Abstract
Reversible lysine acetylation is a widespread post-translational modification controlling the activity of proteins in different subcellular compartments. We previously demonstrated that a class II histone deacetylase (HDAC), HDAC4, and a histone acetyltransferase, PCAF, associate with cardiac sarcomeres, and a class I and II HDAC inhibitor, trichostatin A, enhances contractile activity of myofilaments. In this study, we show that a class I HDAC, HDAC3, is also present at cardiac sarcomeres. By immunohistochemical and electron microscopic analyses, we found that HDAC3 was localized to the A band of sarcomeres and was capable of deacetylating myosin heavy chain (MHC) isoforms. The motor domains of both cardiac α- and β-MHC isoforms were found to be reversibly acetylated. Biomechanical studies revealed that lysine acetylation significantly decreased the Km for the actin-activated ATPase activity of both α- and β-MHC isoforms. By an in vitro motility assay, we found that lysine acetylation increased the actin sliding velocity of α-myosin by 20% and β-myosin by 36%, compared to their respective non-acetylated isoforms. Moreover, myosin acetylation was found to be sensitive to cardiac stress. During induction of hypertrophy, myosin isoform acetylation increased progressively with duration of stress stimuli, independent of isoform shift, suggesting that lysine acetylation of myosin could be an early response of myofilaments to increase contractile performance of the heart. These studies provide the first evidence for localization of HDAC3 at myofilaments and uncover a novel mechanism modulating the motor activity of cardiac MHC isoforms.
Keywords: Cardiac Hypertrophy, Histone Acetylase, Histone Deacetylase, Myosin, Signal Transduction
Introduction
Lysine acetylation is a reversible post-translational modification process in which histone acetyltransferases (HATs)2 transfer the acetyl moiety from acetyl coenzyme A to the ϵ-amino group of lysine within a protein (1). The opposite reaction is carried out by another group of enzymes called histone deacetylases (HDACs). Although these names suggest that HATs and HDACs are specific for histones, this is not the case. Recently, lysine acetylation of a multitude of non-histone proteins, including transcription factors, kinases, microtubules, and mitochondrial proteins, has been identified (2). It has also been recognized that convergence between acetylation and other post-translation modifications, such as phosphorylation, methylation, ubiquitination, and oxidation, can form multisite modification programs to regulate activity of a target protein (3).
HAT activity is intrinsic to several transcription co-activators, including p300, a functional homologue of CREB-binding protein (CBP), and p300/CBP-associated factor (PCAF). HATs are generally phosphoproteins, and their activity is regulated by different signaling kinases (4). HDACs belong to a large family of co-repressors, which can be classified into three main classes (I, II, and III), based on their homology to three biochemically and structurally different yeast histone deacetylases, Rpd3, Hda1, and Sir2, respectively (5, 6). All members of the HDAC family contain a highly homologous catalytic domain; however, sequences outside the catalytic domain are highly divergent, suggesting that these enzymes have different biological functions and a much broader substrate repertoire beyond histones. The class I HDACs (HDAC1, HDAC2, HDAC3, HDAC8, and HDAC11) are ubiquitously expressed and are generally localized to the nucleus, with the exception of HDAC3 and HDAC8, which are also present in the cytoplasm (7, 8). The class II HDACs (HDAC4, HDCA5, HDAC6, HDAC7, HDAC9, and HDAC10) are generally cytoplasmic and have the ability to shuttle in and out of the nucleus, as needed (5). The class III HDACs, also called sirtuins (SIRTs), are NAD-dependent deacetylases. In mammals, seven different SIRT isoforms (SIRT1 to -7) have been identified. They are ubiquitously expressed and are implicated in the regulation of various biological functions, including cell growth, metabolism, and genetic control of aging (9).
Myosin is a motor protein capable of converting biochemical energy (ATP) into mechanical energy by interaction with actin filaments (10). In cardiac myocytes, two MHC isoforms (α and β) are expressed. The α-MHC isoform has higher ATPase activity and accounts for a faster rate of actin sliding velocity than the β-MHC isoform with low ATPase activity. The relative distribution of two cardiac MHC isoforms is regulated developmentally and by various pathophysiological stimuli (11). In young adult rodents, the α-MHC/β-MHC isoform ratio is nearly 95:5, which changes with growth and age of the heart (11). During exercise-induced physiologic hypertrophy, there is no significant change in MHC isoform distribution, whereas during pathologic hypertrophy, β-MHC levels are elevated at the expense of the α-MHC isoform. This MHC isoform shift plays a major role in regulating the contractile function of rodents' hearts (11). However, the functional significance of MHC isoform shift in large mammals is controversial, because they express mainly one isoform, β-MHC (12). It is also not known how in a stressed heart, when the MHC isoform shift takes at least a week to manifest, an existing MHC isoform withstands the initial stages of cardiac stress. Many studies conducted with skeletal muscle MHC isoforms have indicated that endurance exercise induces myosin ATPase activity of slow muscle fibers without a change of MHC isoform distributions (13, 14). These studies have also reported that increased ATPase activity of MHC isoforms was unrelated to change in composition and/or phosphorylation of myosin light chains. However, a mechanism other than isoform shift and light chain phosphorylation regulating myosin ATPase activity is not yet understood.
We have previously demonstrated that treatment of skinned fibers with a class I and II HDAC inhibitor, TSA, increases the contractile activity of myofilaments (15). We have localized PCAF and HDAC4 to different regions of the cardiac sarcomeres and shown previously that cardiac sarcomeric proteins are acetylated at lysine residues (15). In this study, we report localization of another HDAC, HDAC3, to the A band of cardiac sarcomeres and report that the head region of MHCs is reversibly acetylated at lysine residues. We also demonstrate that this post-translational modification decreases the Km for the actin-activated ATPase of MHC isoforms and increases the motility of cardiac myosin motors.
MATERIALS AND METHODS
Antibodies
The following antibodies and conjugates were used in this study: rabbit anti-acetyl lysine (9441, Cell Signaling/Millipore; 06-933, Upstate/Millipore); goat anti-actin (sc-1616, Santa Cruz Biotechnology), mouse anti-α-actinin (A7811, Sigma); rabbit anti-HDAC3 (ab2379 and ab16047, Abcam); HDAC3 blocking peptide (ab16279, Abcam), anti-histone 2A (2578, Cell Signaling), and anti-cardiac MHCs (ab15, Abcam). All other appropriate secondary (conjugated) antibodies used here were as described before (15).
