Abstract
We demonstrate that the twin arginine translocation (Tat) system contributes to bacterial resistance to cationic antimicrobial peptides (CAMPs). Our results show that a deletion at the tatC gene, which encodes a subunit of the Tat complex, caused Salmonella and Escherichia coli to become susceptible to protamine. We screened chromosomal loci that encode known and predicted Tat-dependent proteins and found that two N-acetylmuramoyl-l-alanine amidases, encoded by amiA and amiC, elevated bacterial resistance to protamine and α-helical peptides magainin 2 and melittin but not to β-sheet defensin HNP-1 and lipopeptide polymyxin B. Genetic analysis suggests that transcription of both amiA and amiC loci in Salmonella is up-regulated by the CpxR/CpxA two-component system when nlpE is overexpressed. A footprinting analysis reveals that CpxR protein can interact with amiA and amiC promoters at the CpxR box, which is localized between the predicted −10 and −35 regions but present on different strands in these two genes. In addition, our results show that activation of the CpxR/CpxA system can facilitate protamine resistance because nlpE overexpression elevates this resistance in the wild-type strain but not the cpxR deletion mutant. Thus, we uncover a new transcriptional regulation pathway in which the Cpx envelope stress response system modulates the integrity of the cell envelope in part by controlling peptidoglycan amidase activity, which confers bacterial resistance to protamine and α-helical CAMPs. Our studies have important implications for understanding transcriptional regulation of peptidoglycan metabolism and also provide new insights into the role of the bacterial envelope in CAMP resistance.
Keywords: Antimicrobial Peptides, Bacterial Signal Transduction, Bacterial Transcription, Cell Wall, Gene Regulation, Peptidoglycan Amidase, CpxR/CpxA Two-component System, Twin Arginine Translocation System, Transcriptional Activation
Introduction
Cationic antimicrobial peptides (CAMPs)3 are short peptides (12–50 amino acids) that carry positive charges in physiological conditions and generally contain abundant hydrophobic residues to allow for interaction with the bacterial membrane as well as other components of the bacterial envelope (1, 2). The specific interaction allows CAMPs to weaken the integrity of the inner and outer membranes and subsequently kill bacterial cells. On the other hand, bacteria have developed a number of mechanisms against CAMPs. For instance, a two-component system in Salmonella, PmrA/PmrB, mediates modification of lipopolysaccharide that reduces the negative charges in lipid A moiety, resulting in a decrease of electrostatic interactions between the outer membrane and CAMPs, thus conferring resistance to these peptides (for a recent review, see Ref. 3). Protamine, an arginine-rich 33-residue CAMP isolated from salmon sperm nuclei (4), as well as magainin 2 and melittin, both α-helical peptides from frog skin and honeybee (5, 6), have been used as model CAMPs to select chromosomal loci required for bacterial resistance to antimicrobial peptides. It is suggested that protamine may exert its bactericidal effect by disrupting cytoplasmic membrane energization (7), whereas magainin 2 and melittin induce leakage of cytoplasmic components by forming pores in the bacterial membrane (8–10).
Several independent pathways have been implicated in resistance to these peptides in the Gram-negative species Salmonella typhimurium based the following observations. (i) The PhoP/PhoQ system plays a critical role in Salmonella resistance to varied CAMPs, including protamine, magainin 2, and melittin (11, 12), mediated in part by the yqjA gene encoding an inner membrane protein of unknown function (13). Transcription of the yqjA gene is regulated in a PhoP- and CpxR-dependent manner (13, 14). The yqjA mutant is susceptible to protamine and α-helical CAMPs, including magainin 2 and melittin (13). In addition, it has been shown that the PhoP-dependent pgtE gene contributes to the resistance to several α-helical CAMPs (15). The Escherichia coli outer membrane protease, OmpT, which shares significant similarity with PgtE, has been demonstrated to confer bacterial resistance to protamine by mediating cleavage of this peptide (16). (ii) A set of sap loci, encoding factors required for potassium and oligopeptide transport, is essential for Salmonella resistance to protamine (17). The sapABCDF operon encodes a periplasmic oligopeptide-binding protein and its inner membrane transporter, which is proposed to transport protamine into the cytoplasm to yield resistance (18). (iii) The yejABEF operon, which encodes a putative ATP-binding cassette transporter, is suggested to be responsible for resistance to antimicrobial peptides with different structures, including protamine, α-helical melittin, lipopeptide polymyxin B, and β-sheet human defensins (19).
The twin arginine translocation (Tat) complex is a transporter responsible for the export of folded proteins across the cytoplasmic membrane (20). This Sec-independent system consists of three major subunits (TatA, TatB, and TatC), and is known for transporting substrates involved for various cellular activities, such as acquisition of metal ions, energy metabolism, cell wall biogenesis, and virulence (for a review, see Ref. 21). In this study, we used a mutant library containing single deletions at Salmonella loci and demonstrated that a Tat-dependent pathway is responsible for resistance to protamine and α-helical CAMPs in Salmonella. With genetic analysis of the known and predicted Tat-dependent loci, we can begin to understand a novel regulatory circuit required for CAMP resistance through modulation of the peptidoglycan metabolism in response to the envelope stress.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
All Salmonella enterica serovar Typhimurium strains were derived from the wild-type strain 14028s (Table 1). Phage P22-mediated transductions were performed as described previously (22). The E. coli Keio collection was derived from wild-type BW25113 (23). Bacteria were grown at 37 °C in Luria-Bertani (LB) broth. When necessary, antibiotics were added at final concentrations of 50 μg/ml for ampicillin, 20 μg/ml for chloramphenicol, or 50 μg/ml for kanamycin. E. coli DH5α was used as host for the preparation of plasmid DNA. E. coli BL21-Gold (Stratagene) was used for protein expression.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| S. enterica serovar Typhimurium | ||
| 14028s | Wild type | ATCC |
| YS13007 | ΔtatC | This work |
| YS13629 | ΔamiA | This work |
| YS13764 | ΔamiB | This work |
| YS13630 | ΔamiC | This work |
| YS13766 | ΔamiA ΔamiC | This work |
| YS11590 | ΔphoP | Ref. 51 |
| YS13644 | ΔcpxR | This work |
| YS13637 | ΔamiA-lacZ | This work |
| YS13640 | ΔamiC-lacZ | This work |
| YS13995 | ΔtatC ΔcpxR | This work |
| E. coli | ||
| DH5α | F−supE44 ΔlacU169 (φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Ref. 52 |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ−rph-1 Δ(rhaD-rhaB)568 hsdR514 | Ref. 23 |
| Plasmids | ||
| pKD3 | repR6Kγ ApR FRT CmR FRT | Ref. 24 |
| pKD46 | reppSC101ts ApR ParaBAD γ β exo | Ref. 24 |
| pCP20 | reppSC101ts ApR CmR cI857 λPR | Ref. 24 |
| pCE37 | repR6Kγ KmR FRT lacZY this | Ref. 25 |
| pUC19 | reppMB1 ApR | Ref. 53 |
| pUHE21–2lacIq | reppMB1 ApRlacIq | Ref. 49 |
| pET28a | repColE1KmRlacI PT7 | Novagen |
| pBAD TOPO | reppBR322 ApRaraC PBAD | Invitrogen |
| pBAD-tatC | reppBR322 ApRaraC PBADtatC | This work |
| pYS2011 | reppMB1 ApRlacIqamiA | This work |
| pYS2012 | reppMB1 ApRlacIqamiC | This work |
| pYS2131 | reppMB1 ApRlacIqyaeJ | This work |
| pYS2132 | reppMB1 ApRlacIqnlpE | This work |
| pYS2135 | repColE1KmRlacI PT7His6-cpxR | This work |
Construction of Salmonella Strains with Chromosomal Mutations and Harboring lac Gene Fusions
Strains harboring deletions were generated as described previously (24). Deletion of the amiA gene was generated using primers 1369 (5′-CTTGAACTTAATTTTCACAACTCAGGCCGTCATATGAATATCCTCCTTAG-3′) and 1370 (5′-CTTTCTGATTATCAAACCAGTGAAAATAACGTGTAGGCTGGAGCTGCTTC-3′) to amplify the CmR cassette from pKD3 and integrate the resulting PCR product into the chromosome. The same strategy was used to construct deletion of other genes: amiB with primers 1461 (5′-GTTTAGCCGATTAGCTATAAAGGTGGCGGGCATATGAATATCCTCCTTAG-3′) and 1462 (5′-CCAGCGGCGATTTGGTTCGCAAGCTGCGGGGTGTAGGCTGGAGCTGCTTC-3′); amiC with primers 1396 (5′-ATCTCTATTTAGTTTTTGCTCGGGAGAAGCCATATGAATATCCTCCTTAG-3′) and 1397 (5′-CCCGCGCAATAAACTCGCCGTCATCTCAGGGTGTAGGCTGGAGCTGCTTC-3′); cpxR with primers 1407 (5′-CGTAATTTCTGCCTCGGAGGTACGTAAACACATATGAATATCCTCCTTAG-3′) and 1408 (5′-TCCTATCATGAAGCGGAAACCATCAGATAGGTGTAGGCTGGAGCTGCTTC-3′). The CmR cassette was removed from resulting mutants using plasmid pCP20 (24), and the lac transcriptional fusion plasmid pCE37 (25) was integrated into the FLP recombination target sequence in the deleted amiA and amiC loci.
