Abstract
ATP-binding cassette (ABC) transporters mediate transport of diverse substrates across membranes. We have determined the quaternary structure and functional unit of the recently discovered ECF-type (energy coupling factor) of ABC transporters, which is widespread among prokaryotes. ECF transporters are protein complexes consisting of a conserved energizing module (two peripheral ATPases and the integral membrane protein EcfT) and a non-conserved integral membrane protein responsible for substrate specificity (S-component). S-components for different substrates are often unrelated in amino acid sequence but may associate with the same energizing module. Here, the energizing module from Lactococcus lactis was shown to form stable complexes with each of the eight predicted S-components found in the organism. The quaternary structures of three of these complexes were determined by light scattering. EcfT, the two ATPases (EcfA and EcfA′), and the S-components were found to be present in a 1:1:1:1 ratio. The complexes were reconstituted in proteoliposomes and shown to mediate ATP-dependent transport. ECF-type transporters are the smallest known ABC transporters.
Keywords: ABC Transporter, ATPases, Bacteria, Membrane Energetics, Membrane Enzymes, Membrane Proteins, Membrane Reconstitution, Protein Structure, Vitamins and Cofactors
Introduction
ATP-binding cassette (ABC)2 transporters catalyze the transport of a wide variety of molecules across lipid bilayers into or out of cells and organelles. They form one of the largest known protein families and are found in organisms from all kingdoms of life (1). ABC transporters consist of two homologous or identical integral membrane domains that form the translocation pore and two peripheral nucleotide-binding domains (ATPases) that energize the transport. ABC importers contain additional extracellular or periplasmic soluble substrate-binding domains or proteins and are found only in prokaryotes (1–3). Recently, a new type of ABC importer was found, which is widespread among prokaryotes and was named the ECF type (for energy coupling factor) (4, 5). Like classical ABC transporters, they consist of peripheral (ATPase) and transmembrane subunits that together form an energizing module. Unlike classical ABC importers, ECF-type transporters employ integral membrane proteins (named “S-components”) as substrate-binding proteins. The S-components are small (20–25 kDa) and hydrophobic (4–6 predicted transmembrane segments) (5). Bioinformatics analyses have predicted that ECF-type transporters are involved in the uptake of vitamins or other nutrients needed in trace amounts (such as Ni2+ or Co2+ ions) (4–7). Recently, the first crystal structure of an S-component was published. The riboflavin-binding protein RibU from Staphylococcus aureus was found to have a new fold, unrelated to the membrane domains of other ABC transporters (8).
ECF-type ABC transporters fall into two groups (5). In group I, S-components are encoded in the same operon as the energizing module (hence designated as “dedicated” energizing modules). The biotin transporter BioMNY from Rhodobacter capsulatus is the best characterized member of this group (4). Group II ECF transporters consist of energizing modules that are encoded in an operon without S-component genes. These energizing modules usually consist of two different homologous ATPases (EcfA and EcfA′), alongside the membrane protein EcfT, and are predicted to interact with various S-components that are encoded by genes scattered over the genome (hence the name “shared” energizing modules) (5). The different S-components are often unrelated at the sequence level. The group II proteins are particularly abundant in Gram-positive organisms, many of which are pathogens.
Here, we present the first genome-wide experimental analysis of an ECF-type transporter with shared energizing modules. We reveal the quaternary structure and minimal functional unit using purified proteins solubilized in detergent or reconstituted in proteoliposomes.