Plasmid Constructs and Reagents
Mouse α- and β-MHC cDNA constructs were a kind gift from J. Robbins (Cincinnati Children's Hospital, Cincinnati, OH). These constructs were used as a template for PCR to amplify the head domain of mouse α- and β-MHC, corresponding to amino acids 1–800. The amplified PCR fragment of each MHC was cloned in BamHI-SalI sites of pBiEx3 vector (Novagen). Active PCAF and p300 HAT domain proteins were purchased from Upstate/Millipore. All other chemicals were purchased from Sigma-Aldrich unless otherwise mentioned.
Animal Studies
Male mice (20–30 g) from CD1 strain were used for all of the animal experiments. All animal protocols followed in this study were in accordance with the University of Chicago Institutional Animal Care and Use Committee. The iodine-deficient diet purchased from Harlan Teklad (Madison, WI) contained 0.15% polythiouracil (PTU). Mice were fed with PTU diet for 6–8 weeks to replace α-MHC with the β-MHC isoform. Miniosmotic pumps (Alzet model 2002) were implanted in adult littermate mice anesthetized with ketamine (100 mg/kg body weight) and xylazine (5 mg/kg body weight). Pumps were filled with either phenylephrine (PE) or isoproterenol (ISO) or vehicle (150 mm NaCl, 1 mm acetic acid) and were set to deliver PE at 75 mg/kg/day or ISO at 8.7 mg/kg/day (16). PE pumps were placed for 14 days in mice fed the PTU diet for six weeks. ISO pumps were applied to mice fed with regular diet, and hearts were harvested at the indicated time points after agonist administration. To produce pressure overload hypertrophy, aortic banding was carried out in adult CD1 mice as described previously (17).
Myosin Extraction from the Mouse Heart
Fresh ventricular tissue from adult mouse heart was homogenized using a Polytron PT2100 tissue homogenizer in 2 ml of ice-cold myosin extraction buffer (0.3 m KCl, 0.09 m KH2PO4, 0.06 m K2HPO4, 1 mm MgCl2, 1 mm ATP, 1 mm DTT, 1 mm PMSF, and mammalian protease inhibitor). The homogenate was extracted at 4 °C on a rotator for 1 h and then centrifuged at 140,000 × g for 30 min at 4 °C. Supernatant was diluted 10-fold with ice-cold 1 mm DTT, and myosin was allowed to precipitate on ice for 1 h. Precipitated myosin was collected by spinning at 26,000 × g for 20 min at 4 °C. Pellet was resuspended in 300 μl of resuspension buffer (0.6 m KCl, 25 mm imidazole, pH 7.5, 1 mm EGTA, 4 mm MgCl2, and 1 mm DTT) (18). Protein concentration was estimated from absorbance measurement at 280 nm using an extinction coefficient of E2801% = 5.6. This myosin was used for all assays, including ATPase activity and in vitro motility assays. A crude myosin was prepared without the addition of PMSF to the buffer according to the method of Uchida et al. (19). This preparation is known to contain full-length MHC (220 kDa) and a cleaved 117-kDa fragment, which constitutes subfragment 1 (S1) of MHC.
For Western blot analysis, myosin was extracted from mouse heart ventricles using a myosin extraction buffer as above containing 50 μm TSA, 50 mm NAM, 1 mm acetyl-CoA where mentioned. Homogenates were incubated for 1 h on a rotator and cleared by centrifugation, and supernatants were used for further analyses. Myosin isoform separation was done as described before (17). S-tagged α- and β-MHC (amino acids 1–800) head domain proteins were expressed and purified from bacteria as described previously (15). Skinned fiber preparations, myofibril protein extraction, Western blot analysis, immunostaining, and immunoelectron microscopy were done as explained elsewhere (15).
In Vitro Acetylation Assay
The acetylation reaction was carried out as follows. Myosin or protein to be acetylated (25–50 μg) was incubated at 30 °C for 30–60 min in 1× HAT buffer (50 mm Tris-HCl, pH 8.0, 0.1 mm EDTA, 1 mm DTT) containing 50 μm TSA, 50 mm NAM, 1 mm acetyl-CoA, and 50 ng of PCAF/μg of myosin. This protocol was modified as per the requirement of downstream application. When the p300 HAT domain was used along with PCAF for acetylation, 25 ng of p300/μg of myosin was used. When myosin was acetylated for ATPase assays, EDTA was excluded from the reaction buffer. For ATPase and motility assays, acetylation of myosin was carried out only for 20 min to avoid losing myosin enzymatic activity. Acetylation of protein was confirmed by Western blotting for each modification of the procedure. Untreated (control) myosin was also incubated at 30 °C in the same buffer for the same incubation period as for the acetylated counterpart.
Mass Spectrometric Analysis of Acetylated Lysines
To purify in vivo acetylated MHCs, the cytoskeletal protein fraction was extracted from rat neonatal cardiomyocytes cultured for 10 days (15) with a 5-h treatment of 5 μm TSA, 50 mm NAM, and 10 μm acetyl-CoA. The acetylated MHC protein was resolved by 10% SDS-PAGE, and the appropriate band was excised from the gel. Tryptic digests of the purified myosin heavy chain isoforms were analyzed by mass spectrometry according to the method described by Chen et al. (20).
Myosin ATPase Assay
The actin-activated MgATPase activity was determined at 30 °C from the rate of Pi release in a reaction buffer containing 15 mm KCl, 6 mm MgCl2, 2 mm EGTA, 20 mm Tris-HCl, pH 7.5, 2 mm Na2ATP, 50 μg/ml acetylated or control myosin, and variable concentrations of F-actin. Incubation time was 20–25 min. The reaction was stopped by adding an equal volume of 2% SDS (21). Liberated orthophosphate (Pi) was determined according to the method of Fiske and Subbarow (22). The following equation (Michaelis-Menten-like kinetics) was used to fit experimentally measured actin-activated ATPase activity data at various actin concentrations ([A]): ATPase = Vmax [A]/Km + [A], where Vmax and Km are the maximal actin-activated ATPase activity and actin concentration corresponding to ATPase activity of Vmax/2, respectively. Least-squares estimates of Km and Vmax were obtained using the SigmaPlot® program (Systat Software Inc., San Jose, CA).