Plasmid Construction
The plasmid pYS2011 was constructed using PCR fragments containing the amiA coding region generated with primers 1327 (5′-CGGGATCCTTTTCACAACTCAGGC-3′) and 1328 (5′-CCCAAGCTTTTACCGTTTCTTCGTG-3′) and Salmonella wild-type 14028s chromosomal DNA as template, which were digested with BamHI and HindIII and then ligated between the BamHI and HindIII sites of pUHE21–2lacIq. The pYS2012 plasmid was constructed by cloning the amiC gene into the HindIII site of pUHE21–2lacIq with primers 1402 (5′-CCCAAGCTTTTGCTCGGGAGAAGC-3′) and 1403 (5′-CCCAAGCTTAACTTCTTCTCGCCAGCG-3′). The plasmid pYS2131 was constructed by cloning the yaeJ gene into BamHI and HindIII sites of pUHE21–2lacIq with primers 1413 (5′-CGGGATCCTGGCAACAGCCCTCATG-3′) and 1414 (5′-CCCAAGCTTCAATCCAGTGGACGAC-3′). The plasmid pYS2132 was constructed by cloning the nlpE gene into BamHI and HindIII sites of pUHE21–2lacIq with primers 1421 (5′-CGGGATCCATTTCATAAGGATTTTATGG-3′) and 1422 (5′-CCCAAGCTTAGTGAGTGCAATCTTTAC-3′). The plasmid pBAD-tatC was constructed by cloning the tatC gene into pBAD TOPO (Invitrogen) with primers tatC-forward (5′-GGGACCGTAAACATGGCTGTA-3′) and tatC-reverse (5′-CGGTTGTGTAAAGTCTTCAGT-3′).
Selection for Genes Required for Protamine Resistance
A Salmonella collection containing more than 1,400 single mutant strains (26) were used to screen for genes required for resistance to protamine. Strains were cultured overnight, reinoculated (1:100) in LB broth, and grown for 4 h at 37 °C. Each was diluted 105 times, and 5 μl (i.e. 102 to 103 cells, depending on growth of individual strains) were dropped onto LB agar plates containing varying concentrations (0.8–1.5 mg/ml) of protamine sulfate (MP Biomedicals) and incubated overnight to screen for sensitivity. Those strains displaying a sensitive phenotype, in comparison with an isogenic wild type, were selected and streaked onto protamine plates to confirm initial findings. A similar approach was used when screening E. coli mutants from the Keio collection, except 0.6–1.2 mg/ml protamine was used to confirm susceptible mutants.
Antimicrobial Peptide Killing Assay
Strains cultured in LB overnight were inoculated 1:100 in fresh medium and grown for 4 h. Cultures were diluted with LB broth to 1–2 × 105 bacteria/ml. Ampicillin (50 μg/ml), l-arabinose (10 mm), and IPTG (0.25 mm) were supplemented when necessary. Susceptibility to antimicrobial peptides was determined as described previously (13) with the following modifications. For protamine, 5 μl of cell suspension was plated onto LB agar plates with determined concentrations of protamine. Plates were incubated overnight at 37 °C, and the number of colony-forming units (cfu) were counted. For magainin 2 (Bachem), melittin (Bachem), and polymyxin B (Sigma), peptides were dissolved and diluted with autoclaved distilled water. 5 μl of peptide solution (concentration 10 times higher than the final concentration) were placed in wells of a microtiter 96-well plate, and 45 μl of the diluted bacterial culture (see above) was added. After a 1-h incubation at 37 °C with aeration, 20 μl was mixed with 180 μl of LB broth, and 50 μl of this solution was plated on to LB agar plates. For defensin HNP-1 (Bachem), bacterial strains were grown as described above, harvested, washed once with TSB medium-sodium phosphate buffer, pH 7.4, and diluted to 1–2 × 105 bacteria/ml in the same buffer. HNP-1 was dissolved in autoclaved distilled water and diluted in 0.01% acetic acid to a concentration 10 times higher than the final concentration. An aliquot of 5 μl of HNP-1 solution was placed in wells in a 96-well plate, and 45 μl of bacterial solution was added. After incubation for 90 min at 37 °C with aeration, 20 μl was mixed with 180 μl of LB broth, and 50 μl was plated on an LB agar plate. The percentage survival was calculated by the formula, (cfu from a plate with a given CAMP concentration/cfu from the LB plate) × 100.
Vancomycin Killing Assay
Salmonella survival after a vancomycin challenge was determined as follows. Overnight cultures were inoculated 1:5 in fresh LB broth. An appropriate inducer IPTG (0.25 mm) or l-arabinose (10 mm) was added when necessary. Strains were shaken for 4 h at 37 °C to allow for induction. Cells were diluted to ∼105 bacteria/ml and added to microtiter wells containing 0 and 0.5 mg/ml (final concentration) vancomycin (Sigma). Strains were challenged overnight with aeration at 37 °C, and survival was determined by measuring the optical density. Survival percentage was calculated as described previously (27) (i.e. A600 nm of culture with vancomycin/A600 nm of cultures without vancomycin) × 100.
Screening for the Regulator That Up-regulates amiA and amiC Transcription
Chromosomal DNA prepared from wild-type strain 14028s was digested with Sau3AI (1 unit; New England Biolabs) for 15, 30, or 45 min. The digested DNA was separated on 0.8% agarose gel, and 2–5-kb fragments were recovered and ligated to BamHI-digested pUC19 plasmid DNA. The ligation mixture was transformed into E. coli DH5α selecting for ampicillin-resistant transformants. Plasmid DNA was isolated from a pool of about 20,000 transformants (∼95% of which carried an inserted chromosomal fragment) and introduced into strains, YS13637 and YS13640, which harbored a chromosomal lac transcriptional fusion at the amiA and amiC loci, respectively. Ampicillin-resistant transformants were selected on an LB ampicillin agar plate containing X-Gal (40 μg/ml). Plasmid DNA was purified from those colonies that were darker blue than others and reintroduced into YS13637 and YS13640 by electroporation. The resulting strains were used to measure β-galactosidase activity and to compare with those that received a control plasmid pUC19. To determine the inserted fragments, the plasmids were sequenced using primers 232 (5′-GAAACAGCTATGACCATG-3′) and 233 (5′-TTCCCAGTCACGACGTTG-3′).
β-Galactosidase Assay
β-galactosidase assays were carried out in triplicate (28), and the activity (Miller units) was determined using a VERSAmax plate reader (Molecular Device). Data correspond to three independent assays conducted in duplicate, and all values are mean ± S.D.
Primer Extension
The primer extension assay was performed using primers 1472 (5′-TTAGGAGTTTAAAAGTGCTCAT-3′) for amiA and 1482 (5′-ATAAAATTTACGCTTGCACAGA-3′) for amiC as described previously (29). Total RNA was isolated from bacterial cells grown in 5 ml of LB medium containing IPTG (0.25 mm) to A600 nm 0.6 with RNAzol (Molecular Research Center) by following the manufacturer's instructions. Samples were analyzed by 6% denaturing polyacrylamide electrophoresis by comparison with DNA sequences amplified from chromosome with primers 32P-1472 and 1567 (5′-ACCAAGATTATGGCGCAAACATC-3′) for amiA, or 32P-1482 and 1484 (5′-CTTCGCCGCCGAGCAT-3′) for amiC and generated using Maxam and Gilbert A + G reactions.