EXPERIMENTAL PROCEDURES
Cloning and Expression of EcfAA′T and EcfAA′T-S-component Complexes
The ecf operon from Lactococcus lactis (consisting of the genes annotated as cbiO, cbiO, and cbiQ2, here renamed as ecfA, ecfA′, and ecfT) was cloned in a pNZ8048 vector (9) for expression in the L. lactis strain NZ9000 (10) and in a pBAD vector (11, 12) for expression in the Escherichia coli strain MC1061 (13). The sequence coding for a His10 tag was added in-frame at the 5′ end of the ecfT gene (ecfT-His) or the 3′ end of the first cbiO (His-ecfA) gene via the ligation independent cloning method (12). For simultaneous expression of the ecf operon and the genes encoding S-components in E. coli MC1061, an expression vector based on pBAD24 was used with two tandem arabinose-inducible promoters, ribosomal-binding sites, multiple cloning sites, and terminator sequences.3 Downstream of the first promoter, the ecfAA′T-His operon was cloned. Downstream of the second promoter, a gene coding an S-component was cloned in-frame with the sequence coding for a C-terminal STREPII tag (WSHPQFEK). Cultivation, induction of expression, and harvesting of E. coli and L. lactis cells as well as preparation of membrane vesicles were done according to standard protocols (see supplemental methods).
Protein Purification
The His-tagged complexes EcfAA′T and EcfAA′T-S-component were solubilized in 0.5% n-dodecyl-β-d-maltoside and purified in two steps, using nickel-Sepharose and size-exclusion chromatography. Details of the protein purification procedure can be found in the supplemental methods.
Light Scattering
The subunit stoichiometries of the complexes were determined by size-exclusion chromatography coupled to multi-angle laser light scattering (SEC-MALLS) (14, 15). SEC-MALLS was performed as described before (6, 14). 200 μl of the purified protein was used in the experiment (∼155 μg). For calculation of the molecular mass of multisubunit complexes of membrane proteins, we made use of the internal consistency method described by Wen et al. (15). This method assumes several different possible subunit stoichiometries and then determines the molecular mass from the light scattering data, which should be consistent with the stoichiometry initially assumed.
Analysis by SDS-PAGE, Western Blotting, and MS/MS
SDS-PAGE, Western blotting, and protein immunodetection were done according to standard protocols (for detailed information, see supplemental methods). For mass spectrometry (tandem MALDI-TOF) analysis, all the visible bands on a Coomassie Brilliant Blue-stained gel were excised. The gel bands were destained and digested overnight with trypsin (Promega V5111), and peptides were extracted as described by Kiel et al. (16). The peptide digests were mixed 1:1 (v/v) with a solution of α-cyano-4-hydroxycinnamic acid matrix (5 mg/ml in 50% acenotrile and 0.1% TFA, LaserBio Labs), spotted onto a stainless steel MALDI target, and analyzed with a 4700 proteomics analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems).
Reconstitution and Transport Assays in Proteoliposomes
The purified complexes EcfAA′T-NiaX and EcfAA′T-RibU were reconstituted into proteoliposomes, using the method described by Geertsma et al. (17). The ECF complexes were reconstituted into liposomes composed of E. coli polar lipids and egg phosphatidylcholine (3:1, w/w) at protein-to-lipid ratios (w/w) of 1:250 (EcfAA′T-NiaX for uptake experiments), 1:100 (EcfAA′T-NiaX for efflux experiments), and 1:333 (EcfAA′T-RibU for uptake experiments). For use in the transport assays, proteoliposomes were thawed, and the solute composition was adjusted to match the desired luminal composition: 50 mm potassium phosphate, with 10 mm ATP and 10 mm MgSO4, 10 mm ADP and 10 mm MgSO4, 10 mm MgSO4 only, or 10 mm AMP-PNP with 10 mm MgSO4. In all cases, the pH was 7. Subsequently, the suspension was frozen in liquid nitrogen and thawed three times. Subsequently, the proteoliposomes were extruded 11 times through a 200-nm pore size polycarbonate filter (Avestin) and centrifuged (267,000 × g, 20 min, 4 °C). For the transport assays, the proteoliposomes were diluted to an estimated protein concentration of 5 μg/ml. 200-μl aliquots were made, one for each time point, and transport was started by the addition of radiolabeled substrate. In the case of EcfAA′T-NiaX, [3H]niacin was added to a final concentration of 375 nm, and in the case of EcfAA′T-RibU, 35 nm [3H]riboflavin was used. At the indicated time points, 2 ml of stop buffer (ice-cold 80 mm potassium phosphate, pH 7) was added. Once the stop buffer had been added, the solution was rapidly filtered over a BA-85 nitrocellulose filter, which was subsequently washed once with 2 ml of stop buffer. Filters were dried for 1 h at 80 °C, 2 ml of Emulsifier-Scintillator Plus liquid (PerkinElmer Life Sciences) was added, the suspension was vortexed, and levels of radioactivity were determined with a PerkinElmer Tri-Carb 2800 TR isotope counter.