Sliding Filament Motility Assays
The portion of myosin was purified further as follows for the motility assay. 10× molar excess of chicken skeletal muscle F-actin and ATP to a final concentration of 2 mm were added to myosin prepared as above. Actin-bound dead motors were pelleted down by spinning the solution at 90,000 × g for 20 min at 4 °C. Live myosin motor in the supernatant was used for the acetylation reaction and motility assays (23). Acetylated and control myosin were concentrated 4-fold using Millipore Microcon columns. Motility assays were performed at 23 °C in flow chambers constructed of a glass slide, two strips of double-sided tape, and a nitrocellulose-coated coverslip. All reagents were prepared in AB buffer (25 mm imidazole, pH 7.5, 25 mm KCl, 1 mm EGTA, 4 mm MgCl2, and 10 mm DTT). Reagents were added to the flow chamber in 10-μl volumes in the following order: myosin (incubated for 10 min and again another 10 μl for 2 min), 1 mg/ml BSA in AB to block the surface, 200 nm TRITC-labeled phalloidin-stabilized actin, AB, and motility buffer. Motility buffer contained 2 mm ATP, 0.86 mg/ml glucose oxidase, 0.14 mg/ml catalase, 9 mg/ml glucose, and 2% methyl cellulose (400 centipoise) as a crowding agent in AB. All solutions were incubated in the flow chamber for 2 min except the assay buffer washes. A single flow cell was used to collect all of the movies for a given condition (acetylated and control). Movies were collected in epifluorescence on a Ziess Axiovert 200 microscope with an Andor Luca CCD camera. Movies were 200 frames in length, with exposures of either 0.1 or 0.25 s/frame. Three locations were selected in each movie at random for the analysis. Each of these selected areas encompassed ∼5% of the visual area of the movie. Filament was counted as moving only if it moved for at least 10 consecutive frames. This method was used starting in frame number 1 and 100 of each movie. Filaments that stalled or moved only part of the time were counted as not moving. Distances were calibrated using a micropatterned standard of known dimensions. Filament tracking was performed using ImageJ. Leading or trailing filament ends were manually tracked using the segmented line tool. The line was measured using built in software. The length was converted from pixels to μm using the calibration from the micropatterned standard. Rates were calculated by dividing the distance moved by the duration of the tracked motion (calculated by the number of frames moved multiplied by the frame rate of the particular movie). Average rates and S.D. values were then calculated. Additional analysis was performed using the Igor Pro software package. Data from the acetylated and control (untreated) motor was compared using histogram analysis and Student's t test.
Statistical Analysis
Student's t test and the analysis of excess variance were applied to calculate statistical significance between groups.
RESULTS
MHCs Are Reversibly Acetylated by HATs and HDACs
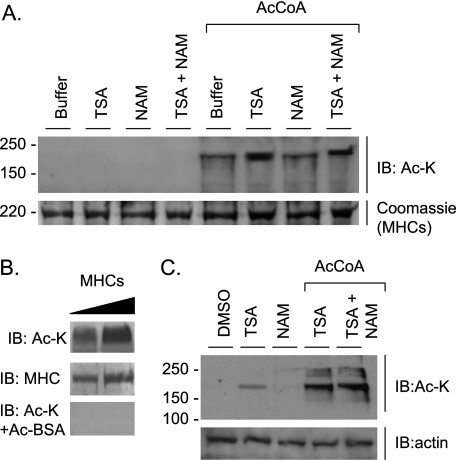
Previously, we have shown that PCAF and HDAC4 are localized to the A band of sarcomeres (15). We therefore explored whether myosin, a constituent A band protein, could be targeted for reversible lysine acetylation. We found that myosin heavy chains were acetylated when myofibrillar proteins extracted from adult mouse heart were incubated with acetyl-CoA, with or without HDAC inhibitors (Fig. 1A). In this experiment, no acetylation of myosin light chains was detected (data not shown). We confirmed the validity of MHC acetylation by blocking the acetyllysine antibody with acetylated BSA (Fig. 1B). To examine MHC acetylation in vivo, we utilized primary cultures of cardiomyocytes that were treated with the HDAC inhibitors TSA and/or NAM (a class III HDAC inhibitor). The results showed that myosin prepared from TSA-treated but not NAM-treated myocytes was substantially acetylated, thus suggesting the presence of class I and II but not class III HDACs on the sarcomeres (Fig. 1C). This MHC acetylation was further enhanced when the myosin preparation was incubated with acetyl-CoA (Fig. 1C). These results are consistent with our earlier observation showing that acetyl-CoA treatment alone is sufficient to increase contractile activity of skinned myofibers, thus confirming the presence of a HAT (PCAF) on myofilaments.
FIGURE 1.
Myosin heavy chains are acetylated at lysine residues. A, myofibrillar proteins of mouse heart (25 μg) were incubated at 30 °C for 1 h in 1× HAT buffer containing 50 μm TSA, 50 mm NAM, 1 mm acetyl-CoA (AcCoA) where mentioned. The acetylation status of MHC was determined by Western blotting with the use of an anti-Ac-K antibody. Unless otherwise mentioned, all Western blots were stained with Coomassie Brilliant Blue to verify protein loading. B, porcine β-myosin (Sigma) (2 and 4 μg) was subjected to acetylation with PCAF in a HAT buffer containing TSA, NAM, and acetyl-CoA (as described in A). MHC acetylation and protein loading were examined by immunoblotting (IB) with the use of anti-Ac-K and anti-MHC antibodies. The lower panel shows loss of acetylation signal when Ac-K antibody was first incubated with acetyl-BSA. C, neonatal rat cardiomyocyte cultures were treated with 5 μm TSA, 50 mm NAM, and 10 μm acetyl-CoA (where mentioned) for 5 h. The myofibrillar fraction was prepared and analyzed for MHC acetylation by IB. The same blot was probed with anti-actin antibody for a loading control.