Purification of His6-CpxR Protein
The Salmonella CpxR protein was fused to a His6 tag at its N terminus by PCR amplification with the primers 1512 (5′-GTTTCATATGAATAAAATCCTG-3′) and 1513 (5′-ACGCGTCGACTCATGAAGCGGAAACCAT-3′) and wild-type 14028s chromosomal DNA as template. The PCR product was digested with NdeI and SalI and then ligated between the NdeI and SalI sites of pET28a. The His6-CpxR protein was purified from E. coli BL21-Gold with His-Select nickel affinity gel (Sigma) according to the manufacturer's instructions. After purification, the fractions containing His6-CpxR protein were desalted and concentrated using an Amicon Ultra centrifugal filter (Millipore).
Electrophoretic Mobility Shift Assay (EMSA)
Primers were labeled using T4 polynucleotide kinase (New England Biolabs) and [γ-32P]ATP (PerkinElmer Life Sciences). 10 nmol of 32P-labeled DNA fragments containing 206-bp amiA and 188-bp amiC promoter regions, amplified by PCR from Salmonella chromosomes with primers 1472 and 32P-labeled 1567 and primers 1482 and 32P-labeled 1484, respectively, were incubated at room temperature for 30 min with 0, 25, or 50 pmol of His6-CpxR protein in 20 μl of an EMSA buffer consisting of 10 mm Tris-HCl (pH 7.5), 1 mm EDTA, 5 mm DTT, 10 mm NaCl, 1 mm MgCl2, and 5% glycerol. After the addition of the DNA dye solution (40% glycerol, 0.05% bromphenol blue, 0.05% xylene cyanol), the mixture was directly subjected to 4% polyacrylamide electrophoresis. Signals were detected by autoradiography.
DNase I Footprinting Assay
DNase I footprinting assays were carried out using the 206-bp amiA promoter region amplified from Salmonella chromosome with primers 32P-1567 and 1472 for the coding strand and primers 1567 and 32P-1472 for the non-coding strand and using the 188-bp amiC promoter region amplified with primers 32P-1484 and 1482 for the coding strand and primers 1484 and 32P-1482 for the non-coding strand. Approximately 25 pmol of 32P-labeled DNA and 0, 50, or 100 pmol of His6-CpxR protein were mixed in a 100-μl reaction containing 20 mm HEPES, pH 8.0, 10 mm KCl, 1 mm DTT, and 0.1 mg/ml BSA. The reaction mixture was incubated at room temperature for 20 min. Then 1 μl of 100 mm CaCl2, 1 μl of 100 mm MgCl2, and 0.005 units of DNase I (Fermentas) were added, and the mixture was incubated at room temperature for 2 min. The DNase I digestion was stopped by phenol treatment, and the DNA was precipitated. Samples were analyzed by 6% polyacrylamide electrophoresis by comparison with a DNA sequence ladder generated with the same primers using a Maxam and Gilbert A + G reaction. The site-directed mutagenesis of DNA fragments was performed by following a two-step PCR method described previously (30). The first step used the mutagenic primers and the reverse universal primer 1472 or 1482 flanking the 3′ end of the amiA or amiC promoter region. The mutagenic primers were as follows: for the CpxR box 1 mutation of amiA, 1595 (5′-ATAATGGCGATGTGTCACGTATTCACATGAAAACACATACAATTCTCATCACCAAC-3′); for the CpxR box mutation of amiC, 1598 (5′-CTTCTACCAGTTCGGTATGTGGTTCCATGTGCATTGCGCGCCCCACTAG-3′). The second step used the product of the first PCR as a primer and the forward universal primer 1567 or 1484 to yield the whole promoter region with the desired mutation. The DNase I footprinting assay was carried out as described above.
RESULTS
The Twin Arginine Translocation System Plays an Important Role in Protamine Resistance in Enteric Bacteria S. typhimurium and E. coli
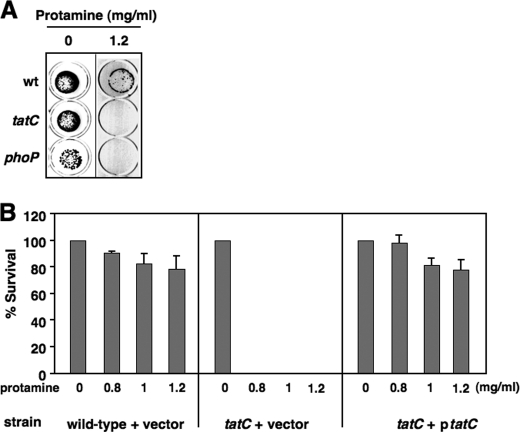
A collection of over 1,400 S. typhimurium mutants (26), each carrying a unique deletion at a chromosomal locus, was used to identify loci responsible for bacterial resistance to the CAMP protamine. Individual strains were grown in LB broth to log phase (4 h), diluted, inoculated (∼102-103 cells) onto LB agar plates with varied concentrations of protamine, and incubated at 37 °C overnight. The candidate mutants that were selected displayed increased susceptibility to protamine and thus could not grow or formed significantly fewer colonies when compared with the wild-type strain (14028s) in the presence of protamine. As expected, phoP and phoQ mutants in the collection were blindly characterized as sensitive strains from the screen because none yielded colonies on a plate supplemented with protamine (1.2 mg/ml), whereas the wild-type colonies remained at levels similar to those of the control plate without protamine (Fig. 1A) (data not shown). A strain carrying a deletion at the tatC locus, which encodes a subunit of the twin arginine translocation complex (Tat) used for protein secretion (20), exhibited increased susceptibility to protamine, because the tatC mutant cells, like the phoP mutant cells, were unable to form colonies on the protamine plate (Fig. 1A). The phenotype of the tatC mutant was solely the result of a lack of the TatC protein because it was reversed by a plasmid containing an arabinose-inducible wild-type copy of the tatC gene (pBAD-tatC) (Fig. 1B). Similarly, E. coli mutants unable to produce respective essential components of the Tat complex (i.e. TatA, TatB, and TatC) (20) were rendered susceptible to protamine (data not shown), demonstrating that the Tat system contributes to resistance of protamine in Salmonella and E. coli.
FIGURE 1.
The Tat system is required for Salmonella resistance to protamine. A, protamine susceptibility screening for wild type (wt; 14028s), tatC (YS13007), and phoP (YS11590) on LB plates containing protamine (0 or 1.2 mg/ml). B, plasmid harboring a wild-type copy of tatC restores resistance to protamine. Shown is percentage survival of wild type carrying plasmid pBAD TOPO (vector) and tatC mutant carrying plasmid vector or pBAD-tatC after incubation with protamine (0, 0.8, 1.0, or 1.2 mg/ml). Data correspond to mean values from at least two independent experiments performed in duplicate. Error bars, S.D.
Two Tat-dependent N-Acetylmuramoyl-l-alanine Amidases Contribute to Bacterial Resistance to Protamine
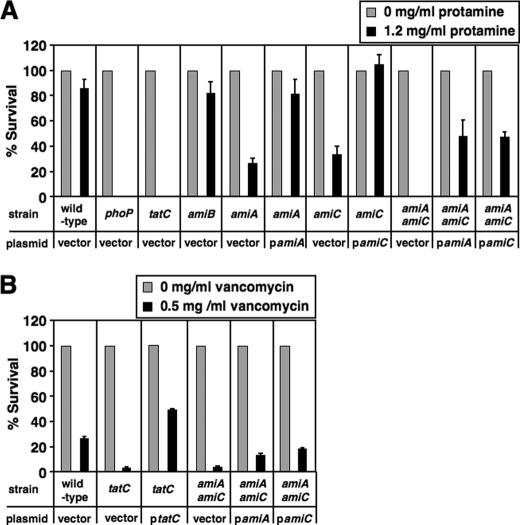
We assume that the Tat system could confer protamine resistance by mediating secretion of specific protein substrate(s); therefore, a strain deficient in the substrate production could display increased susceptibility to protamine. We used the mutants from the Keio collection, an E. coli library with in-frame, single-gene deletion mutants (23), and examined 34 individual mutants, each of which carried a deletion at one Tat-dependent locus, identified or predicted previously (31, 32). We found that the amiA and amiC mutants exhibited increased susceptibility to protamine (0.8 mg/ml), and the survival rate was reduced to 30 and 46%, respectively, when compared with their wild-type parent (BW25113). Both amiA and amiC genes encode N-acetylmuramoyl-l-alanine amidases, AmiA and AmiC, which cleave the bond between the l-alanine and N-acetylmuramoyl residues in the cell wall component peptidoglycan (33, 34). AmiA and AmiC should be translocated in Salmonella similarly as in E. coli (27, 35) because the amino acid sequence is 100% conserved between E. coli and Salmonella in the signal peptide, which is recognized by the Tat system. Therefore, we carried out one-step gene disruption procedure to construct Salmonella single mutants with deletions at the amiA and amiC genes. Similar to E. coli, both Salmonella amiA and amiC mutants displayed increased susceptibility to protamine when challenged by protamine (1.2 mg/ml), and the survival rate was 27 and 36%, respectively, when compared with wild type (85%) (Fig. 2A), confirming that Salmonella and E. coli share the mechanism of peptidoglycan amidase-dependent resistance to protamine. Resistance could be restored to wild-type levels in both amiA and amiC mutants when they harbored plasmids carrying an IPTG-inducible wild-type open reading frame (ORF) of amiA (pYS2011) and amiC (pYS2012), respectively (Fig. 2A). The tatC mutant, like a phoP mutant, was hypersensitive to protamine and was more susceptible than amiA or amiC mutant because it could not grow on the protamine-containing plate (Fig. 2A), suggesting that these Tat-dependent amidases have an additive effect on resistance to protamine. Consistent with this notion, an amiA amiC double mutant exhibited much higher susceptibility to protamine (0% survival) than either the amiA or amiC single mutant in Salmonella (Fig. 2A). The heterologous production of an amidase, either AmiA or AmiC, did not rescue the protamine resistance to a wild-type level because the susceptibility of the double mutant could only be rescued partly because the survival rate increased to 47 or 43% when AmiA or AmiC was expressed from plasmid pYS2011 (i.e. pamiA) or pYS2012 (i.e. pamiC), respectively (Fig. 2A). Our results demonstrate that the Tat-dependent peptidoglycan amidases AmiA and AmiC contribute to bacterial resistance to protamine.
FIGURE 2.
Tat-dependent amidases are required for Salmonella resistance to protamine and vancomycin. A, percentage survival of wild-type (14028s), phoP (YS11590), tatC (YS13007), amiB (YS13764), amiA (YS13629), amiC (YS13630), and amiA amiC (YS13766) strains after incubation with protamine (0 or 1.2 mg/ml). Each strain carries pUHE21 (vector). Additionally, amiA and amiA amiC each carry pamiA (pYS2011), whereas amiC and amiA amiC each carry pamiC (pYS2012). B, percentage survival of wild-type, tatC, and amiA amiC strains after incubation with vancomycin (0 or 0.5 mg/ml). Each strain carries pUHE21. Additionally, tatC mutant carries pBAD-tatC, whereas amiA amiC mutant carries pamiA or pamiC. Data correspond to mean values from at least two independent experiments performed in duplicate. Error bars, S.D.
Salmonella tatC and amiA amiC Mutants Have a Defective Outer Membrane
E. coli tatC and amiA amiC mutants displayed a pleiotropic lesion in the outer membrane and became sensitive to hydrophobic antibiotics and detergents (27, 35). We found that Salmonella tatC and amiA amiC mutants exhibited increased susceptibility to the high molecular weight antibiotic vancomycin (Fig. 2B), implying that the outer membrane of these strains, like the E. coli mutants, was defective, which allowed this antibiotic to pass through and target the cell wall. The phenotype was fully restored in the tatC mutant harboring pBAD-tatC but partly complemented in amiA amiC mutant harboring either pamiA or pamiC (Fig. 2B).
Both amiA and amiC Genes Are Required for Resistance to α-Helical Magainin 2 and Melittin but Not β-Sheet Defensin HNP-1 and Lipopeptide Polymyxin B
We examined the susceptibility of the amiA and amiC mutants to more physiologically relevant peptides belonging to various families. The amiA and amiC mutants displayed increased susceptibility to magainin 2 and melittin (data not shown), both of which can adopt amphipathic α-helical structures and form pores in bacterial cytoplasmic membrane (8, 36). Consistently, the tatC mutant exhibited hypersensitivity to magainin 2, similarly as the phoP mutant. Resistance to magainin 2 could be restored in amiA and amiC mutants by pamiA or pamiA, respectively (data not shown). The amiA amiC mutant also displayed much higher susceptibility to magainin 2 than the single mutants, and the resistance could only be partly rescued by pamiA or pamiA (data not shown). On the other hand, the amiA and amiC loci were not required for the resistance to lipopeptide polymyxin B or β-sheet defensin HNP-1 because the amiA amiC double mutant strain exhibited a wild-type resistance to these peptides, whereas the phoP mutant cells were killed (data not shown).
Transcription of the amiA and amiC Genes Is Up-regulated through nlpE Overexpression in a CpxR-dependent Manner
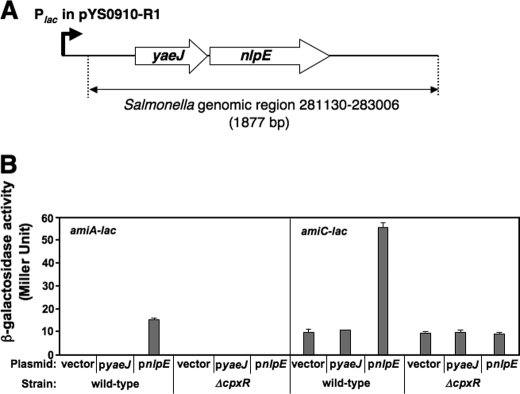
To explore the possibility that a transcriptional regulator may stimulate resistance to protamine by activating transcription of these amidase loci, we constructed Salmonella strains harboring a chromosomal lac transcriptional fusion at the amiA and amiC loci, YS13637 and YS13640, respectively, which could form pale and light blue colonies when grown on a LB agar plate supplemented with 40 μg/ml X-Gal (data not shown). We prepared a genomic library from the wild-type strain (14028s) in the multicopy number plasmid pUC19, introduced the library into YS13637 and YS13640, and cultured transformants on LB agar plates containing X-Gal. This strategy was based on the premise that overexpression of a positive transcriptional regulator from the Plac promoter in pUC19 may activate transcription of amiA-lac in YS13637 or amiC-lac in YS13640, resulting in dark blue bacterial colonies. An 1877-bp chromosomal fragment covering a genomic region from 281130 to 283006 of Salmonella typhimurium LT2 was characterized in four identical plasmids (pYS0910-R1 and others) recovered from individual dark blue colonies of the amiA-lac strain. This region carried two intact open reading frames, yaeJ and nlpE, under the control of the Plac promoter in the plasmid (Fig. 3A). These two genes, along with yaeQ, should form an operon (i.e. yaeQ-yaeJ-nlpE) in the S. typhimurium chromosome (37). To determine which gene was responsible for amiA activation, we constructed two plasmids, pYS2131 and pYS2132, which carried a wild-type copy of IPTG-inducible yaeJ and nlpE genes, respectively, and introduced them into the amiA-lac strain. β-Galactosidase activity in this strain harboring pnlpE (pYS2132), but not the vector or pyaeJ (pYS2131), significantly increased when transformants were grown in LB medium supplemented with IPTG (Fig. 3B). This result indicates that overexpression of nlpE, but not yaeJ, is able to elevate amiA transcription. Meanwhile, plasmid pYS0910-R1 was isolated again from darker blue colonies obtained from a screen using the amiC-lac strain (YS13640) in a manner analogous to that described above. β-Galactosidase activity from the amiC-lac strain harboring pnlpE grown in IPTG-containing LB medium was ∼5.6-fold higher than that harboring the vector or pyaeJ (Fig. 3B). Together, our results show that nlpE overexpression can activate transcription of both amiA and amiC.
FIGURE 3.
Overexpression of nlpE up-regulates transcription of amiA and amiC. A, Salmonella genomic region of the insert present in the plasmid, pYS0910-R1, identified as increasing transcription of amiA in the amiA-lac strain (YS13637) and amiC in the amiC-lac strain (YS13640). B, expression of amiA and amiC is up-regulated by the overexpression of nlpE, but not yaeJ, in a CpxR-dependent manner. Shown is β-galactosidase activity of amiA-lac and amiC-lac strains, each harboring pUHE21 (vector), pyaeJ (pYS2131), or pnlpE (pYS2132), grown in LB supplemented with IPTG for 4 h. Assays were conducted in triplicate. Error bars, S.D.
The nlpE gene encodes an outer membrane lipoprotein that is suggested to be involved in copper homeostasis and adhesion (38, 39); however, its biochemical function remains elusive. The fact that nlpE overexpression stimulates the regulatory activity of the CpxR/CpxA system (40) raised the possibility that amiA and amiC transcription could be activated by this two-component system. Thus, we deleted the cpxR gene from the amiA-lac and amiC-lac strains to construct their isogenic mutants, YS13731 and YS13733. In contrast to the observation from wild type, β-galactosidase activity in YS13731 and YS13733 harboring pnlpE remained as low as those harboring the empty vector (Fig. 3B). These observations collectively demonstrate that the CpxR/CpxA system up-regulates transcription of both of the Tat-dependent amidase genes, amiA and amiC.
The CpxR Protein Binds to the amiA and amiC Promoters
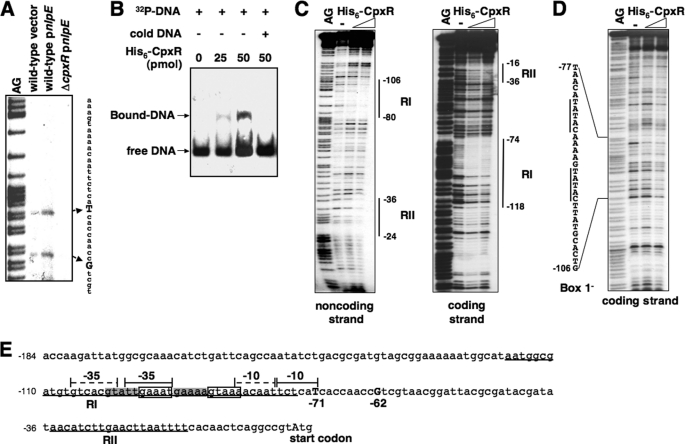
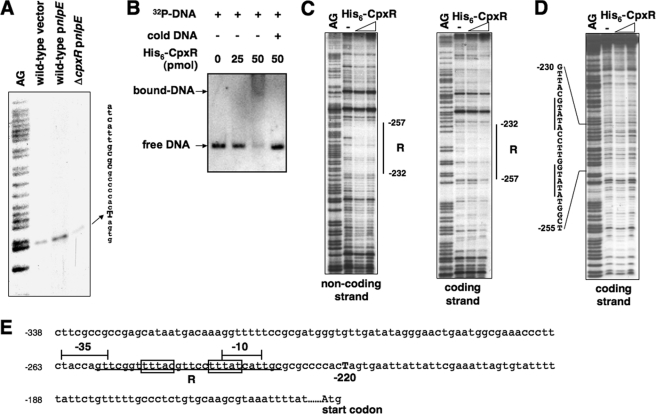
We carried out primer extension analysis to map the transcription start of the amiA and amiC genes using total mRNA samples isolated from Salmonella wild type and the cpxR mutant harboring plasmid vector or pnlpE. Two transcription products of amiA were observed from wild type harboring the vector, indicating that transcription could start at the nucleotides located 71 and 62 bp upstream of the amiA start codon (Fig. 4A; summarized in Fig. 4E). The mRNA level of both transcripts increased when nlpE was overexpressed in wild-type but not in the cpxR mutant, which further demonstrated that the CpxR/CpxA system could facilitate amiA transcription. Meanwhile, we found that amiC transcription was initiated 220 bp upstream of the putative start codon, and the transcript level increased significantly in a CpxR-dependent manner when nlpE was overexpressed (Fig. 5A; summarized in Fig. 5E), similarly to amiA transcription.
FIGURE 4.
The CpxR protein enhances amiA transcription by binding to the amiA promoter. A, primer extension for mapping the transcription start site of amiA. The cDNA products were synthesized using 32P-labeled primer 1472 and total RNA templates isolated from wild-type (14028s) carrying pUHE21 (vector) (lane 2) and pnlpE (pYS2132) (lane 3) and cpxR mutant carrying pnlpE (lane 4). Transcription starts at −71 and −62 are shown in boldface capital letters. B, EMSA. A 32P-labeled DNA fragment of wild-type amiA promoter was incubated with different amounts (0, 25, and 50 pmol) of His6-CpxR, shown in lanes 1–3. Lane 4, same as lane 3 but supplemented with “cold” amiA promoter fragment. His6-CpxR-DNA mixtures were subjected to 4% polyacrylamide electrophoresis. The location of DNA migration was detected by autoradiography. C, DNase I footprinting analysis of the wild-type amiA promoter with probes for the coding and noncoding strands and increasing amounts of His6-CpxR protein (50 and 100 pmol). Solid vertical lines correspond to the CpxR-binding sites, RI and RII. D, DNase I footprinting analysis of the CpxR box (box 1)-substituted amiA promoter with probes for the coding strand and increasing amounts of His6-CpxR protein (50 and 100 pmol). Solid vertical lines correspond to the substituted CpxR box. E, DNA sequence of the amiA promoter region. The underlines correspond to the CpxR-binding sites, RI and RII. The boxes correspond to sequences (box 1) resembling the consensus CpxR box. The putative −35 and −10 boxes for the transcription started from −62 described in A are labeled with solid braces. The highlighted sequences correspond to an alternative CpxR box (box 2), and the putative −35 and −10 regions for the transcription started from −71 are labeled with dashed braces. Numbering in C–E is from the predicted start codon of amiA.
FIGURE 5.
The CpxR protein enhances amiC transcription by binding to the amiC promoter. A, primer extension for mapping the transcription start site of amiC. The cDNA products were synthesized using 32P-labeled primer 1482 and total RNA templates isolated from wild-type (14028s) carrying pUHE21 (vector) (lane 2) and pnlpE (pYS2132) (lane 3) and cpxR mutant carrying pnlpE (lane 4). Transcription start at −220 is shown in boldface capital letters. B, EMSA. A 32P-labeled DNA fragment of the wild-type amiC promoter was incubated with different amounts (0, 25, and 50 pmol) of His6-CpxR, shown in lanes 1–3. Lane 4, same as lane 3 but supplemented with “cold” amiC promoter fragment. His6-CpxR-DNA mixtures were subjected to 4% polyacrylamide electrophoresis. The location of DNA migration was detected by autoradiography. C, DNase I footprinting analysis of the wild-type amiC promoter with probes for the coding and noncoding strands and increasing amounts of His6-CpxR protein (50 and 100 pmol). Solid vertical lines correspond to the CpxR-binding site. D, DNase I footprinting analysis of the CpxR box-substituted amiC promoter with probes for the coding strand and increasing amounts of His6-CpxR protein (50 and 100 pmol). Solid vertical lines correspond to the substituted CpxR box. E, DNA sequence of the amiC promoter region. The underline corresponds to the CpxR-binding site, R. The boxes correspond to sequences resembling the consensus CpxR box. The putative −35 and −10 boxes for the transcription start at −220 described in A are labeled with braces. Numbering in C–E is from the predicted start codon of amiC.
An EMSA was performed using purified His6-CpxR protein and 206- and 188-bp DNA fragments corresponding to an upstream region of the amiA and amiC start codons, respectively. We determined that this CpxR protein, whose phosphorylated ratio remained unknown, could shift these two DNA fragments (Figs. 4B and 5B), indicating the presence of CpxR-binding sites in the amiA and amiC promoter regions. We carried out DNase I footprinting analysis to determine the DNA sequences recognized by CpxR. The His6-CpxR protein protected the amiA promoter at the −118 to −74 and −36 to −16 regions (numbering from the amiA start codon) in the coding strand and the −106 to −80 and −36 to −24 regions in the noncoding strand (Fig. 4C). The region protected by CpxR located upstream of the amiA transcription starts (labeled as RI) includes an imperfect direct repeat sequence, 5′-GAAATN5GTAAA-3′ (from −96 to −82 boxed sequences, named box 1; Fig. 4E), which is similar to the consensus CpxR box 5′-GTAAAN5GTAAA-3′ described previously (41). It seems that the CpxR box in the amiA promoter is located upstream of the putative −10 region and partly overlaps with the putative −35 region for σ70 that initiates transcription from −62 (Fig. 4E). Furthermore, a CpxR box-like sequence, 5′-GTATTN5GAAAA-3′, is located 5 bp upstream of, and overlapping with, the CpxR box in the amiA promoter (from −101 to −87, highlighted sequences, named box 2; Fig. 4E), which is located between the alternative putative −10 and −35 regions for σ70 that initiates transcription from −71. Another region (labeled as RII), which is weakly protected by CpxR (Fig. 4C), is located downstream of the transcription start and does not contain a sequence homologous to the CpxR box (Fig. 4E). However, this weakly CpxR-protected sequence is unlikely to be involved in amiA regulation in vivo because a site-directed substitution of this sequence did not affect CpxR-dependent transcription of amiA (data not shown).
To investigate the specificity of the identified sequence for CpxR binding, we synthesized the amiA promoter fragment with substituted CpxR box sequence and carried out a footprinting assay described above. When the box 1 sequence was substituted, the CpxR protein failed to protect both the R1 and RII regions in the amiA promoter region (Fig. 4D). Thus, it is possible that CpxR either first binds to box 1 and then initiates transcription from −62 or moves from box 1 to box 2 and initiates transcription from −71.
The CpxR protein protected the amiC promoter at the −257 to −232 region of the coding strand and the −257 to −232 region of the noncoding strand (Fig. 5C). We identified a sequence, 5′-ATAAAN5GTAAA-3′, in this CpxR-protected region (labeled R), which resembles the CpxR box but is located on the opposite strand and embedded between the putative −35 and −10 regions (from −250 to −236, boxed sequences, numbering from the amiC start codon; see Fig. 5E). Also, the CpxR protein did not protect the amiC promoter region containing the substituted reverse CpxR box (Fig. 5D). Taken together, these results suggest that the CpxR box identified is essential for CpxR binding in the amiA and amiC promoters.
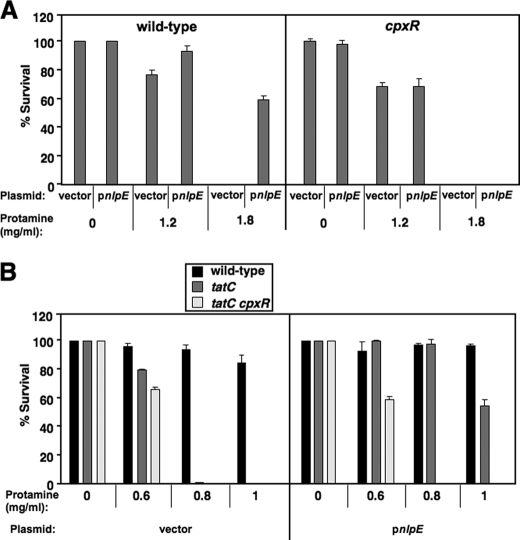
The CpxR/CpxA System Facilitates Salmonella Resistance to Protamine
We compared the survival rate of wild type harboring plasmid vector and pnlpE (pYS2132) to determine protamine resistance facilitated by nlpE overexpression. Bacterial cells with pnlpE were rescued by 61% in a plate supplemented with protamine (1.8 mg/ml), whereas those with vector were completely killed (Fig. 6A). However, a cpxR mutant harboring either vector or pnlpE was killed completely in the same protamine plate (Fig. 6A), demonstrating that the resistance stimulated by nlpE overexpression is CpxR-dependent. Interestingly, nlpE overexpression could still facilitate resistance of the tatC mutant to protamine in a CpxR-dependent manner because the tatC mutant harboring pnlpE survived by 54% in a plate supplemented with protamine (1.0 mg/ml), whereas the tatC mutant harboring vector or the tatC cpxR double mutant harboring pnlpE was completely killed (Fig. 6B). We conclude that the CpxR/CpxA system also confers protamine resistance in a Tat-independent manner. We are currently identifying the chromosomal loci contributing to this resistance.
FIGURE 6.
Overexpression of nlpE enhances Salmonella resistance to protamine in a CpxR-dependent manner. A, percentage survival of Salmonella wild-type (14028s) and cpxR (YS13644), each harboring either the pUHE21 (vector) or pnlpE (pYS2132) after incubation with protamine (0, 1.2, or 1.8 mg/ml). B, percentage survival of 14028s, tatC (YS13007), and tatC cpxR (YS13995), each harboring either pUHE21 or pnlpE after incubation with protamine (0, 0.6, 0.8, or 1.0 mg/ml). Data correspond to mean values from at least two independent experiments performed in duplicate. Error bars, S.D.
DISCUSSION
According to the results shown in this study, we present a transcriptional regulation pathway that contributes to resistance of Gram-negative bacteria Salmonella and E. coli to specific CAMPs. We demonstrate that (i) the Tat system is required for the resistance to protamine and α-helical CAMPs magainin 2 and melittin; (ii) the Tat-dependent resistance is in part mediated by two of its amidase substrates, AmiA and AmiC; (iii) the CpxR/CpxA two-component system up-regulates amiA and amiC transcription; (iv) the CpxR protein binds to divergent CpxR boxes in the amiA and amiC promoters; and (v) the CpxR/CpxA system facilitates resistance to protamine.
The Tat-dependent Cell Wall Amidases Contribute to Resistance to Protamine and α-Helical Antimicrobial Peptides in Enteric Bacteria
Enzymes that can cleave peptidoglycan and damage the integrity of bacterial cell wall play a role in cell division, growth, and cell wall remodeling. The Tat-dependent N-acetylmuramoyl-l-alanine amidases, AmiA and AmiC, catalyze cleavage of the cross-link formed by the peptide strand and polysaccharide strand of peptidoglycan (21, 33, 34). We have shown that tatC, amiA, and amiC mutants exhibit increased susceptibility to protamine and α-helical CAMPs magainin 2 and melittin (Fig. 2A) (data not shown). This phenotype is probably caused by an altered outer membrane that allows penetration of more peptides to the sites of action (e.g. cytoplasmic membrane) that are located within this barrier. This hypothesis is based on the observation that interference of the AmiA and AmiC function by either disruption of the Tat activity or mutation of the amiA and amiC genes renders the integrity of the E. coli cell envelope highly defective, resulting in increased permeability of the outer membrane to hydrophobic antibiotics and detergents (27). Consistent with this notion, both tatC and amiA amiC mutants of Salmonella displayed increased susceptibility to the antibiotic vancomycin (Fig. 2B), which targets the peptidoglycan of Gram-positive bacteria. However, this antibiotic is prohibited, probably due to its high molecular weight, by the outer membrane of Gram-negative bacteria. Furthermore, Gram-negative bacteria could be sensitized to many antibiotics when pretreated with polymyxin B nonapeptide, a polymyxin B analog that could enhance the permeability of bacterial outer membrane but not kill bacteria by itself (42).
Notably, antimicrobial peptide nisin exhibits bactericidal activity against Gram-positive bacteria by specifically binding to lipid II, a membrane-bound component involved in peptidoglycan synthesis (43). In Gram-negative bacteria, the pentapeptide moiety of the lipid II intermediates, l-alanyl-γ-d-glutamyl-diaminopimelyl-d-alanyl-d-alanine, should carry negative charges in the physiological condition due to the carboxyl group of the glutamate residue. We hypothesize that the defective outer membrane in the tatC and amidase mutants may allow more CAMP molecules to pass through and interact with the pentapeptide domain via electrostatic force. Thus, an alternative mechanism for protamine and α-helical CAMPs to implement their bactericidal activities might be to bind to the glycan chain during peptidoglycan biosynthesis in the periplasm. It is possible that a pentapeptide in the lipid II intermediate, once bound to protamine, cannot integrate into the peptidoglycan meshwork via the transpeptidation step. On the other hand, the periplasmic amidases, AmiA and AmiC, could probably cleave the pentapeptides to prevent the protamine-glycan complex accumulation, thus conferring resistance to this CAMP. It remains to be investigated why AmiA and AmiC are not required for Salmonella resistance to defensins and polymyxin B although they are also CAMPs.
Interestingly, a Salmonella mutant carrying deletion at the amiB locus displayed wild-type resistance to protamine (Fig. 2A). It is possible that AmiB, a Tat-independent periplasmic amidase (44), has a different specificity to the target bond formed between the l-alanine and N-acetylmuramoyl residues, which is located in a particular location of the peptidoglycan meshwork. Although AmiA and AmiC both contribute to resistance to CAMPs, the AmiC amidase appears to act on the peptidoglycan at the septum and is located exclusively at the septal ring during cell division, whereas the AmiA amidase can be detected throughout the periplasm (44). N-acetylmuramoyl-l-alanine amidases are widely distributed among bacterial species; therefore, the amidase-dependent mechanism characterized in this study may represent a generalized mechanism for bacterial resistance to α-helical CAMPs and probably other peptides with similar features.
The CpxR/CpxA Two-component System Contributes to Resistance to Protamine and α-Helical Antimicrobial Peptides in Enteric Bacteria
The CpxR/CpxA two-component system senses perturbations in the cell envelope and up-regulates the expression of genes encoding factors that combat the damage (for a review, see Ref. 45). In agreement with its function, we demonstrate that the CpxR/CpxA system contributes to Salmonella resistance to protamine and α-helical CAMPs in this study. Our results further show that the amiA and amiC genes are required for resistance to these peptides (Fig. 2A) (data not shown) and are up-regulated by the CpxR/CpxA system (Fig. 3B). Although the direct action of these Tat-dependent amidases is to modulate cell wall remodeling, they should play a role in the release of bacterial envelope stress because mutations of the amiA and amiC loci or the tatC locus caused a loss of integrity of the bacterial cell envelope (27). Thus, it is plausible that the CpxR/CpxA system confers the resistance to CAMPs by combating damage of the cell envelope caused by these peptides or by modifying the outer membrane to prevent CAMPs from binding.
Activation of the CpxR/CpxA system by overexpressing nlpE is still able to elevate resistance of the tatC mutant to protamine, indicating that some other CpxR-dependent loci contribute to the peptide resistance in a Tat-independent manner. One of these loci is the yqjA gene, whose transcription is up-regulated by both the PhoP/PhoQ and CpxR/CpxA systems (13, 14). The yqjA gene encodes a protein with unknown function but unlikely to be a Tat substrate and is required for magainin 2 and protamine resistance (13). In addition to the CpxR/CpxA system, other regulatory mechanism(s) should also be responsible for activation of the amiA and amiC genes. Both amiA and amiC are transcribed to similar levels in wild type and the cpxR mutant when grown in LB medium without nlpE overexpression (Fig. 3B), and the cpxR mutant does not display increased susceptibility to protamine when compared with the wild-type parent (Fig. 6A).
It is shown that CAMPs are able to induce regulatory activity of some two-component systems. A number of antimicrobial peptides, including polymyxin B and LL-37 (46), activate the PmrA/PmrB system, thus elevating resistance to these peptides in Pseudomonas aeruginosa (47). In addition, LL-37, as well as other CAMPs, was shown to induce the PhoP/PhoQ system, resulting in up-regulation of the PhoP-activated gene, phoN, in Salmonella (48). However, CAMPs are unlikely to activate the CpxR/CpxA system because β-galactosidase activity remained similar to that of the amiA-lac and amiC-lac strains grown in LB medium supplemented with or without sublethal concentrations of protamine and magainin 2 (data not shown).
The CpxR Regulator Protein Binds to the amiA and amiC Promoters via the CpxR Box
The CpxR-dependent regulation of the amiA and amiC genes is validated by identifying the CpxR-binding sites in their promoters. Although the CpxR protein used was not further phosphorylated in vitro using acetyl phosphate, the identified CpxR-binding sequences in these genes share similarity to the CpxR box, previously characterized as the CpxR-binding sequence from demonstrated or predicted CpxR-regulated genes (41). The CpxR box in both amiA and amiC promoters is localized within the −35 and −10 regions. A preliminary result showed that substitutions of the CpxR box abolished CpxR-dependent activation of amiA transcription (data not shown). However, we could not rule out the possibility that the amiA promoter was damaged due to these substitutions being located near the −35 and −10 regions. A previous study showed that the PhoP box, the consensus DNA sequence for the PhoP binding (29), was characterized from a region that overlapped with the −35 region in many PhoP-activated genes (29). Thus, it is plausible that binding of these two-component regulators to the regions located near −35 and −10 could facilitate, but not inhibit, interaction of the RNA polymerase to some specific promoter regions they regulate. On the other hand, the CpxR box, similarly to the amiA promoter, is also located between the −35 and −10 regions of the aer gene in E. coli, which mediates down-regulation of its transcription (41). Further study is ongoing to investigate how CpxR can coordinate RNA polymerase in transcription of amiA and aer.
Although the CpxR box is located in the opposite strand at the amiC promoter (Fig. 5E), its transcription is up-regulated in a CpxR-dependent manner (Fig. 3B). A comparable example was found in the P2 promoter of the rpoE operon, in which a CpxR box in the reverse direction was overlapped with the −35 for σE; however, it was responsible for negative regulation of rpoE transcription (41). According to these observations, it is likely that CpxR interacts with different σ factors, thus facilitating σ70 binding or inhibiting σE binding when it is located on the opposite strand of a promoter region. Furthermore, it has been shown that the CpxR box is located far upstream of the −35 region in three CpxR-activated loci, degP, yihE, and ppiA (50). Cumulatively, these results indicate divergent models for the CpxR regulatory function, which provide possibilities for further investigation of this stress response regulator.
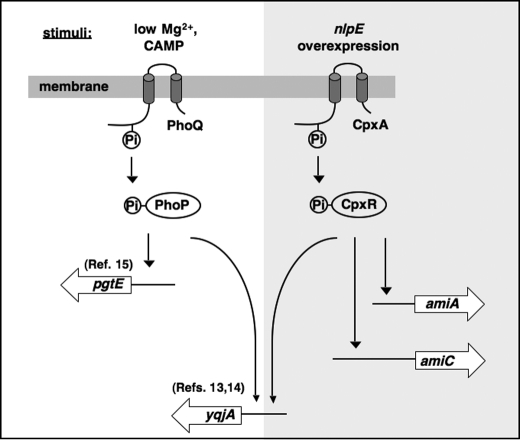
It is very interesting that bacteria such as Salmonella use multiple signaling systems to confer resistance to antimicrobial peptides. A possibility that might explain this phenomenon is that these systems can modify different cellular components in separate parts of bacterial cells under various stresses, thus protecting different targets from attack by CAMPs. In summary, we demonstrate that the CpxR/CpxA system confers Salmonella resistance to protamine and α-helical CAMPs partially dependent on the Tat pathway via amidases, AmiA and AmiC. As illustrated in Fig. 7, our findings provide new insights into the regulatory networks that contribute to bacterial resistance to antimicrobial peptides and greatly enrich our understanding of the new mechanism for envelope stress response governed by the CpxR/CpxA system.
FIGURE 7.
Model illustrating the CpxR-dependent resistance to protamine and α-helical CAMPs. In Salmonella, induction of the regulatory activity of the CpxR/CpxA system facilitates transcription of the amiA, amiC, and yqjA genes, which confers resistance to protamine and α-helical CAMPs (highlighted pathways). The yqjA gene is also activated by the PhoP/PhoQ system, which is a master signaling system controlling bacterial resistance to multiple CAMPs.
Acknowledgments
We thank Josephine Clark-Curtiss, Rajeev Misra, and Cheryl Nickerson for thoughtful discussion as well as the anonymous reviewers for helpful comments.
This work was supported by National Institutes of Health Grants U01A152237-05, R01AI07397-01, R01AI039557-11, and R01AI075093-01) (to M. M.) and R21AI083964-01, 1R01AI083646-01, 1R56AI077645, and R01 AI075093 (to H. A.-P.). This study was also supported by research funds from the Center for Infectious Diseases and Vaccinology in the Biodesign Institute (to G. Z.) and Arizona State University (to Y. S.) and National Natural Science Foundation of China Grants 30770483 and 30970646 (to Y. K.) and United States Department of Agriculture Grant AFRI CSREES 2009-03579 (to H. A.-P.).
- CAMP
- cationic antimicrobial peptide
- IPTG
- isopropyl β-d-1-thiogalactopyranoside
- Tat
- twin arginine translocation.
REFERENCES
- 1. Brogden K. A. (2005) Nat. Rev. Microbiol. 3, 238–250 [DOI] [PubMed] [Google Scholar]
- 2. Zasloff M. (2002) Nature 415, 389–395 [DOI] [PubMed] [Google Scholar]
- 3. Gunn J. S. (2008) Trends Microbiol. 16, 284–290 [DOI] [PubMed] [Google Scholar]
- 4. Ando T., Yamasaki M., Suzuki K. (1973) Mol. Biol. Biochem. Biophys. 12, 1–114 [PubMed] [Google Scholar]
- 5. Zasloff M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 5449–5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Habermann E. (1972) Science 177, 314–322 [DOI] [PubMed] [Google Scholar]
- 7. Aspedon A., Groisman E. A. (1996) Microbiology 142, 3389–3397 [DOI] [PubMed] [Google Scholar]
- 8. Yang L., Harroun T. A., Heller W. T., Weiss T. M., Huang H. W. (1998) Biophys. J. 75, 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang L., Harroun T. A., Weiss T. M., Ding L., Huang H. W. (2001) Biophys. J. 81, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee M. T., Chen F. Y., Huang H. W. (2004) Biochemistry 43, 3590–3599 [DOI] [PubMed] [Google Scholar]
- 11. Fields P. I., Groisman E. A., Heffron F. (1989) Science 243, 1059–1062 [DOI] [PubMed] [Google Scholar]
- 12. Gunn J. S., Miller S. I. (1996) J. Bacteriol. 178, 6857–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi Y., Cromie M. J., Hsu F. F., Turk J., Groisman E. A. (2004) Mol. Microbiol. 53, 229–241 [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto K., Ishihama A. (2006) Biosci. Biotechnol. Biochem. 70, 1688–1695 [DOI] [PubMed] [Google Scholar]
- 15. Guina T., Yi E. C., Wang H., Hackett M., Miller S. I. (2000) J. Bacteriol. 182, 4077–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stumpe S., Schmid R., Stephens D. L., Georgiou G., Bakker E. P. (1998) J. Bacteriol. 180, 4002–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parra-Lopez C., Lin R., Aspedon A., Groisman E. A. (1994) EMBO J. 13, 3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parra-Lopez C., Baer M. T., Groisman E. A. (1993) EMBO J. 12, 4053–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eswarappa S. M., Panguluri K. K., Hensel M., Chakravortty D. (2008) Microbiology 154, 666–678 [DOI] [PubMed] [Google Scholar]
- 20. Berks B. C., Sargent F., Palmer T. (2000) Mol. Microbiol. 35, 260–274 [DOI] [PubMed] [Google Scholar]
- 21. Palmer T., Sargent F., Berks B. C. (2005) Trends Microbiol. 13, 175–180 [DOI] [PubMed] [Google Scholar]
- 22. Davis R. W., Bolstein D., Roth J. R. (1980) Advanced Bacterial Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 23. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellermeier C. D., Janakiraman A., Slauch J. M. (2002) Gene 290, 153–161 [DOI] [PubMed] [Google Scholar]
- 26. Santiviago C. A., Reynolds M. M., Porwollik S., Choi S. H., Long F., Andrews-Polymenis H. L., McClelland M. (2009) PLoS Pathog. 5, e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ize B., Stanley N. R., Buchanan G., Palmer T. (2003) Mol. Microbiol. 48, 1183–1193 [DOI] [PubMed] [Google Scholar]
- 28. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 29. Lejona S., Aguirre A., Cabeza M. L., García Véscovi E., Soncini F. C. (2003) J. Bacteriol. 185, 6287–6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landt O., Grunert H. P., Hahn U. (1990) Gene 96, 125–128 [DOI] [PubMed] [Google Scholar]
- 31. Palmer T., Berks B. C. (2007) in The Periplasm (Ehrmann M. ed) pp. 16–29, American Society for Microbiology Press, Washington, D. C. [Google Scholar]
- 32. Dilks K., Rose R. W., Hartmann E., Pohlschröder M. (2003) J. Bacteriol. 185, 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomioka S., Nikaido T., Miyakawa T., Matsuhashi M. (1983) J. Bacteriol. 156, 463–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heidrich C., Templin M. F., Ursinus A., Merdanovic M., Berger J., Schwarz H., de Pedro M. A., Höltje J. V. (2001) Mol. Microbiol. 41, 167–178 [DOI] [PubMed] [Google Scholar]
- 35. Stanley N. R., Findlay K., Berks B. C., Palmer T. (2001) J. Bacteriol. 183, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bechinger B. (1997) J. Membr. Biol. 156, 197–211 [DOI] [PubMed] [Google Scholar]
- 37. McClelland M., Sanderson K. E., Spieth J., Clifton S. W., Latreille P., Courtney L., Porwollik S., Ali J., Dante M., Du F., Hou S., Layman D., Leonard S., Nguyen C., Scott K., Holmes A., Grewal N., Mulvaney E., Ryan E., Sun H., Florea L., Miller W., Stoneking T., Nhan M., Waterston R., Wilson R. K. (2001) Nature 413, 852–856 [DOI] [PubMed] [Google Scholar]
- 38. Gupta S. D., Lee B. T., Camakaris J., Wu H. C. (1995) J. Bacteriol. 177, 4207–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otto K., Silhavy T. J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2287–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snyder W. B., Davis L. J., Danese P. N., Cosma C. L., Silhavy T. J. (1995) J. Bacteriol. 177, 4216–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Wulf P., McGuire A. M., Liu X., Lin E. C. (2002) J. Biol. Chem. 277, 26652–26661 [DOI] [PubMed] [Google Scholar]
- 42. Vaara M., Vaara T. (1983) Antimicrob. Agents Chemother. 24, 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bauer R., Dicks L. M. (2005) Int. J. Food Microbiol. 101, 201–216 [DOI] [PubMed] [Google Scholar]
- 44. Bernhardt T. G., de Boer P. A. (2003) Mol. Microbiol. 48, 1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raivio T. L., Silhavy T. J. (1999) Curr. Opin. Microbiol. 2, 159–165 [DOI] [PubMed] [Google Scholar]
- 46. De Yang Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., Oppenheim J. J., Chertov O. (2000) J. Exp. Med. 192, 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McPhee J. B., Lewenza S., Hancock R. E. (2003) Mol. Microbiol. 50, 205–217 [DOI] [PubMed] [Google Scholar]
- 48. Bader M. W., Sanowar S., Daley M. E., Schneider A. R., Cho U., Xu W., Klevit R. E., Le Moual H., Miller S. I. (2005) Cell 122, 461–472 [DOI] [PubMed] [Google Scholar]
- 49. Soncini F. C., Véscovi E. G., Groisman E. A. (1995) J. Bacteriol. 177, 4364–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pogliano J., Lynch A. S., Belin D., Lin E. C., Beckwith J. (1997) Genes Dev. 11, 1169–1182 [DOI] [PubMed] [Google Scholar]
- 51. Kong W., Weatherspoon N., Shi Y. (2008) J. Biol. Chem. 283, 16612–16621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hanahan D. (1983) J. Mol. Biol. 166, 557–580 [DOI] [PubMed] [Google Scholar]
- 53. Yanisch-Perron C., Vieira J., Messing J. (1985) Gene 33, 103–119 [DOI] [PubMed] [Google Scholar]