RESULTS
The genome of the Gram-positive bacterium L. lactis contains one operon coding for a shared energizing module (ecfAA′T, annotated as cbiOOQ2). In addition, it contains eight different genes coding for predicted S-components with confirmed or predicted specificity for vitamins and their precursors (5–7) (Table 1).
TABLE 1.
S-components found in L. lactis and predicted to interact with the shared energizing module (5)
a.a., amino acids.
| Name | (Probable) substratea | Size | Size | Predicted number of TM helicesb | Accession code in genome |
|---|---|---|---|---|---|
| a.a. | kDa | ||||
| BioY | Biotin | 189 | 20.5 | 6 | llmg_1964 |
| BioY 2 | Biotin | 182 | 19.7 | 6 | llmg_0332 |
| HmpT | Thiamine precursor | 166 | 18.1 | 4 or 5 | llmg_0464 |
| NiaX | Niacin | 222 | 24.6 | 5 or 6 | llmg_1330 |
| PanT | Pantothenic acid | 196 | 21.1 | 6 | llmg_0542 |
| QueT | Queuosine precursor | 169 | 19.1 | 4 or 5 | llmg_1760 |
| RibU | Riboflavin | 206 | 23.0 | 5 or 6c | llmg_1195 |
| ThiT | Thiamine | 182 | 19.9 | 5 or 6 | llmg_0334 |
b Protein sequences were analyzed by topcons, which runs five different prediction programs. The number of predicted transmembrane helices (TM) often depends on which prediction program is used.
c The crystal structure of RibU from Staphylococcus aureus shows that there are six transmembrane helices (8).
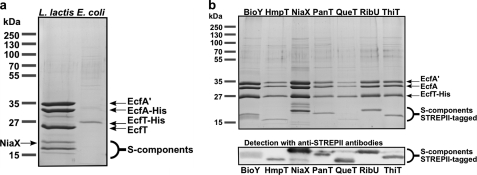
To investigate the quaternary structure of the ECF-type ABC transporters, we initially aimed to isolate the energizing module EcfAA′T from L. lactis (18). His-tagged EcfAA′T was produced in L. lactis, solubilized, and purified by nickel affinity and size-exclusion chromatography. Fig. 1a shows an SDS-PAGE analysis of the purified complex. Besides the three proteins from the energizing module (His-EcfA, EcfA′, and EcfT), we consistently co-purified several proteins with molecular masses around 20 kDa. We hypothesized that these proteins could be the endogenous S-components from L. lactis that had formed stable complexes with the energizing modules. Indeed, one of the proteins (indicated in Fig. 1a) was identified by MALDI mass spectrometry as the S-component NiaX. We could not reveal the identities of the other co-purified proteins, presumably because the physicochemical properties of the S-components (small and very hydrophobic proteins) precluded identification by in-gel protein digestion, peptide extraction, and mass spectrometry.
FIGURE 1.
Purification of EcfAA′T complexes. Coomassie Blue-stained SDS-polyacrylamide gels showing the purified fractions after nickel-Sepharose and size-exclusion chromatography. a, EcfAA′T was expressed in L. lactis (lane 1) or in E. coli (lane 2). b, EcfAA′T was co-produced with seven S-components in E. coli. BioY2 is not shown but behaved in the same way as BioY. The identities of the S-components were confirmed by Western blotting and detection using anti-STREPII tag antibodies (bottom panel). The Western blot was used only for qualitative purposes, and the amounts of protein loaded on the corresponding SDS-polyacrylamide gel were not the same as on the Coomassie Blue-stained gel.
Co-purification of the different endogenous S-components with the energizing module introduced heterogeneity and complicated structural analysis. Therefore, we decided to overproduce the energizing module in E. coli MC1061, a strain devoid of endogenous ECF-type ABC transporters or S-components. The His-tagged energizing module EcfAA′T was again purified by nickel affinity and size-exclusion chromatography. Analysis by SDS-PAGE (Fig. 1a) revealed that only the band corresponding to the His-tagged subunit (EcfT) was visible, indicating that the energizing module had not formed a stable complex or that the complex had fallen apart during the purification in detergent solution.
Possibly, the energizing module can form stable complexes only if an S-component is attached. To test this hypothesis, we co-produced each of the eight S-components (containing a C-terminal STREPII tag) with the EcfAA′T-His module in E. coli MC1061. Membrane solubilization followed by metal affinity and size-exclusion chromatography resulted in co-purification of the entire complex containing both the energizing module and the co-produced S-component (Fig. 1b).
The subunit stoichiometry of the complexes containing the EcfA, EcfA′, and EcfT and the S-components is not known (e.g. see Ref. 19). It is possible that the S-component and EcfT together form the membrane pore (in a 1:1 stoichiometry) and associate with a heterodimer of the two ATPases (EcfA and EcfA′). In this case, the S-component would form an integral part of the complex. On the other hand, it is also possible that two EcfT subunits form a complex with EcfA and EcfA′ (2:1:1 stoichiometry) and that the S-component is attached only peripherally, possibly in multiple copies. The latter organization could resemble sulfonylurea receptors (SURs). SURs are complete ABC transporters, with two transmembrane domains and two nucleotide-binding domains, that associate with an unrelated membrane protein (in this case with KATP channels) (20).
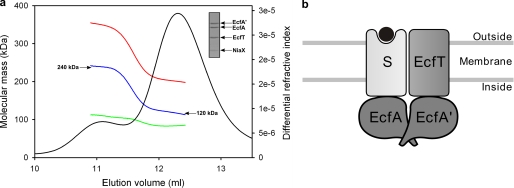
To determine the molecular weight of the complexes (and thus the subunit stoichiometry), it was not possible to use the elution volumes from a size-exclusion column, which had been calibrated with globular protein markers because the amount of attached detergent was not known. Instead, we determined the subunit stoichiometries of three complexes (EcfAA′T-NiaX, EcfAA′T-BioY, and EcfAA′T-ThiT) by SEC-MALLS. SEC-MALLS explicitly accounts for the amount of detergent bound to a membrane protein and allows for determination of the absolute molecular mass of a protein in a protein-lipid-detergent mixed micelle. The technique does not make use of the elution volume from the size-exclusion column. SEC is used only to separate the protein of interest from different species (contaminants/excess empty detergent micelles/aggregated proteins) (14, 15). The mass of protein complex was determined throughout the elution peak and was found to be ∼119 kDa (shown in Fig. 2a for EcfAA′T-NiaX). The only subunit stoichiometry consistent with the data was a 1:1:1:1 ratio between the ATPases EcfA and EcfA′, the transmembrane protein EcfT, and the S-component NiaX, which has a calculated mass of 120.5 kDa (Table 2). The same quaternary structure was found for EcfAA′T-BioY and EcfAA′T-ThiT (supplemental Tables 1 and 2). In all three cases, a stoichiometry in which two EcfT subunits would be present, in addition to one or more S-components, was not consistent with the data.
FIGURE 2.
Subunit stoichiometry of the EcfAA′T-S-component complex. a, SEC-MALLS analysis of the EcfAA′T-NiaX complex. The chromatogram of a size-exclusion chromatography run is shown. The black trace is the signal from the differential refractive index detector. The calculated masses of protein (blue), detergent (green), and total (red) of the protein-detergent micelle are shown in the chromatogram. b, schematic representation of an ECF-type importer. The positions of EcfA and EcfA′ relative to the membrane subunits are not known. S indicates S-component; the black circle indicates substrate.
TABLE 2.
Subunit stoichiometry of EcfAA′T-NiaX
| EcfAa | EcfA′a | EcfTa | NiaXa | Extinction coefficientb | In silico calculated MWb | MW from SEC-MALLSc | Difference |
|---|---|---|---|---|---|---|---|
| liter/(g cm) | kDa | kDa | kDa | ||||
| 1 | 1 | 1 | 1 | 0.683 | 120.5 | 119 ± 3 | −1.5 |
| 1 | 1 | 2 | 0 | 0.768 | 127.2 | 107 ± 4 | −20.2 |
| 1 | 1 | 1 | 2 | 0.689 | 146.2 | 118 ± 3 | −28.2 |
| 1 | 1 | 2 | 1 | 0.760 | 152.9 | 108 ± 4 | −44.9 |
| 2 | 1 | 1 | 1 | 0.646 | 151.4 | 127 ± 4 | −24.8 |
| 1 | 2 | 1 | 1 | 0.636 | 152.1 | 129 ± 4 | −23.1 |
| 2 | 2 | 2 | 1 | 0.679 | 215.3 | 121 ± 4 | −94.3 |
a Stoichiometric coefficient of each subunit in the EcfAA′T-NiaX complex.
b Calculated via the ProtParam tool on the ExPASy Proteomics Server. Extinction coefficients were calculated on the assumption that all cysteines were reduced.
c Mw is the weight-averaged molecular mass of the protein without the detergent contribution. The values are the averages of two independent experiments and the error indicates the range.
For EcfAA′T-BioY and EcfAA′T-ThiT, the complexes with 1:1:1:1 stoichiometry fitted best to the experimental data, although hypothetical complexes consisting of three ATPase subunits also fitted reasonably well (supplemental Tables 1 and 2). However, we do not expect three ATPases to be present in the complex. The EcfA and EcfA′ are typical ABC transporter ATPases, and it is well known that these ATPases form dimers, with the active sites on the dimer interface. Furthermore, on the Coomassie Brilliant Blue-stained SDS-polyacrylamide gels (Fig. 1), the bands for EcfA and EcfA′ were equally intense. The two proteins are soluble and homologous (identity is 35%) and therefore likely to stain in a similar way. It is thus likely that EcfA and EcfA′ are present in a 1:1 ratio.
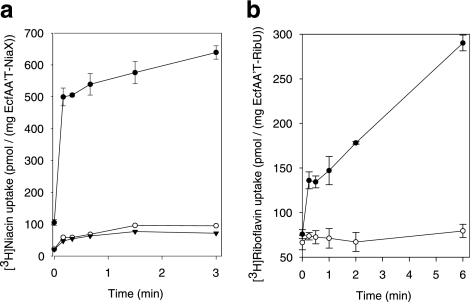
The purified complexes EcfAA′T-NiaX and EcfAA′T-RibU were reconstituted in liposomes to investigate whether the protein complexes found in detergent solution sufficed for transport. Uptake of radiolabeled niacin (by EcfAA′T-NiaX) or riboflavin (by EcfAA′T-RibU) into the proteoliposomes was indeed observed. Transport depended on the presence of Mg-ATP in the lumen of the liposomes. The accumulation levels of niacin and riboflavin were 8.7- and 26-fold, respectively. Inclusion of only Mg2+ ions or Mg-ADP in the liposome lumen did not result in substrate transport (Fig. 3). Also, the presence of 10 mm Mg-AMP-PNP, a slowly hydrolyzable ATP analogue, did not support substrate accumulation, showing that ATP hydrolysis was required (data not shown). As expected, riboflavin was not transported by the EcfAA′T-NiaX complex.
FIGURE 3.
Transport of [3H]niacin (a) and [3H]riboflavin (b) into proteoliposomes containing EcfAA′T-NiaX and EcfAA′T-RibU, respectively. Error bars indicate the S.E. of three measurements. The proteoliposomes were loaded with 50 mm potassium phosphate, supplemented with 10 mm MgSO4 and 10 mm ATP (closed circles), 10 mm MgSO4 only (open circles), or 10 mm MgSO4 and 10 mm ADP (closed triangles). The pH was 7. The accumulation levels of niacin (at the 3-min time point) and riboflavin (at the 6-min time point) are 8.7- and 26-fold, respectively. The non-zero levels of radioactivity (riboflavin or niacin) counted using the proteoliposomes loaded with Mg-ADP or MgSO4 only are due to nonspecific binding of the label to the proteoliposomes. Similar levels were observed when proteoliposomes of an unrelated protein were used (the secondary active aspartate transporter GltPh, data not shown).
In a complementary experiment, we measured transport by an efflux assay using proteoliposomes in which the orientation of the EcfAA′T-NiaX complexes (right-side-out or inside-out) was deliberately scrambled (17). Importantly, upon the addition of external Mg-ATP, luminal niacin was rapidly released from the liposomes (supplemental Fig. 1), confirming that the reconstituted complexes mediated substrate translocation in the presence of Mg-ATP.
DISCUSSION
For the first time, we have shown in a comprehensive genome-wide analysis that all predicted S-components in a single organism (BioY, BioY2, HmpT, NiaX, PanT, QueT, RibU, and ThiT from L. lactis) indeed interact with the same energizing module. With the exception of BioY and BioY2, the S-components do not share significant sequence similarity, raising the question how these proteins recognize the same EcfAA′T module. Presumably, there is a structurally conserved motif in the S-components that is responsible for the interaction with either the EcfT protein or a nucleotide-binding domain or both. It is not clear from the amino acid sequences whether the S-components contain a motif related to the coupling helix that mediates binding between the membrane domains and ATPase subunits in classical ABC transporters. The crystal structure of RibU from S. aureus also did not reveal a coupling helix or any obvious binding sites for the T-component (8). Nonetheless, from our work, it is clear that the interactions between the energizing module and the S-components are strong and that the EcfAA′T module is stable in detergent only when an S-component is attached.
The observed subunit stoichiometry of the purified complexes (1:1:1:1 EcfA:EcfA′:EcfT:S-component) shows that the S-component is an integral part of the ECF transporter taking the place of one of the two transmembrane domains found in classical ABC transporters (Fig. 2b), which may explain why EcfAA′T alone did not form a stable complex in the absence of an S-component (Fig. 1a). The quaternary structure makes ECF transporters clearly different from the SURs, to which the KATP channels are attached peripherally (20).
The presence of a single S-component in the complex is consistent with previous results showing that the S-component ThiT is monomeric in detergent solution in the absence of the energizing module and that monomeric ThiT binds its substrate thiamin in a 1:1 ratio (6). It also agrees with the recent crystal structure of RibU that has a monomeric functional unit (8). The results are not directly compatible with FRET lifetime measurements in E. coli cells expressing the biotin transporter BioMNY (which has a dedicated energizing module). A multimeric state was found for the S-component BioY, both alone and in complex with its dedicated energizing module (19). It is possible that the structure of BioMNY is different from the ECF-type transporters with shared energizing modules studied here. On the other hand, it is also possible that the basic unit with a 1:1:1:1 subunit stoichiometry forms higher order arrays as part of the transport cycle. Our SEC-MALLS analysis indicated that the complexes have a tendency to form higher order aggregates in detergent solution. Besides the main protein peak in the chromatogram (at 12.3 ml), a shoulder (at 11.1 ml) was also present that eluted earlier. The protein complex in this peak had a calculated molecular mass of exactly twice the mass of the protein in the main peak (Fig. 2a). Likely, the protein peak eluting at 11.1 ml represents a dimer of two EcfAA′T-NiaX complexes, and the data indicate that the complex has a tendency to aggregate in detergent solution.
The uptake and efflux experiments revealed that the reconstituted complexes with a 1:1:1:1 quaternary structure (EcfA:EcfA′:EcfT:S-component) mediated ATP-dependent transport without the need for any soluble substrate-binding domains, which are employed by classical ABC import proteins. Thus, the ECF-type ABC transporters described here represent the smallest functional unit of any ABC importer found so far, with a total molecular mass of around 120 kDa. The use of eight different S-components and a single energizing module further adds to the minimalist properties of these transporters.
Supplementary Material
Acknowledgments
We thank Wim Huibers for help with the mass spectrometry, Michael Verhoeven and Pranav Puri for help with cloning, and Bert Poolman for critical reading of the manuscript.
This work was supported by a toptalent grant from the Netherlands Organisation for Scientific Research (NWO) (to J. t. B.) and a vidi grant from the NWO (to D. J. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental methods, references, Tables 1 and 2, and Fig. 1.
J. P. Birkner, A. Kocer, and B. Poolman, manuscript submitted.
- ABC
- ATP-binding cassette
- ECF
- energy coupling factor
- SEC-MALLS
- size-exclusion chromatography coupled to multi-angle laser light scattering
- SUR
- sulfonylurea receptor
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
REFERENCES
- 1. Higgins C. F. (1992) Annu. Rev. Cell Biol. 8, 67–113 [DOI] [PubMed] [Google Scholar]
- 2. Rees D. C., Johnson E., Lewinson O. (2009) Nat. Rev. Mol. Cell Biol. 10, 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson A. L., Dassa E., Orelle C., Chen J. (2008) Microbiol. Mol. Biol. Rev. 72, 317–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hebbeln P., Rodionov D. A., Alfandega A., Eitinger T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodionov D. A., Hebbeln P., Eudes A., ter Beek J., Rodionova I. A., Erkens G. B., Slotboom D. J., Gelfand M. S., Osterman A. L., Hanson A. D., Eitinger T. (2009) J. Bacteriol. 191, 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erkens G. B., Slotboom D. J. (2010) Biochemistry 49, 3203–3212 [DOI] [PubMed] [Google Scholar]
- 7. Duurkens R. H., Tol M. B., Geertsma E. R., Permentier H. P., Slotboom D. J. (2007) J. Biol. Chem. 282, 10380–10386 [DOI] [PubMed] [Google Scholar]
- 8. Zhang P., Wang J., Shi Y. (2010) Nature 468, 717–720 [DOI] [PubMed] [Google Scholar]
- 9. de Ruyter P. G., Kuipers O. P., de Vos W. M. (1996) Appl. Environ. Microbiol. 62, 3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuipers O. P., de Ruyter P. G. G. A., Kleerebezem M., de Vos W. M. (1998) J. Biotechnol. 64, 15–21 [Google Scholar]
- 11. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) J. Bact. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geertsma E. R., Poolman B. (2007) Nature Methods 4, 705–707 [DOI] [PubMed] [Google Scholar]
- 13. Wertman K. F., Wyman A. R., Botstein D. (1986) Gene 49, 253–262 [DOI] [PubMed] [Google Scholar]
- 14. Slotboom D. J., Duurkens R. H., Olieman K., Erkens G. B. (2008) Methods 46, 73–82 [DOI] [PubMed] [Google Scholar]
- 15. Wen J., Arakawa T., Philo J. S. (1996) Anal. Biochem. 240, 155–166 [DOI] [PubMed] [Google Scholar]
- 16. Kiel J. A., van den Berg M. A., Fusetti F., Poolman B., Bovenberg R. A., Veenhuis M., van der Klei I. J. (2009) Funct. Integr. Genomics 9, 167–184 [DOI] [PubMed] [Google Scholar]
- 17. Geertsma E. R., Nik Mahmood N. A., Schuurman-Wolters G. K., Poolman B. (2008) Nature Protocols 3, 256–266 [DOI] [PubMed] [Google Scholar]
- 18. Kunji E. R., Slotboom D. J., Poolman B. (2003) Biochim. Biophys. Acta 1610, 97–108 [DOI] [PubMed] [Google Scholar]
- 19. Finkenwirth F., Neubauer O., Gunzenhäuser J., Schoknecht J., Scolari S., Stöckl M., Korte T., Herrmann A., Eitinger T. (2010) Biochem. J. 431, 373–380 [DOI] [PubMed] [Google Scholar]
- 20. Bryan J., Muñoz A., Zhang X., Düfer M., Drews G., Krippeit-Drews P., Aguilar-Bryan L. (2007) Pflugers Arch. 453, 703–718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.