HDAC3 Deacetylates MHC
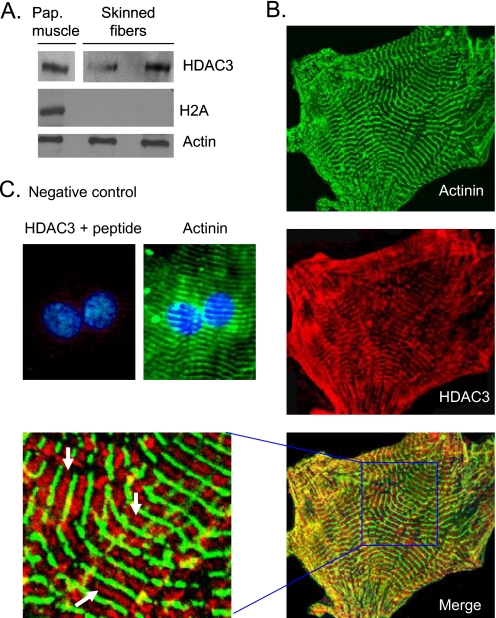
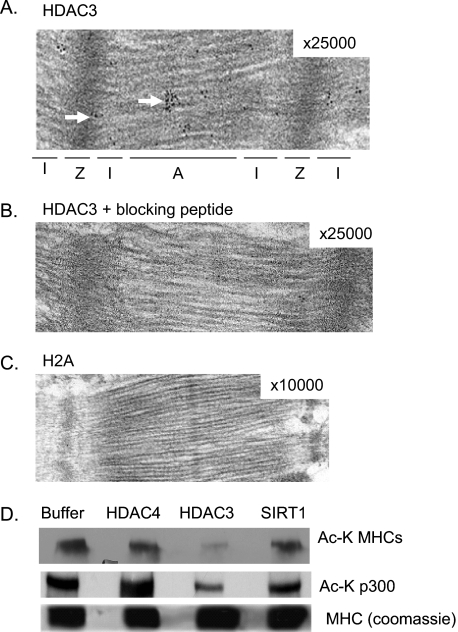
HDAC4, which was previously localized on sarcomeres, has little or no deacetylase activity of its own (24). However, the discovery that TSA (an inhibitor of class I and II HDACs) is capable of increasing contractile activity of skinned myofibers suggests that other members of the HDAC family must also be localized to cardiac sarcomeres. To identify these HDACs, we performed a Western blot analysis of skinned cardiac myofibers with the use of antibodies against different members of the HDAC family. We found that a class I HDAC, HDAC3, was associated with cardiac myofilaments (Fig. 2A). To confirm these results, we carried out immunohisto(cyto)chemical analysis of mouse heart sections and cardiomyocytes, cultured for 7 days to enhance sarcomeric organization. Confocal imaging of these cells revealed that HDAC3 is localized not only to the nucleus but also to cardiac sarcomeres, which were identified by staining of a Z disc protein α-actinin (Fig. 2B). At higher magnification, HDAC3 was found to be highly localized between two Z discs (Fig. 2B). The validity of HDAC3 staining was confirmed by use of a blocking peptide (Fig. 2C). To substantiate these results, we carried out immunoelectron microscopic analysis of mouse heart sections. The results showed that high density black dots representing HDAC3 were mostly localized to the A band, with a few scattered dots present at the Z disc, but not in the I band region (Fig. 3A). As a negative control, we stained heart sections with anti-histone 2A antibody or with HDAC3 antibody blocked with peptide, which showed no staining of sarcomeres (Fig. 3, B and C). These results thus demonstrated that HDAC3 is localized to the A band and to a lesser extent on the Z disc of sarcomeres.
FIGURE 2.
HDAC3 is localized to sarcomeres. A, a Western blot showing HDAC3 was associated with skinned fibers derived from mouse heart papillary (Pap.) muscles, whereas histone 2A (H2A) was not. Actin was used as a loading control. B, immunolocalization of HDAC3 (red) on sarcomeres of rat neonatal cardiomyocytes cultured for 7 days. α-Actinin antibody (green) was used to visualize the sarcomeric pattern of cardiomyocytes. The enlarged section of the merged picture shows HDAC3 localization between two Z discs (white arrows). C, a negative control, in which HDAC3 antibody was incubated with the blocking peptide. DAPI staining was used to localize nuclei.
FIGURE 3.
HDAC3 is localized to the A band and Z disc of sarcomeres. Shown are representative electron micrographs of mouse heart sections stained with HDAC3 antibody (A), antibody blocked with peptide (B), and histone 2A antibody (C). Numbers in the right-hand corners of pictures indicate magnification. High density black dots (arrows) indicate localization of HDAC3 to A band (A) and Z discs (Z). D, a Western blot showing deacetylation of MHCs by HDAC3. In vitro acetylated porcine β-myosin was incubated with HDAC3, SIRT1, or HDAC4 in a deacetylation buffer. Deacetylation of autoacetylated p300 in the reaction was used as a positive control. The same blot was stained with Coomassie Brilliant Blue to visualize protein loading in the reactions.
To test whether HDAC3 was capable of deacetylating A band proteins, we tested reversible acetylation of MHCs. Proteins were acetylated in vitro with PCAF and then tested for deacetylation with different HDACs, HDAC4, HDAC3, or SIRT1. The results showed that HDAC3 substantially deacetylated MHCs, whereas HDAC4 and SIRT1 did not (Fig. 3D). In this assay, deacetylation of p300 was used as an internal positive control. These results together demonstrated that reversible lysine acetylation of MHCs is regulated by PCAF and HDAC3.
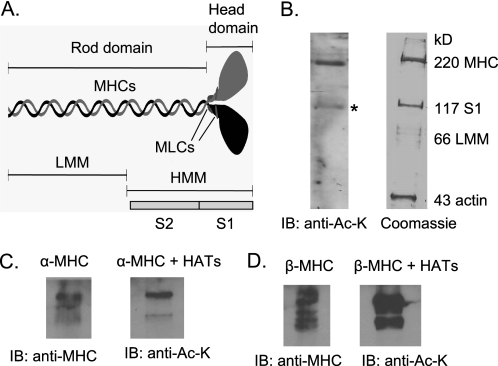
The Head Region of MHC Is Acetylated
To determine which region of myosin is acetylated (Fig. 4A), we first analyzed a preparation of adult mouse heart myosin, which was prepared without the addition of PMSF to the buffer (19). This preparation is known to contain full-length MHC (220 kDa) and a cleaved 117-kDa fragment, which constitutes S1 of MHC (Fig. 4B, right). By Western analysis with anti-acetyllysine (Ac-K) antibody, we found that both MHC bands (220 and 117 kDa) were acetylated, suggesting that the head region of MHC was a target of lysine acetylation (Fig. 4B, left). To further confirm these results, we examined acetylation of the in vitro synthesized S1 fragment of MHCs. The results showed that S1 fragments of both α- and β-MHCs (amino acids 1–800) were acetylated by the HAT PCAF (Fig. 4, C and D).
FIGURE 4.
S1 fragment of MHCs is acetylated. A, schematic representation of a myosin molecule. MLCs, myosin light chains; HMM, heavy meromyosin; LMM, light meromyosin. B, a crude preparation of adult mouse heart myosin, which contains full-length MHC (220 kDa) and a cleaved 117-kDa S1 fragment, was subjected to immunoblotting (IB) with anti-Ac-K antibody. Note that the 117 kDa band containing S1 (*) of MHC was acetylated. C and D, the S1 fragments of the mouse α- and β-MHCs were synthesized in vitro and subjected to acetylation with PCAF. Acetylation of S1 fragments was examined by immunoblotting with use of the indicated antibodies.
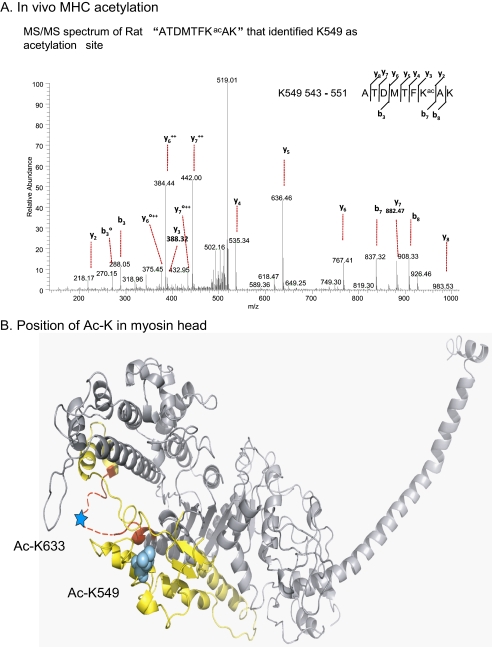
To identify acetylated lysine residues of S1, we carried out mass spectrometric (MS/MS) analysis of MHC acetylated in vitro and in vivo. By MS/MS analysis of cardiac β-MHC (porcine β-myosin, Sigma), acetylated in vitro, we identified Lys549 and Lys633 as acetylated residues (supplemental Fig. 1). To validate acetylation of these residues in vivo, we prepared acetylated myosin from 10-day-old rat neonatal cardiomyocytes expressing both α- and β- MHC isoforms. MHC was subjected to MS/MS analysis, which again confirmed the presence of both isoforms of myosin in the sample. We found that MHC was also acetylated at Lys549 in vivo (Fig. 5A). This residue is conserved between both α- and β-MHC isoforms among all species. However, under our culture conditions, we could not detect MHC acetylation at Lys633 in vivo. These studies confirmed acetylation of the myosin head and suggested that this post-translational modification is likely to be occurring at Lys549 and Lys633 residues, which are part of the actin-interacting surface of the myosin (Fig. 5B).
FIGURE 5.
Analysis of acetylated lysine residues in MHCs. A, annotation of representative tandem mass spectra of trypsin digested MHCs of rat cardiomyocytes acetylated in vivo (as explained under “Materials and Methods”) showing Lys549 as an acetylated residue. The degree symbol designates b or y ions with water and/or ammonia loss. B, visualization of myosin acetylation sites in a three-dimensional structure of myosin subfragment 1, closely mimicking the structure of cardiac myosin molecules examined in this study (31). The actin binding domain as defined by Geeves and Holmes (32) is composed of the yellow and red regions. Red highlights the Loop 2 region. The dotted line indicates a disordered loop that is not seen in the crystal structure. Cardiac myosin lysine 633 (supplemental Fig. 1) falls in this disordered loop, as indicated by the cyan star. Cardiac myosin lysine 549 closely aligns with skeletal lysine 551, highlighted in the cyan atom fill model. Both of these residues clearly fall within the actin-interacting surface of the myosin.
Cardiac Stress Induces MHC Acetylation
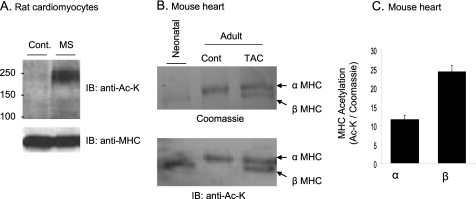
Having shown that MHCs are acetylated, we next asked whether this post-translational modification of myosin is sensitive to cardiac stress. We subjected rat cardiomyocytes to cyclic mechanical stretch for 4 h. Subsequently, cell lysate was prepared and analyzed by Western blotting with use of an anti-Ac-K antibody. The results showed significant acetylation of cardiac MHCs in mechanically stretched cells, compared with controls (Fig. 6A). We then examined MHC acetylation during pressure overload hypertrophy of the heart. Adult mice were subjected to a sham operation or thoracic aortic constriction (TAC), and MHC acetylation was examined 8 weeks postsurgery. In this experiment, neonatal rat heart expressing the β-MHC isoform was used as a positive control. As shown in Fig. 6B, sham-operated mouse hearts expressed only α-MHC, whereas the hearts with TAC expressed both α- and β-MHC isoforms, as expected. By Western blotting with anti-Ac-K antibody, both α- and β-MHC isoforms were found to be acetylated, and between two isoforms, β-MHC was acetylated more than the α-MHC isoform (Fig. 6, B and C).
FIGURE 6.
Stress induces acetylation of α- and β-MHC isoforms. A, cardiomyocytes were mechanically stretched (MS) (10%) for 4 h. Cell lysate was analyzed by Western blotting (IB) with the use of anti-Ac-K antibody. The same blot was also probed with anti-MHC antibody to verify equal protein loading. B, Coomassie-stained picture (top) of a representative gel showing separation of cardiac α- and β-MHC isoforms from neonate, adult sham-operated (cont.), and TAC-carrying mice. Bottom, the same blot was subjected to Western blotting with anti-Ac-K antibody. C, quantification of α- and β-MHC acetylation (mean ± S.D. (error bars), n = 3). β-MHC was relatively more acetylated compared to α-MHC. MS/MS, mass spectrometry.
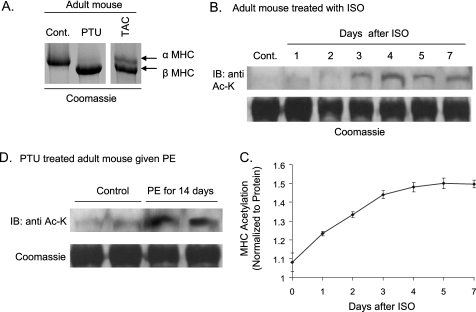
To confirm these findings and to examine stress-dependent acetylation of individual MHC isoforms, we studied young adult mice for α-MHC and PTU-treated mice for β-MHC. Mice fed with a PTU diet for 6–8 weeks had complete replacement of α-MHC with the β-MHC isoform (Fig. 7A). Adult mice were infused with a hypertrophy agonist, isoproterenol, for 1–7 days, and a time course study of MHC acetylation was carried out. ISO infusion increased lysine acetylation of α-MHC progressively reaching a maximum level by the fourth day of treatment (Fig. 7, B and C), when no shift in MHC isoforms could be detected. Similarly, β-MHC was found to be acetylated when PTU-treated mice were infused with the hypertrophy agonist PE (Fig. 7D). These experiments confirmed our observation that cardiac stress induces and modulates acetylation of both α- and β-MHC isoforms.
FIGURE 7.
During cardiac stress MHC acetylation precedes isoform shift. A, separation of cardiac MHC isoforms of control and PTU-treated mice. Expression of both α- and β-MHC isoforms in a TAC mouse was used as a reference control. B, control mice were infused with the hypertrophic agonist ISO for 7 days. Animals were sacrificed at different days after ISO infusion, their hearts were isolated, and MHCs were separated. MHC acetylation was examined by Western blotting (IB). The same blot was stained with Coomassie Brilliant Blue to verify equal protein loading. C, graphical representation of α-MHC acetylation after ISO infusion. Two or three mice were used for each time point to quantify (arbitrary units) myosin acetylation (mean ± S.D. (error bars)). D, PTU-treated mice were infused with PE for 14 days, and β-MHC acetylation was determined as in B. Results are shown for two mice in each group.
Lysine Acetylation Decreases the Km for the Actin-activated ATPase Activity of MHC Isoforms
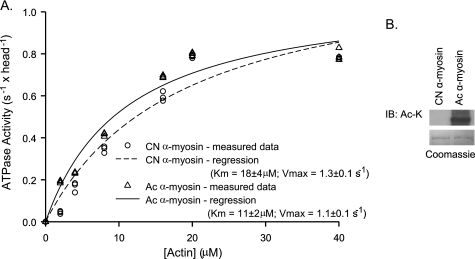
We next evaluated whether lysine acetylation could influence the actin-activated ATPase activity of myosin. Fig. 8A shows a comparison between actin-activated ATPase activity for control and PCAF-mediated in vitro acetylated α-myosin of mouse heart. In this experiment, the control and acetylated myosin for each set of experiments was derived from the same purified preparation of myosin. Enzymatic activity was measured for three independent myosin preparations. Values for Km and Vmax (mean ± S.E.) of actin-activated ATPase were as follows: Km, 18 ± 4 μm (control) and 11 ± 2 μm (acetylated); Vmax, 1.3 ± 0.1 s−1 (control) and 1.1 ± 0.1 s−1 (acetylated). The analysis of excess variance showed that although Km for acetylated myosin was significantly lower (p = 0.024), Vmax was not affected by myosin acetylation. In this ATPase assay, we also tested the effect of acetylated actin, which did not influence the actin-activated ATPase activity of myosin isoforms (data not shown). These results suggested that lysine acetylation of MHC isoforms apparently increased affinity between myosin and actin molecules. The PCAF-mediated acetylation of MHC isoform was confirmed by Western blotting (Fig. 8B).
FIGURE 8.
Myosin acetylation reduces the Km of the actin-activated ATPase activity. A, actin-activated ATPase activity of control and in vitro acetylated mouse α-MHC isoform. Activity was measured at 30 °C using 50 μg/ml α-myosin and variable concentrations of actin (2–40 μm). Data sets were corrected for myosin ATPase activity in the absence of actin and background ATPase activity of actin. The data were fitted to Michaelis-Menten-like kinetics to determine Km and Vmax. The data of three different preparations of control (CN) and acetylated (Ac) myosin are shown here. The Km value (mean ± S.E., n = 3) for control α-myosin is 18 ± 4 μm; Km for acetylated α-myosin is 11 ± 2 μm. These Km values are statistically different (p = 0.024; analysis of excess variance). The Vmax for control and acetylated myosin, however, was not significantly changed. B, a representative Western blot (IB) showing PCAF- mediated acetylation of α-myosin used in our ATPase and motility assays.
Lysine Acetylation Enhances in Vitro Motility of the Actin-Myosin Motor
Next we performed in vitro sliding filament motility assays on acetylated and control myosin to determine if the change in actin affinity altered the sliding behavior. These assays were performed using non-acetylated and in vitro acetylated myosin prepared from adult mice (α-MHC isoform) and mice treated with PTU (β-MHC isoform). Fig. 9, A and B, shows that at 2 mm ATP concentration, the acetylated pool of α-MHC isoform showed a 20% increase in sliding velocity above control, whereas for β-MHC isoform, there was a 36% increase upon acetylation. Table 1 shows the percentage change in motility for each preparation of α- and β-myosin. Student's t test showed that in both cases, the behavior of the acetylated motor population was distinct from that of the non-acetylated motor population (p = 7.3 × 10−5 for α-MHC and p = 4.04 × 10−10 for β-MHC isoform). It should be noted that the pool of acetylated MHC isoform used in this assay is likely to be a mixture of acetylated and non-acetylated myosins. If so, then that would imply that the effect of acetylation on motor activity of myosin would be higher than what we observed here.
FIGURE 9.
Acetylation increases motility rate of both α- and β-MHC isoforms in vitro. In vitro sliding filament motility assays were performed on control and acetylated forms of α- and β-MHC isoforms purified from mouse ventricles. Four independent myosin preparations were used for the assays. In all cases, the acetylation caused an increase in observed sliding filament velocity. A, a representative histogram for control (untreated) and acetylated α-MHC isoform (Ac-myosin). These populations were statistically distinct (Student's t test, p = 7.3 × 10−5). n = 40 for each population. The velocity of control α-myosin was 2.19 ± 0.1, and velocity of Ac-α-myosin was 2.64 ± 0.1 (20% increase). B, histogram of control β-myosin and Ac-β-myosin. These populations were statistically distinct (Student's t test, p = 4.04 × 10−10). n = 60 for each population. The velocity of control β-myosin was 0.87 ± 0.02, and velocity of Ac-β-myosin was 1.18 ± 0.03 (36% increase). Bin width was 0.25 μm/s.
TABLE 1.
Data for percentage change in motility rates of acetylated myosin compared with untreated controls
The first row of data was used to draw the histograms shown in Fig. 9.
| Preparation number | Ac-α-myosin % | Ac-β-myosin % |
|---|---|---|
| 1 | 20 | 36 |
| 2 | 16 | 29 |
| 3 | 31 | 29 |
| 4 | 11 | 44 |
DISCUSSION
In this study, we report two novel findings. First, HDAC3, a class I HDAC, is localized to cardiac sarcomeres, and second, cardiac MHC isoforms are reversibly acetylated at lysine residues. We also demonstrate that these acetylated lysines are present in the actin-binding interface of the myosin head domain with an apparent effect on the actin-binding affinity of the myosin motor. Studies carried out to examine functional consequences of MHC acetylation revealed that this modification increases actin sliding velocity of myosin motors. Finally, we demonstrated that lysine acetylation of both α- and β-MHC isoforms was sensitive to stress of cardiomyocytes, and it increased proportionately with the intensity of stress during hypertrophy of the heart. These studies reveal a novel post-translational mechanism that may modulate contractile function of cardiac actin-myosin motors.
Role of HDAC3 on Cardiac Sarcomeres
In our previous studies, we have demonstrated that TSA treatment was capable of enhancing contractile activity of skinned myofilaments, suggesting that members of class I and II HDACs are localized to sarcomeres. We have reported earlier that a class II HDAC, HDAC4, is localized to the Z disc and A and I bands of sarcomeres (15). Because HDAC4 has little or no intrinsic deacetylase activity (24), it was apparent from TSA experiments that other members of class I and II HDACs are also localized to sarcomeres. In this study, by use of different techniques and antibodies from different sources, we demonstrated that a class I HDAC, HDAC3, is also present at cardiac sarcomeres. However, whereas HDAC4 localizes to different regions of sarcomeres (15), we found that HDAC3 was present mainly at the A band and, to a lesser extent, at Z discs of sarcomeres and had the capability to target MHC isoforms. In this study, we also tested sarcomeric localization of other members of class I HDACs, including HDAC8, which has been shown before to bind to cytoskeletal proteins (smooth muscle α-actin and β-actin) (8); however, our results were negative, and we found no other class I HDAC binding to sarcomeres besides HDAC3.
Among class I HDACs, HDAC3 has been shown to exhibit unique properties. HDAC3 can shuttle in and out of the nucleus, whereas other class I HDACs are found primarily in the nucleus (7). HDAC3 also possesses some distinct structural features that are not present in other members of class I HDACs. The catalytic domain of HDAC3 is located much nearer to the C terminus than other HDACs, suggesting that HDAC3 might possess unique binding partners and activity (7). A recent report shows that HDAC3 localizes at the plasma membrane, where it forms a complex with the tyrosine kinase, c-Src, adding to its role outside the nucleus (25). In the nucleus, HDAC3 forms a complex with a transcriptional co-repressor, NCoR-SMRT, which also includes class II HDACs like HDAC4 and HDAC7 (26, 27). Based on this characteristic, it has been suggested that HDAC3 might be functionally related to class II HDACs. Because HDAC4 is a part of the HDAC3 complex in the nucleus and we have found previously that HADC4 is localized at sarcomeres (15), we tested the possibility that HDAC3 associates with HDAC4 and forms a complex on sarcomeres. However, even after concerted efforts, we failed to co-precipitate HDAC4 with HDAC3 from myofilaments, suggesting that both HDACs might be associating independently on sarcomeres and that they might target different proteins.
Regulation of Actin-Myosin Motor Activity by Lysine Acetylation
We observed that MHC acetylation did not alter the maximal actin-activated ATPase activity (Vmax). However, acetylation did reduce the actin-activated Km, implying that there is an increase in the apparent affinity between actin and myosin. Because acetylation also increases sliding velocity, we expect that it also decreases the lifetime of the strongly bound actomyosin state. However, because exit from this strongly bound state is not rate-limiting for the ATPase cycle, this change in lifetime would not change the maximal ATPase activity for the acetylated myosin.
In the heart, MHC isoform shift is considered a hallmark of cardiac hypertrophy (11). Although this mechanism plays an important role in regulation of contractile activity of the rodent's heart, its value in the control of the heart function of large mammals remains disputed because they express mainly one isoform, β-MHC (11). In the human heart, 2–7% α-MHC expression has been reported, but its distribution in the myocardium is not yet known (28). In rodent hearts, the α- to β-MHC ratio is ∼95:5, which is nearly opposite to the human isoform ratio. Recently, it was demonstrated that 5% β-MHC of the adult mouse heart is distributed to specific regions of the myocardium, including the base of the heart and the tip of papillary muscles (29). If a similar distribution pattern exists for 2–7% of α-MHC of the human heart, that will indicate that between two cardiac MHC isoforms, the majority of work of the adult human heart is carried out by modulation of the activity of only one isoform, which is β-MHC. How β-MHC of the human heart deals with changing work load on the myocardium, however, is not understood. Our results presented here demonstrate that under stress conditions, lysine acetylation might adjust the motor activity of the existing MHC isoform, which may be physiologically relevant for the performance of the human heart (and hearts of other large animals) during increased workload, where MHC isoform shift does not seem to play a major role (11).
There are many other studies where post-translational modification of MHCs has been suggested to regulate the motor activity of myosin molecule. Roels et al. (13) have reported that endurance training significantly increases myofilament ATPase activity of soleus muscle despite an increase in slow type MHC-I. Similar observations were noticed by others who reported increased maximal shortening velocity and ATPase activity of slow type 1 fibers of the soleus after forced exercise (14). On the other hand, during chronic hypoxia, despite the slow-to-fast transition in MHC isoform distribution, no increase in myofilament ATPase activity was noticed, suggesting that some sort of modification in MHC molecules led to the suppression of myofilament ATPase activity (13). Consistent with these findings, Kamitomo et al. (30) have reported that after chronic hypoxia, a significant decrease in MHC ATPase activity occurs in ventricles of sheep, although no change in myosin isoform is detected. These reports also indicate that changes in expression of myosin light chain isoforms and their levels of phosphorylation cannot explain the dissociation between MHC ATPase activity and MHC isoform distribution (13, 30). Exercise and hypoxia are well established stimuli affecting lysine acetylation of cellular proteins. Our data suggest that some of the effects reported earlier might be linked with lysine acetylation of myosin motors. Although we have not measured the effect of exercise or hypoxia in this study, we have found that cardiac stress induced by hypertrophic agonists increased acetylation of both cardiac MHC isoforms.
In summary, we have demonstrated that cardiac MHCs are acetylated at lysine residues of the head region, leading to a shift in the biomechanical activity of myosin motors. Based on our observations, we propose that during mild stress or the initial stages of cardiac hypertrophy where MHCs get acetylated, an increase in myosin's affinity for actin and escalation in actin sliding velocity may reflect on the consequent increase in heart function. In rodents, this post-translational modification may be followed by a shift in the MHC isoform if there is further progression of cardiac hypertrophy. However, in large mammals where MHC isoform shift is not a major event to regulate myosin ATPase activity, it is likely that MHC acetylation contributes to spatial or focused increase in the motor activity of myosin. Because the process of protein modification is more economical than an isoform shift, bypassing transcription/translation of the protein, we believe that in higher mammals, this mechanism of MHC acetylation may be better adapted to make the system more energy-efficient. Although more studies, particularly loss of function with lysine mutation, are required to establish a role of acetylation in regulation of MHC motor function, our studies lay the foundation for a novel mechanism modulating the activity of contractile proteins.
Supplementary Material
Acknowledgments
We thank Dr. J. Robbins for providing mouse α- and β-MHC cDNA plasmids. We also thank C. Labno, S. Bond, and Y. Chen for technical assistance in microscopic analyses.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL-77788 and HL-83423.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- HAT
- histone acetyltransferase
- HDAC
- histone deacetylase
- ISO
- isoproterenol
- PCAF
- p300/CBP-associated factor
- PTU
- polythiouracil
- PE
- phenylephrine
- TAC
- thoracic aortic constriction
- TSA
- trichostatin A
- NAM
- nicotinamide
- CREB
- cAMP-response element-binding protein
- CBP
- CREB-binding protein
- SIRT
- sirtuin
- S1
- subfragment 1
- Ac-K
- acetyllysine.
REFERENCES
- 1. Kouzarides T. (2000) EMBO J. 19, 1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glozak M. A., Sengupta N., Zhang X., Seto E. (2005) Gene 363, 15–23 [DOI] [PubMed] [Google Scholar]
- 3. Yang X. J., Seto E. (2008) Mol. Cell 31, 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKinsey T. A., Zhang C. L., Olson E. N. (2002) Curr. Opin. Cell Biol. 14, 763–772 [DOI] [PubMed] [Google Scholar]
- 5. de Ruijter A. J., van Gennip A. H., Caron H. N., Kemp S., van Kuilenburg A. B. (2003) Biochem. J. 370, 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khochbin S., Verdel A., Lemercier C., Seigneurin-Berny D. (2001) Curr. Opin. Genet. Dev. 11, 162–166 [DOI] [PubMed] [Google Scholar]
- 7. Yang W. M., Tsai S. C., Wen Y. D., Fejer G., Seto E. (2002) J. Biol. Chem. 277, 9447–9454 [DOI] [PubMed] [Google Scholar]
- 8. Waltregny D., Glénisson W., Tran S. L., North B. J., Verdin E., Colige A., Castronovo V. (2005) FASEB J. 19, 966–968 [DOI] [PubMed] [Google Scholar]
- 9. Blander G., Guarente L. (2004) Annu. Rev. Biochem. 73, 417–435 [DOI] [PubMed] [Google Scholar]
- 10. Ruppel K. M., Spudich J. A. (1996) Annu. Rev. Cell Dev. Biol. 12, 543–573 [DOI] [PubMed] [Google Scholar]
- 11. Gupta M. P. (2007) J. Mol. Cell Cardiol. 43, 388–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark W. A., Jr., Chizzonite R. A., Everett A. W., Rabinowitz M., Zak R. (1982) J. Biol. Chem. 257, 5449–5454 [PubMed] [Google Scholar]
- 13. Roels B., Reggiani C., Reboul C., Lionne C., Iorga B., Obert P., Tanguy S., Gibault A., Jougla A., Travers F., Millet G. P., Candau R. (2008) Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1911–R1918 [DOI] [PubMed] [Google Scholar]
- 14. Schluter J. M., Fitts R. H. (1994) Am. J. Physiol. 266, C1699–C1673 [DOI] [PubMed] [Google Scholar]
- 15. Gupta M. P., Samant S. A., Smith S. H., Shroff S. G. (2008) J. Biol. Chem. 283, 10135–10146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sundaresan N., Isbatan A., Samant S., Pillai V., Rajamohan S., Gupta M., Gupta M. (2009) Circulation 119, 3603 [Google Scholar]
- 17. Pillai J. B., Chen M., Rajamohan S. B., Samant S., Pillai V. B., Gupta M., Gupta M. P. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H1388–H1397 [DOI] [PubMed] [Google Scholar]
- 18. Jacques A. M., Briceno N., