min), and cells were washed three times in PBS and resuspended in PBS/BSA (100 μg/ml) to initial concentration. 100 μl of each cell suspension were transferred into individual wells of a 96-well flat-bottomed plate. Plates were then directly read in a FARCyte ELISA reader (Amersham Biosciences) at 485 nm (excitation) and 650 nm (emission).
Morphology of Cells
Cells were collected onto object glasses by cytospin (500 rpm, 5 min) and stained with May-Grünwald/Giemsa (Merck). Briefly, cells were fixed in methanol (3 min) and then stained in May-Grünwald solution (5 min) followed by Giemsa (15 min), with H2O washing steps after staining. Digital images were acquired using a Leica CTRMIC microscope (Leica Microsystems, Wetzlar, Germany). Transmission electron microscopy was performed as described previously (4).
Caspase-3-like Activity
BMMCs (106 cells/ml) were either left untreated or induced to undergo apoptosis with different concentrations of LLME in complete culture medium. At different time points (as specified in the figure legends), cells were harvested by centrifugation and washed once in PBS, and caspase-3-like activity was measured according to the EnzChek® caspase-3 assay kit number 2 procedure (Molecular Probes/Invitrogen). Triplicates were transferred into individual wells of a 96-well flat-bottomed plate, and incubated for 30 min at room temperature. Fluorescence intensity was measured at 496 nm (excitation) and 520 nm (emission) using a FARCyte ELISA reader.
Flow Cytometry
Flow cytometry was performed using an annexin V-FITC apoptosis detection kit (Sigma) according to the protocol provided by the manufacturer. After staining, the cells were analyzed using a FACScan® flow cytometer and the CELLQuestTM 3.3 software (BD Biosciences). Data from 10,000 events/sample were collected.
Western Blot Analysis
Samples of 0.5–2 × 106 BMMCs were solubilized in 1× SDS-PAGE sample buffer containing 5% dithiothreitol. Samples corresponding to an equal number of cells were subjected to SDS-PAGE on 12% gels. Proteins were subsequently blotted onto nitrocellulose membranes, followed by blocking with 5% milk powder in Tris-buffered saline (TBS), 0.1% Tween 20 (1 h, room temperature). Next, the membranes were incubated with polyclonal antibodies (diluted 1:500–1:1000 in TBS, 5% milk powder, 0.1% Tween 20), at 4 °C overnight. After washing the membranes extensively with TBS, 0.1% Tween 20, the membranes were incubated with anti-rabbit or anti-goat Ig, both conjugated to horseradish peroxidase (diluted 1:2000–1:2500 in TBS/0.1% Tween 20). After 1 h of incubation at room temperature, the membranes were washed extensively with TBS, 0.1% Tween 20. The membranes were developed with ECL (GE Healthcare) according to the protocol provided by the manufacturer.
Cytosolic Extract Preparation and Measurement of Z-Phe-Arg-AMC Cleaving Activity
BMMCs (106 cells) were collected by centrifugation (1,200 rpm, 8 min, 4 °C) in 1.5-ml Eppendorf tubes and then resuspended in 300 μl of ice-cold digitonin extraction buffer (10 μg/ml digitonin, 250 mm sucrose, 20 mm HEPES, pH 7.5, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA). After 10 min of incubation on ice, the supernatant was quickly removed by centrifugation (13,000 rpm; 2 min, 4 °C), and 20 μl of each sample were transferred in triplicates into individual wells of a 96-well flat-bottomed plate, followed by addition of 20 μl of H2O and 50 μl of 100 mm phosphate buffer containing 1 mm EDTA and 1 mm dithiothreitol, pH 6.0. Samples were kept for 15 min at 37 °C, and 10 μl of 200 μm Z-Phe-Arg-AMC was then added, followed by incubation for 30 min at 37 °C. Finally, AMC release was measured by determining fluorescence at 390 nm (excitation) and 460 nm (emission) (Hitachi F-4000, Tokyo, Japan).
DNA Degradation
BMMCs (106 cells) previously washed in PBS were incubated with 0.5 ml of lysing buffer (PBS, 0.2% Nonidet P-40, 0.9 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, 0.4 mm EDTA, 80 μm bestatin, 12 μm pepstatin A, 12 μm E64d) for 10 min at 4 °C. Next, the supernatant was recovered after centrifugation (12,000 rpm, 20 min, 4 °C), and RNA was digested using RNase (0.1 mg/ml at 37 °C for 1 h) followed by proteinase K digestion (20 μg/ml at 37 °C for 1 h). DNA was precipitated by adding an equal volume of isopropyl alcohol, incubated overnight at −20 °C, and centrifuged at 12,000 × g for 15 min at 4 °C. The pellets were air-dried, resuspended in 20 μl of Tris acetate EDTA buffer supplemented with 2 μl of sample buffer (0.25% bromphenol blue, 30% glycerol), and electrophoretically separated on 2% agarose gels containing 1 μg/ml ethidium bromide and visualized under ultraviolet transillumination.
Statistical Analyses
Statistical significance was tested using one-way analysis of variance (ANOVA) performed by using Origin 7.5 (OriginLab Corp., Northampton, MA). All data shown are from individual experiments, representative of several (up to five) independent experiments.
RESULTS
MC Apoptosis Induced by a Secretory Granule-mediated Pathway Is SG-dependent
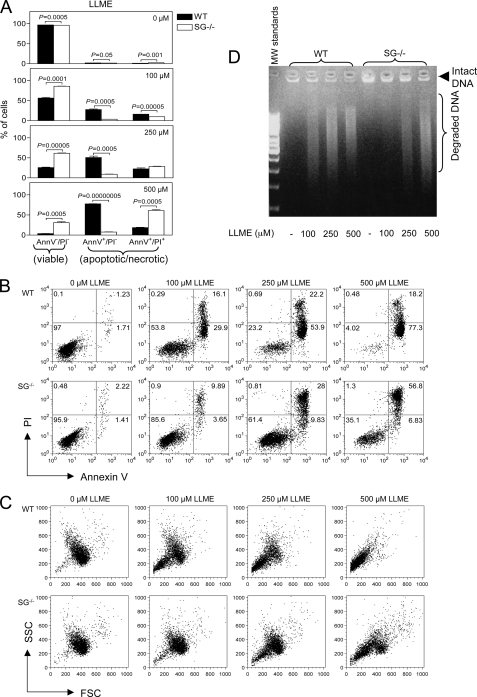
To assess the susceptibility of MCs to apoptosis, we used a lysosomotropic agent, H-Leu-Leu-OMe (LLME). LLME is well known to induce apoptosis by causing lysosomal membrane permeabilization and has previously been shown to trigger apoptosis in multiple cell lineages, in particular of hematopoietic type (33). Because secretory granules and lysosomes share many features (see Introduction), we reasoned that lysosomotropic agents also could induce permeabilization of secretory granules. LLME was added to bone marrow-derived MCs (BMMCs), at different concentrations and for different time periods, followed by assessment of apoptosis/necrosis by annexin V (apoptosis) and PI (necrosis/late stage apoptosis) staining.
Initial experiments showed that a 24-h culture of wild-type (WT) BMMCs with 500 μm LLME caused 97% apoptosis, suggesting that MCs are highly susceptible to LLME-induced apoptosis (data not shown). To establish conditions in which only limited apoptosis was induced, we instead measured apoptosis after a 4-h culture period. As shown in Fig. 1A, apoptosis of WT BMMCs was efficiently induced at LLME concentrations as low as 100 μm, and almost 100% apoptosis was seen at 500 μm. Strikingly, SG−/− BMMCs showed a marked resistance to LLME-induced apoptosis, indicating a prominent role for SG in apoptosis induced by LLME. SG is a main component of secretory granules, rather than being a typical component of conventional lysosomes. Therefore, the most likely explanation for the differential sensitivity of WT versus SG−/− BMMCs to apoptosis is that LLME induces cell death in BMMCs via targeting of the secretory granules, rather than targeting of conventional lysosomes.
FIGURE 1.
SG−/− BMMCs are less susceptible than WT BMMCs to apoptosis induced by a secretory granule-mediated pathway. A, WT (black bars) or SG−/− (white bars) BMMCs (0.5 × 106 cells/ml) were either left untreated or treated with different concentrations of LLME for 4 h and were then stained with annexin V (AnnV)-FITC and PI, followed by flow cytometry analysis. Data are expressed as mean ± S.E. (n = 3), and statistical differences were analyzed by ANOVA. B, dot plots for representative samples showing staining with annexin V-FITC (FL-1) and PI (FL-3). The % of cells is indicated within each quadrant. C, dot plots showing forward scatter (FSC) and side scatter (SSC). Note that LLME causes a higher extent of cell shrinking in WT than in SG−/− BMMCs, as shown by a reduction in forward scatter after addition of 100–500 μm LLME. D, effect of SG deficiency on DNA degradation in response to LLME. DNA was extracted from WT and SG−/− BMMCs 4 h after addition of LLME at the indicated concentrations, followed by analysis by agarose gel electrophoresis.
In contrast to apoptosis induced by LLME, apoptosis induced by camptothecin was unaffected by the absence of SG (data not shown), indicating that apoptosis induced by inhibition of nucleic acid synthesis is not SG-dependent. Notably, among the nonresistant population of the SG−/− BMMCs, a major portion was double annexin V/PI-positive rather than solely annexin V-positive (Fig. 1, A and B), suggesting necrosis/late stage apoptosis. Typically, induction of apoptosis induces a reduction in cellular size, and in agreement with this notion, LLME caused a marked reduction in the size of WT BMMCs (Fig. 1C). In contrast, but in line with the lower susceptibility of SG−/− BMMCs to LLME-induced apoptosis, a large portion of the SG−/− BMMCs showed no signs of cellular shrinking (Fig. 1C). Reduced sensitivity of SG−/− BMMCs to apoptosis was also supported by a considerably less extent of DNA degradation in SG−/− versus WT cells in response to LLME (Fig. 1D).
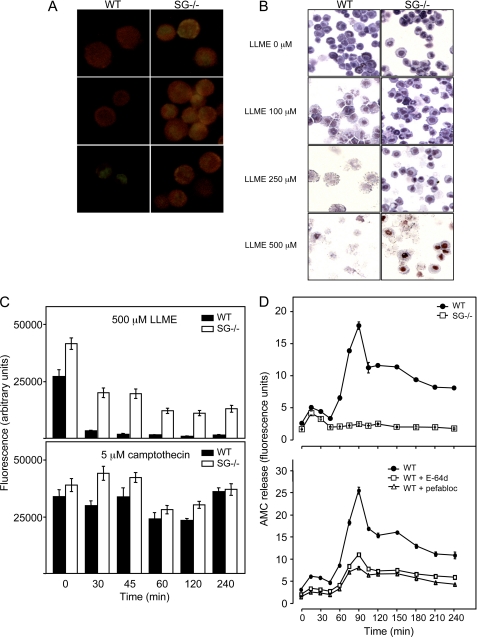
Granule Damage Is Reduced in MCs Lacking SG
To assess granule integrity, we used staining with acridine orange, a dye that yields strong fluorescence when present in acidic compartments such as lysosomes/secretory granule (34). Following lysosomal damage, the pH of the lysosomal/granule compartment is raised, and acridine orange therefore shows reduced fluorescence. As shown in Fig. 2A, untreated BMMCs, both WT and SG−/−, showed granular staining with acridine orange as detected by fluorescence microscopy. Upon treatment with LLME, WT BMMCs showed clear signs of lost cellular integrity accompanied by reduced acridine orange staining (Fig. 2A), an effect seen at LLME concentrations down to 100 μm. Extensive cellular decomposition was also apparent when cells were inspected by light microscopy after staining with May Grünwald/Giemsa (Fig. 2B). In contrast, SG−/− BMMCs showed markedly lower susceptibility to lysosomal damage, with acridine orange-positive cells clearly detectable even at 250 μm LLME (Fig. 2A) and less signs of cellular decomposition as assessed by light microscopy (Fig. 2B). Quantification of the acridine orange fluorescence confirmed that LLME caused an almost complete loss of fluorescence in WT BMMCs, although this effect was markedly reduced in BMMCs lacking SG (Fig. 2C, upper panel). Notably, when the cells were treated with camptothecin, no significant difference in acridine orange staining was seen when comparing WT with SG−/− BMMCs (Fig. 2C, lower panel). As an additional approach to evaluate the contribution of SG to lysosomal/granule damage, we measured the release of granule/lysosomal proteases into the cytosol during LLME-induced apoptosis. As depicted in Fig. 2D (upper panel), addition of LLME to WT cells resulted in extensive release of protease activity into the cytosol, as measured by Z-Phe-Arg-AMC cleaving activity in cytosolic extracts. In contrast, only minimal Z-Phe-Arg-AMC cleaving activity was detected in cytosolic extracts prepared from SG−/− cells treated with LLME (Fig. 2D, upper panel). Z-Phe-Arg-AMC is a substrate for various cysteine cathepsins, and to assess whether the Z-Phe-Arg-AMC cleaving activity was due to cysteine cathepsin activity, samples were treated with a specific cysteine cathepsin inhibitor (E64d), followed by measurement of residual Z-Phe-Arg-AMC cleaving activity. Indeed, the Z-Phe-Arg-AMC cleaving activity was inhibited to a large extent by E64d (Fig. 2D, lower panel). As judged by Western blot analysis, the total levels of cathepsin B and L were similar in WT and SG−/− cells (data not shown), indicating that SG affects the rate of cysteine cathepsin release into the cytosol rather than affecting the total levels of stored cysteine cathepsins. Notably, ∼50% of the total Z-Phe-Arg-AMC cleaving activity was nonsensitive to E64d, suggesting that also other types of proteases are translocated into the cytosol. Because MCs store a number of MC-specific serine proteases in their secretory granules (see Introduction), we assessed whether the residual Z-Phe-Arg-AMC cleaving activity could be inhibited by a specific serine protease inhibitor (Pefabloc SC). In agreement with this notion, ∼50% of the total Z-Phe-Arg-AMC cleaving activity was inhibited by Pefabloc SC. Hence, BMMC apoptosis induced by the secretory granule/lysosomal pathway involves extensive release of both cysteine cathepsins and serine proteases into the cytosol, and SG is essential for this process.
FIGURE 2.
LLME causes more extensive granule damage in WT than in SG−/− BMMCs. A and B, WT or SG−/− BMMCs were treated with LLME for 4 h at the concentrations indicated, followed by staining with acridine orange and analysis by fluorescence microscopy (A) or staining with May Grünwald/Giemsa and analysis by light microscopy (B) (original magnification ×40). C, WT (black bars) or SG−/− (white bars) BMMCs (0.5 × 106 cells/ml) were either left untreated or treated with 500 μm LLME (upper panel) or 5 μm camptothecin (lower panel) for different time points, followed by staining with acridine orange. 70% of the WT and 73% of the SG−/− BMMCs were annexin V+ and/or PI+ after treatment with 5 μm camptothecin for 4 h (data not shown). D, upper panel, release of Z-Phe-Arg-AMC cleaving protease activity into the cytosol after treatment of WT and SG−/− BMMCs (106 cells) with LLME (100 μm). At the time points indicated, cytosolic extracts were prepared and analyzed for Z-Phe-Arg-AMC cleaving activity. D, lower panel, residual Z-Phe-Arg-AMC cleaving activities after incubation (15 min) of cytosolic extracts with specific inhibitors of either cysteine cathepsins (E64d; 10 μm) or serine proteases (Pefabloc SC; 1 mm). Data are expressed as mean ± S.D. of triplicates.
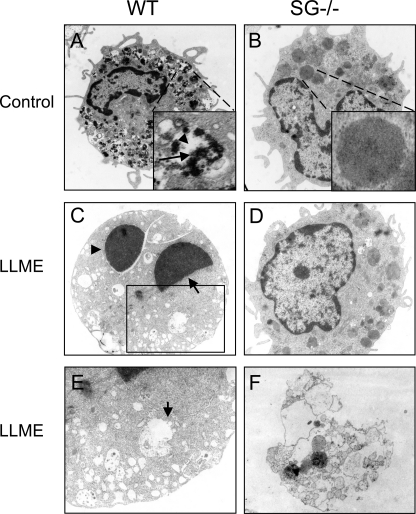
Ultrastructural Evidence for a Role of SG in Promoting Apoptosis
To provide ultrastructural insight into the differential susceptibility of WT versus SG−/− BMMCs to LLME, we employed transmission electron microscopy analysis. WT BMMCs showed typical morphology, containing numerous cytoplasmic granules with characteristic dense core formation (Fig. 3A) (4). In line with previous studies (4, 35), SG−/− BMMCs contained approximately equal numbers of granules as did WT cells, but the granules of SG−/− BMMCs were morphologically distinguished from those of WT cells, with substantially less dense core formation and with granules instead filled with evenly distributed amorphous material of moderate electron density (Fig. 3B). As displayed in Fig. 3C, LLME-treated WT BMMCs displayed clear signs of apoptosis, showing nuclear condensation and generation of apoptotic bodies. Moreover, after addition of LLME, the granules of WT BMMCs showed a dramatically decreased electron density, i.e. appearing empty, suggesting that the granule contents had been translocated into the cytosol (Fig. 3C). In line with this notion, damage of granule membranes was evident (Fig. 3E). In contrast, a majority of the SG−/− BMMCs was intact after addition of LLME (Fig. 3D), in agreement with their lower susceptibility to apoptosis as judged by annexin V staining (see Fig. 1). Only a small fraction of the SG−/− cells showed signs of cell death as judged by transmission electron microscopy analysis. Intriguingly, however, the occasional nonviable SG−/− cells were to a major extent necrotic rather than apoptotic (Fig. 3F).
FIGURE 3.
Transmission electron microscopy analysis showing more extensive apoptosis and granule damage in WT than in SG−/− BMMCs. A and B, morphology of untreated WT (A) and SG−/− (B) BMMCs, showing typical dense core formation (arrow, inset in A) interspersed with electron translucent areas (arrowhead, inset in A) in granules of WT BMMCs, whereas granules in SG−/− cells have an amorphous appearance with evenly distributed, moderately electron dense material, without dense core formation (inset in B). C, signs of apoptosis in a representative WT BMMC after 4 h of treatment with 250 μm LLME; note the extensive nuclear condensation (arrow) and formation of apoptotic bodies (arrowhead). Note also that granules are electron-translucent, indicative of translocation of granule content into the cytosol. D, representative morphology of LLME-treated SG−/− cells, without signs of apoptosis or lysosomal/granule damage. E, larger magnification of boxed region (see C) of WT cell showing decomposed granule membranes (arrow). F, SG−/− cell with signs of cell death; note the absence of signs of apoptosis and the extensive signs of necrosis, i.e. decomposed cell membrane. Original magnifications, ×12,500.
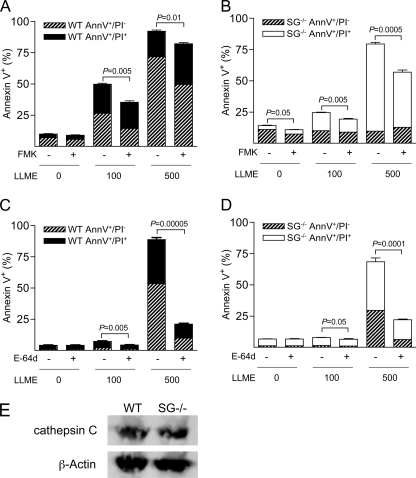
MC Apoptosis Induced by LLME Is Caspase- and Cysteine Cathepsin-dependent
All apoptotic pathways eventually converge at the level of activation of effector caspases (caspase-3, -6, and -7), of which caspase-3 occupies a central position (13, 14). To investigate whether MC apoptosis via secretory granule damage involved caspase activation, we used an inhibitor of caspase-3, -6–8, and -10, Z-DEVD-FMK. Addition of Z-DEVD-FMK to both WT and SG−/− BMMCs resulted in significantly reduced susceptibility to LLME-induced cell death, indicating a role for caspase activation (Fig. 4, A and B). Previous studies have revealed a prominent role for lysosomal cysteine cathepsins in apoptosis via the lysosomal pathway (36–38). In line with this notion, LLME-induced cell death of BMMCs was prevented in the presence of E64d, a membrane-permeable inhibitor of cysteine cathepsins (Fig. 4, C and D). In contrast, inhibition of lysosomal aspartic acid proteases (cathepsin D and E) by pepstatin A did not cause any reduction in sensitivity to apoptosis (data not shown). Thus, secretory granule damage-induced cell death in BMMCs is strongly dependent on cysteine cathepsins.
FIGURE 4.
BMMC apoptosis induced by secretory granule damage is cysteine cathepsin- and caspase-dependent. WT and SG−/− BMMCs (0.5 × 106 cells/ml) were either left untreated or treated for 30 min with 40 μm Z-DEVD-FMK (A and B, FMK) or for 120 min with 20 μm E64d (C and D), followed by incubation with 0, 100, or 500 μm LLME for 4 h, staining with annexin V (AnnV)/PI, and flow cytometry analysis. Data are from a representative experiment, expressed as mean ± S.E. (n = 3). E, Western blot analysis showing equal levels of cathepsin C in WT and SG−/− BMMCs.
Because cathepsin C has been implicated in LLME-induced cell death (39), a possible explanation for the reduced sensitivity of SG−/− BMMCs to LLME could be that cathepsin C storage in BMMCs is dependent on SG. However, the levels of cathepsin C were similar in WT and SG−/− BMMCs (Fig. 4E), suggesting that the effects of SG on LLME-induced apoptosis are explained by effects that are unrelated to cathepsin C.
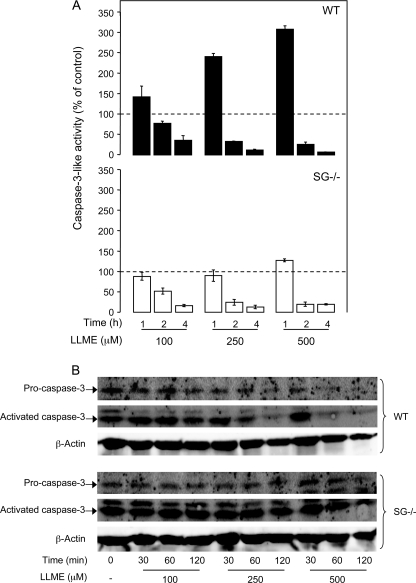
Caspase-3 Activation Requires SG
To analyze the effect of SG on effector caspase activation, a fluorescent substrate detecting caspase-3-like activity (Z-DEVD-R110) was used. As shown in Fig. 5A (upper panel), LLME induced a robust induction of caspase-3-like activity above base-line levels in WT cells (base-line activity indicated by dashed line). In contrast, caspase-3 activation was undetectable in SG−/− cells at LLME concentrations up to 250 μm, and only minimal caspase-3 activation was seen at 500 μm LLME (Fig. 5A, lower panel). Moreover, Western blot analysis showed that the addition of LLME to WT cells caused a marked reduction in the band corresponding to pro-caspase-3, compatible with processing of pro-caspase-3 (Fig. 5B). In addition, there was an increase in the band corresponding to active caspase-3 at early time points after addition pf LLME. In striking contrast, the levels of pro- and active caspase-3 remained fairly constant in SG−/− cells after the addition of LLME at increasing concentrations, in line with defective processing in SG−/− cells (Fig. 5B). These data indicate that optimal effector caspase activation requires SG.
FIGURE 5.
Induction of caspase-3 activity in response to apoptosis via secretory granule damage is dependent on SG. WT and SG−/− BMMCs (106 cells/ml) were either left untreated or treated with LLME at the concentrations and time periods indicated. A, triplicates of each sample were transferred into individual wells of a 96-well flat-bottomed plate and incubated with 10 μm Z-DEVD-R110 for 30 min. Caspase-3-like activity was measured by R110 release as described under “Experimental Procedures.” Base-line caspase-3-like activities, i.e. in the absence of added LLME, are indicated by dashed lines. Data are expressed as mean ± S.D. (n = 3). B, Western blot showing extensive processing of pro-caspase-3 in WT BMMCs but only minimal processing in SG−/− cells.
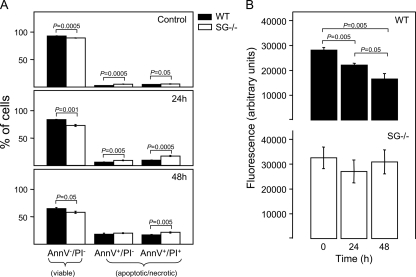
Apoptosis Induced by Growth Factor Deprivation Is SG-independent
Previous studies of the mechanisms involved in MC apoptosis have mainly focused on apoptosis induced by deprivation of growth factors essential for MC survival (40–42). To examine whether SG has a role also in this pathway of apoptosis, WT and SG−/− BMMCs were cultured in medium without MC growth factors and were assessed for extent of apoptosis at various time points. As seen in Fig. 6A, both WT and SG−/− cells underwent extensive cell death during these conditions, with ∼50% of the cells being apoptotic/necrotic after 48 h of culture. However, in contrast to apoptosis induced by the secretory granule/lysosomal pathway, SG−/− cells were not protected from cell death; in fact, SG−/− BMMCs showed a slightly decreased survival following growth factor deprivation as compared with WT cells. Nevertheless, when assessing granule damage (by acridine orange staining), WT cells showed clear signs of reduced granule integrity, whereas SG−/− cells were largely protected (Fig. 6B). Together, these data indicate that SG regulates secretory granule integrity also during growth factor deprivation-induced apoptosis, although the actual extent of apoptosis is not affected by the absence of SG.
FIGURE 6.
SG affects the secretory granule damage but not apoptosis in BMMCs deprived of growth factors. WT and SG−/− BMMCs were cultured in culture medium deprived of growth factors necessary for cell survival. At the time points indicated, cells were assessed for extent of apoptosis using annexin V (AnnV)/PI staining (A) and lysosomal damage as judged by acridine orange staining (B).
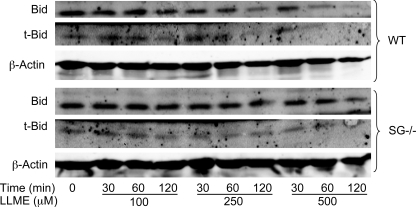
SG Affects the Levels of Bid
One of the major players in apoptosis induced by the lysosomal pathway is Bid (38, 43), a pro-apoptotic protein belonging to the BH3-only subfamily of BCL-2 proteins (13). Following proteolytic processing, truncated Bid (t-Bid) induces the release of pro-apoptotic molecules from the mitochondria (44). To investigate whether the differential sensitivity of WT and SG−/− BMMCs to apoptosis could be reflected by effects on Bid, we analyzed for levels and cleavage of Bid. In untreated cells, intact Bid dominated, both in WT and SG−/− cells (Fig. 7), but low levels of t-Bid were also detected. Upon addition of LLME, the band corresponding to intact Bid was reduced in WT BMMCs, accompanied by a slight increase in the levels of t-Bid at early time points after addition of LLME. In contrast, the levels of both Bid and t-Bid were largely unaffected in SG−/− cells after the addition of LLME, indicating defective Bid processing (Fig. 7). Hence, these data indicate that the absence of SG causes altered levels of Bid/t-Bid following induction of apoptosis through the secretory granule-mediated pathway, findings that are well in line with an apoptosis-promoting effect of SG.
FIGURE 7.
Differential processing of Bid in WT and SG−/− LLME-treated BMMCs. WT and SG−/− BMMCs (0.5 × 106 cells/ml) were either left untreated or incubated for the time periods indicated with different concentrations of LLME. Samples corresponding to equal number of cells were subjected Western blot analysis for Bid and t-Bid, with β-actin as loading control. The presented data are representative of three independent experiments. Densitometric scanning of gels is shown in supplemental Fig. 1.
Apoptosis Induced by Secretory Granule Damage Is Reduced in MCs Lacking SG-dependent MC Proteases
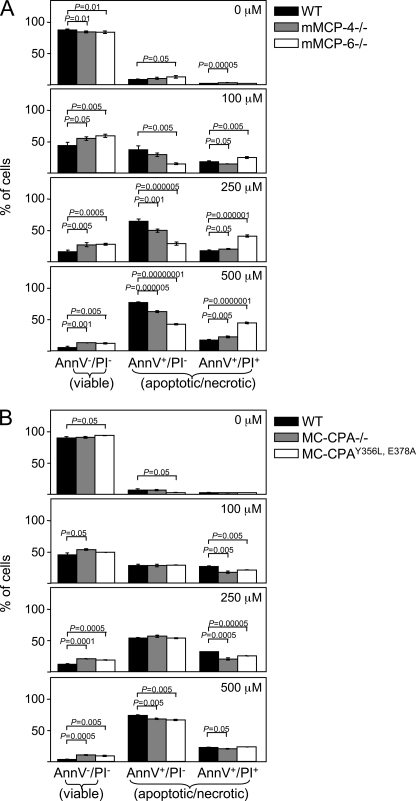
Mechanistically, it is conceivable that the apoptosis-promoting effect of SG could be explained by direct interaction of the proteoglycan with compounds involved in the pro-apoptotic machinery. Alternatively, its apoptosis promoting activity could be explained by indirect effects related to any of the multiple proteases that are stored in complex with SG, and whose storage in MCs is critically dependent on SG. These include mMCP-4, mMCP-5, mMCP-6, and MC-CPA (5, 7). To investigate the latter possibility, we assessed whether BMMCs deficient in either of these proteases showed reduced sensitivity to LLME-induced apoptosis. As shown in Fig. 8A, mMCP-4−/− BMMCs were significantly less susceptible to apoptosis than were WT cells, as shown by a higher percentage of viable cells as compared with WT cells. Thus, SG-bound mMCP-4 may partly account for the apoptosis-promoting effect of SG. Also, mMCP-6-deficient BMMCs showed a slightly but significantly reduced sensitivity to LLME-induced cell death as compared with corresponding WT cells (Fig. 8A). Notably, and similar to SG−/− cells, the portion of mMCP-6−/− BMMCs that were sensitive to LLME was to a higher extent annexin V/PI double-positive rather than solely annexin V-positive than were the corresponding WT cells (Fig. 8A), indicating necrosis/late stage apoptosis.
FIGURE 8.
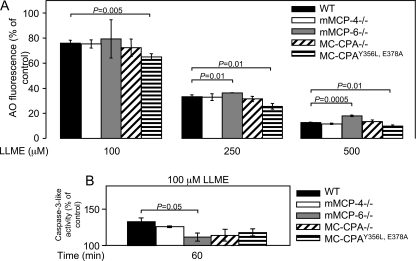
LLME-mediated apoptosis in BMMCs involves mMCP-4 (chymase), mMCP-6 (tryptase), and MC-CPA. BMMCs (0.5 × 106 cells/ml) from WT, mMCP-4−/−, and mMCP-6−/− mice (A); WT, MC-CPA−/−, and MC-CPAY356L, E378A mice (B) were either left untreated or incubated for 4 h with different concentrations of LLME, followed by staining with annexin V (AnnV)-FITC/PI and flow cytometry analysis. Data are from a representative experiment and are expressed as mean ± S.E. (n = 3).
To evaluate the contribution of MC-CPA, we assessed apoptosis in MC-CPA−/− BMMCs and in BMMCs developed from mice in which the active site of MC-CPA is mutated (MC-CPAY356L, E378A). Importantly, the absence of MC-CPA (MC-CPA−/−) leads to a secondary loss of mMCP-5 protein (31), whereas the active site mutation of MC-CPA does not lead to any secondary effects on mMCP-5 (32). Thus, the MC-CPA−/− strain combined with the MC-CPAY356L, E378A strain can also be used to indirectly assess the function of mMCP-5. As shown in Fig. 8B, both MC-CPA−/− and MC-CPAY356L, E378A BMMCs were slightly but significantly less susceptible to LLME-induced apoptosis than were WT BMMCs. However, there was no major difference in susceptibility when comparing the MC-CPA−/− and MC-CPAY356L, E378A strains. This indicates that MC-CPA contributes significantly to apoptosis induced by the secretory granule-mediated pathway, whereas the contribution of mMCP-5 is minimal.
To examine the mechanism by which the MC proteases contribute to apoptosis, we first assessed their contribution to the granule damage. These experiments revealed a slight but significant protection toward granule damage, as assessed by acridine orange staining, in mMCP-6−/− BMMCs but no significant protection in mMCP-4−/−, MC-CPA−/−, or MC-CPAY356L, E378A cells (Fig. 9A). The role of the various MC proteases in caspase-3 activation was also assessed. As shown in Fig. 9B, there was a tendency of reduced caspase-3 activation in mMCP-4−/−, MC-CPA−/−, and MC-CPAY356L, E378A BMMCs, but this did not reach statistical significance. In contrast, activation of caspase-3 was significantly reduced in BMMCs lacking mMCP-6, with caspase-3 activity in response to treatment with LLME only barely exceeding base-line levels (Fig. 9B). These data thus suggest a role for mMCP-6 in inducing secretory granule damage and in caspase-3 activation.
FIGURE 9.
Effect of MC proteases on granule damage and caspase-3 activation in response to LLME-induced apoptosis. A, BMMCs (0.5 × 106 cells/ml) derived from WT, mMCP-4−/−, mMCP-6−/−, MC-CPA−/−, or MC-CPAY356L, E378A mice were treated for 4 h with LLME at the concentrations indicated, followed by staining with acridine orange. B, BMMCs (106 cells/ml) derived from WT, mMCP-4−/−, mMCP-6−/−, MC-CPA−/−, or MC-MC-CPAY356L, E378A mice were treated for 1 h with 100 μm LLME, followed by measurement of caspase-3-like activity in cell extracts. Data are from a representative experiment and are expressed as mean ± S.D. (n = 3).
DISCUSSION
It has been established for many years that MCs play a major role in allergic disease, but more recent research has implicated MCs in a number of additional disorders, e.g. atherosclerosis (45), cancer (46), experimental autoimmune encephalitis (47), arthritis (48), and obesity (49). MCs are therefore emerging as potential therapeutic targets in numerous pathological settings. Possible regimens to treat MC-dependent disease include the use of agents that stabilize MCs, i.e. prevent their degranulation, and regimens in which pathogenic MC components are identified and specifically targeted. A third strategy to counteract MC-dependent disease is to selectively induce MC apoptosis, a notion that is currently being discussed (50).
Previous research has identified a number of pro-apoptotic pathways in MCs. For example, it has been shown that the pro-apoptotic BH-3 only proteins, Puma (40) and Bim (41), have key roles in MC apoptosis induced by growth factor deprivation, and it was recently demonstrated that increased sensitivity of IL-15−/− MCs to growth factor deprivation-induced or endogenous acid sphingomyelinase-induced apoptosis involved cathepsin D (42). It has also been demonstrated that human MCs express tumor necrosis factor-related apoptosis-inducing ligand receptor, i.e. one of the death receptors (51). Here, we explore an alternative pathway for inducing MC apoptosis. MC secretory granules are known to contain extraordinarily large amounts of various proteases. Potentially, these proteases could have the ability to proteolytically activate pro-apoptotic compounds, and we therefore hypothesized that their release from the granule compartment into the cytosol would cause MC apoptosis. Moreover, we have previously shown that the storage of several of the MC granule proteases is critically dependent on SG (5), and we therefore hypothesized that the absence of SG may render MCs less sensitive to apoptosis induced by targeting the secretory granules. Indeed, we show here for the first time that MCs are sensitive to apoptosis via a secretory granule-mediated pathway, and we also identify SG as an essential component of this pathway of apoptosis.
The mechanism by which SG promotes apoptosis is intriguing. Our data strongly indicate that the extent of granule damage in response to LLME is markedly diminished in cells lacking SG. In addition, granule damage in response to growth factor deprivation was also dependent on SG, even though SG did not affect the actual extent of apoptosis. Collectively, these data point to a key role for SG in regulating the integrity of secretory granules in response to both exogenous (LLME) and endogenous (induced by growth factor deprivation) apoptotic stimuli. SG is composed of a relatively small (∼17 kDa) protein core, which is densely substituted with heavily sulfated and thereby strongly anionic glycosaminoglycans. The SG molecule is thus dominated by its glycosaminoglycan moieties, and it is therefore most likely that any effects attributed to SG are mediated by its glycosaminoglycan chains rather than by the protein core. Indeed, SG has been shown to bind in an electrostatic fashion to a large number of compounds, which in many cases affects their activities and other properties, in particular their storage (reviewed in Ref. 2). We therefore favor the possibility that the effects of SG on granule integrity are related to electrostatic interactions between SG and other cellular compounds. For example, it is conceivable that SG could interact directly with membrane compounds at the luminal side of the granule, thereby promoting events that lead to granule damage.
In addition to promoting the granule damage, our data indicate that SG is required for optimal caspase-3 activation, i.e. one of the hallmark events occurring in apoptosis induced by multiple pathways. Our data also indicate that the processing of Bid is dependent on SG. Hence, SG may promote a number of proteolytic events affecting cytosolic components, which collectively can contribute to efficient induction of apoptosis. As regards the exact mechanism by which SG promotes the proteolysis of these components, several possibilities are conceivable. One possibility is that SG enhances the catalytic activity of proteases responsible for the respective proteolytic event. In line with this, SG has been shown to enhance the catalytic activity of MC chymase (52). Another possibility would be that SG promotes the assembly of pro-apoptotic components in MCs, and this possibility is in line with the known ability of SG to promote tryptase tetramer assembly (53) and with the known co-receptor function of cell-surface proteoglycans (3). A third possibility would be that the apoptosis-promoting effects of SG are indirect, being mediated by any of the various MC proteases that are stored in complex with SG and whose storage is critically dependent on SG (reviewed in Ref. 7). To assess the latter possibility, we evaluated the sensitivity of MCs lacking either of the SG-dependent MC proteases (mMCP-4, -5, -6, and MC-CPA) to apoptosis induced by granule damage. Indeed, we found that MCs lacking mMCP-4, mMCP-6, and, to a somewhat lesser extent, MC-CPA showed significant protection toward LLME-induced cell death, thus in support of this notion. Notably, the contribution of each of these proteases was relatively modest, but it appears likely that their additive activities may account for a large part of the apoptosis-promoting effect of SG. Possibly, the SG-dependent MC proteases could contribute at the level of granule permeabilization, for example by degrading membrane components. In line with this possibility, mMCP-6−/− BMMCs showed a slight but significant protection toward granule damage induced by LLME. Furthermore, it is clearly conceivable that the SG-dependent proteases could be translocated into the cytosol and there contribute to the proteolytic processing events occurring downstream of the granule permeabilization, e.g. processing of caspase-3. In favor of the latter notion, LLME was shown to induce the release of tryptase-like serine protease activity, compatible with the known catalytic activity of mMCP-6 (54), into the cytosol. Moreover, the absence of mMCP-6 resulted in significantly reduced rates of caspase-3 activation, suggesting that the SG-dependent enhancement of caspase-3 processing, at least partly, could be attributed to SG-bound mMCP-6.
An interesting finding was that not only were SG−/− cells more resistant to apoptosis induced by LLME than were WT cells, the occasional SG−/− cells actually undergoing cell death were predominantly annexin V/PI double-positive rather than only annexin V+. Importantly, this phenomenon was seen at all tested LLME concentrations and after both short (4 h) and long (24 h; data not shown) incubation times with LLME. Because PI positivity is a typical sign of necrosis/late stage apoptosis, this indicates that SG-deficient cells, when eventually undergoing cell death, die by a mechanism dominated by necrosis rather than by classical apoptosis. Furthermore, the failure of SG−/− cells to enter apoptosis was also underscored by ultrastructural analysis, demonstrating that the occasional SG−/− cells showing signs of cell death were necrotic rather than apoptotic. Notably, the dominance of annexin V/PI double positivity over sole annexin V+ positivity was also seen in BMMCs lacking mMCP-6, suggesting that SG-bound mMCP-6 may partly account for this finding.
The sensitivity to apoptosis via the lysosomal pathway varies to a large extent between different cell types. For example, a number of cancer cell lines are largely resistant, although several cells of hematopoietic origin are more sensitive. The reason for this differential sensitivity has not been fully clarified. Interestingly, however, many of the cell types that are susceptible to apoptosis via the lysosomal/granule pathway, e.g. NK cells, cytotoxic T lymphocytes, monocytes/macrophages (55, 56), and MCs (this study) are known to express high levels of SG (2). A possible explanation for the sensitivity of these cells to apoptosis could therefore be that they contain large amounts of intracellular SG. Hence, the level of SG expression may be a predictor of sensitivity to apoptosis induced by the lysosomal/granule pathway. Furthermore, we may suggest that induction of apoptosis via the lysosomal/granule pathway can constitute a possible regimen for therapy in which cells abundant in SG-containing granules, such as MCs, are targeted.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01 AI 059746. This work was also supported by grants from Swedish Research Council, Formas, King Gustaf V 80-Year Anniversary Fund, Torsten and Ragnar Söderberg Foundation, Swedish Cancer Foundation, CAPES-Brazil, Vårdal Foundation, Swedish Society of Medicine, Slovene Research Agency Grants P1-0140 and J1-711, European Research Council (FP7/2007-2013)/ERC grant agreement no. 233074, and Deutsche Forschungsgemeinschaft 754-2-2.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- SG
- serglycin
- BMMC
- bone marrow derived mast cell
- MC
- mast cell
- MC-CPA
- mast cell carboxypeptidase A
- LLME
- H-Leu-Leu-Ome
- Z
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone
- AMC
- aminomethylcoumarin
- PI
- propidium iodide
- t-Bid
- truncated Bid.
REFERENCES
- 1. Kolset S. O., Tveit H. (2008) Cell. Mol. Life Sci. 65, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pejler G., Abrink M., Wernersson S. (2009) Biofactors 35, 61–68 [DOI] [PubMed] [Google Scholar]
- 3. Bishop J. R., Schuksz M., Esko J. D. (2007) Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 4. Braga T., Grujic M., Lukinius A., Hellman L., Abrink M., Pejler G. (2007) Biochem. J. 403, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrink M., Grujic M., Pejler G. (2004) J. Biol. Chem. 279, 40897–40905 [DOI] [PubMed] [Google Scholar]
- 6. Pejler G., Rönnberg E., Waern I., Wernersson S. (2010) Blood 115, 4981–4990 [DOI] [PubMed] [Google Scholar]
- 7. Pejler G., Abrink M., Ringvall M., Wernersson S. (2007) Adv. Immunol. 95, 167–255 [DOI] [PubMed] [Google Scholar]
- 8. Grujic M., Braga T., Lukinius A., Eloranta M. L., Knight S. D., Pejler G., Abrink M. (2005) J. Biol. Chem. 280, 33411–33418 [DOI] [PubMed] [Google Scholar]
- 9. Niemann C. U., Abrink M., Pejler G., Fischer R. L., Christensen E. I., Knight S. D., Borregaard N. (2007) Blood 109, 4478–4486 [DOI] [PubMed] [Google Scholar]
- 10. Woulfe D. S., Lilliendahl J. K., August S., Rauova L., Kowalska M. A., Abrink M., Pejler G., White J. G., Schick B. P. (2008) Blood 111, 3458–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wernersson S., Braga T., Sawesi O., Waern I., Nilsson K. E., Pejler G., Abrink M. (2009) J. Leukocyte Biol. 85, 401–408 [DOI] [PubMed] [Google Scholar]
- 12. Grujic M., Christensen J. P., Sørensen M. R., Abrink M., Pejler G., Thomsen A. R. (2008) J. Immunol. 181, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 13. Taylor R. C., Cullen S. P., Martin S. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 231–241 [DOI] [PubMed] [Google Scholar]
- 14. Elmore S. (2007) Toxicol. Pathol. 35, 495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turk B., Turk V. (2009) J. Biol. Chem. 284, 21783–21787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boya P., Kroemer G. (2008) Oncogene 27, 6434–6451 [DOI] [PubMed] [Google Scholar]
- 17. Kirkegaard T., Jäättelä M. (2009) Biochim. Biophys. Acta 1793, 746–754 [DOI] [PubMed] [Google Scholar]
- 18. Bird P. I., Trapani J. A., Villadangos J. A. (2009) Nat. Rev. Immunol. 9, 871–882 [DOI] [PubMed] [Google Scholar]
- 19. van Nierop K., Muller F. J., Stap J., Van Noorden C. J., van Eijk M., de Groot C. (2006) J. Histochem. Cytochem. 54, 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michallet M. C., Saltel F., Flacher M., Revillard J. P., Genestier L. (2004) J. Immunol. 172, 5405–5414 [DOI] [PubMed] [Google Scholar]
- 21. Tran T. M., Temkin V., Shi B., Pagliari L., Daniel S., Ferran C., Pope R. M. (2009) Apoptosis 14, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ida H., Nakashima T., Kedersha N. L., Yamasaki S., Huang M., Izumi Y., Miyashita T., Origuchi T., Kawakami A., Migita K., Bird P. I., Anderson P., Eguchi K. (2003) Eur. J. Immunol. 33, 3284–3292 [DOI] [PubMed] [Google Scholar]
- 23. Laforge M., Bidère N., Carmona S., Devocelle A., Charpentier B., Senik A. (2006) J. Immunol. 176, 3966–3977 [DOI] [PubMed] [Google Scholar]
- 24. Dragonetti A., Baldassarre M., Castino R., Démoz M., Luini A., Buccione R., Isidoro C. (2000) J. Cell Sci. 113, 3289–3298 [DOI] [PubMed] [Google Scholar]
- 25. Wolters P. J., Laig-Webster M., Caughey G. H. (2000) Am. J. Respir. Cell Mol. Biol. 22, 183–190 [DOI] [PubMed] [Google Scholar]
- 26. Henningsson F., Wolters P., Chapman H. A., Caughey G. H., Pejler G. (2003) Biol. Chem. 384, 1527–1531 [DOI] [PubMed] [Google Scholar]
- 27. Henningsson F., Yamamoto K., Saftig P., Reinheckel T., Peters C., Knight S. D., Pejler G. (2005) J. Cell Sci. 118, 2035–2042 [DOI] [PubMed] [Google Scholar]
- 28. Blott E. J., Griffiths G. M. (2002) Nat. Rev. Mol. Cell Biol. 3, 122–131 [DOI] [PubMed] [Google Scholar]
- 29. Tchougounova E., Pejler G., Abrink M. (2003) J. Exp. Med. 198, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., Lee D. M. (2008) J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feyerabend T. B., Hausser H., Tietz A., Blum C., Hellman L., Straus A. H., Takahashi H. K., Morgan E. S., Dvorak A. M., Fehling H. J., Rodewald H. R. (2005) Mol. Cell. Biol. 25, 6199–6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider L. A., Schlenner S. M., Feyerabend T. B., Wunderlin M., Rodewald H. R. (2007) J. Exp. Med. 204, 2629–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charley M., Thiele D. L., Bennett M., Lipsky P. E. (1986) J. Clin. Invest. 78, 1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivanova S., Repnik U., Bojic L., Petelin A., Turk V., Turk B. (2008) Methods Enzymol. 442, 183–199 [DOI] [PubMed] [Google Scholar]
- 35. Henningsson F., Hergeth S., Cortelius R., Abrink M., Pejler G. (2006) FEBS J. 273, 4901–4912 [DOI] [PubMed] [Google Scholar]
- 36. Cirman T., Oresiæ K., Mazovec G. D., Turk V., Reed J. C., Myers R. M., Salvesen G. S., Turk B. (2004) J. Biol. Chem. 279, 3578–3587 [DOI] [PubMed] [Google Scholar]
- 37. Blomgran R., Zheng L., Stendahl O. (2007) J. Leukocyte Biol. 81, 1213–1223 [DOI] [PubMed] [Google Scholar]
- 38. Droga-Mazovec G., Bojic L., Petelin A., Ivanova S., Romih R., Repnik U., Salvesen G. S., Stoka V., Turk V., Turk B. (2008) J. Biol. Chem. 283, 19140–19150 [DOI] [PubMed] [Google Scholar]
- 39. Thiele D. L., Lipsky P. E. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ekoff M., Kaufmann T., Engström M., Motoyama N., Villunger A., Jönsson J. I., Strasser A., Nilsson G. (2007) Blood 110, 3209–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alfredsson J., Puthalakath H., Martin H., Strasser A., Nilsson G. (2005) Cell Death Differ. 12, 136–144 [DOI] [PubMed] [Google Scholar]
- 42. Mirghomizadeh F., Winoto-Morbach S., Orinska Z., Lee K. H., Schütze S., Bulfone-Paus S. (2009) Exp. Cell Res. 315, 3064–3075 [DOI] [PubMed] [Google Scholar]
- 43. Stoka V., Turk B., Schendel S. L., Kim T. H., Cirman T., Snipas S. J., Ellerby L. M., Bredesen D., Freeze H., Abrahamson M., Bromme D., Krajewski S., Reed J. C., Yin X. M., Turk V., Salvesen G. S. (2001) J. Biol. Chem. 276, 3149–3157 [DOI] [PubMed] [Google Scholar]
- 44. Desagher S., Martinou J. C. (2000) Trends Cell Biol. 10, 369–377 [DOI] [PubMed] [Google Scholar]
- 45. Sun J., Sukhova G. K., Wolters P. J., Yang M., Kitamoto S., Libby P., MacFarlane L. A., Mallen-St Clair J., Shi G. P. (2007) Nat. Med. 13, 719–724 [DOI] [PubMed] [Google Scholar]
- 46. Soucek L., Lawlor E. R., Soto D., Shchors K., Swigart L. B., Evan G. I. (2007) Nat. Med. 13, 1211–1218 [DOI] [PubMed] [Google Scholar]
- 47. Secor V. H., Secor W. E., Gutekunst C. A., Brown M. A. (2000) J. Exp. Med. 191, 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee D. M., Friend D. S., Gurish M. F., Benoist C., Mathis D., Brenner M. B. (2002) Science 297, 1689–1692 [DOI] [PubMed] [Google Scholar]
- 49. Liu J., Divoux A., Sun J., Zhang J., Clément K., Glickman J. N., Sukhova G. K., Wolters P. J., Du J., Gorgun C. Z., Doria A., Libby P., Blumberg R. S., Kahn B. B., Hotamisligil G. S., Shi G. P. (2009) Nat. Med. 15, 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karra L., Berent-Maoz B., Ben-Zimra M., Levi-Schaffer F. (2009) Curr. Opin. Immunol. 21, 708–714 [DOI] [PubMed] [Google Scholar]
- 51. Berent-Maoz B., Piliponsky A. M., Daigle I., Simon H. U., Levi-Schaffer F. (2006) J. Immunol. 176, 2272–2278 [DOI] [PubMed] [Google Scholar]
- 52. Pejler G., Sadler J. E. (1999) Biochemistry 38, 12187–12195 [DOI] [PubMed] [Google Scholar]
- 53. Hallgren J., Bäckström S., Estrada S., Thuveson M., Pejler G. (2004) J. Immunol. 173, 1868–1875 [DOI] [PubMed] [Google Scholar]
- 54. Huang C., Friend D. S., Qiu W. T., Wong G. W., Morales G., Hunt J., Stevens R. L. (1998) J. Immunol. 160, 1910–1919 [PubMed] [Google Scholar]
- 55. Thiele D. L., Lipsky P. E. (1986) J. Immunol. 136, 1038–1048 [PubMed] [Google Scholar]
- 56. Kobayashi Y., Takasaki A., Kurosaka K., Sakurai Y., Iwamura M., Watanabe N. (2000) Biochem. Biophys. Res. Commun. 272, 687–690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.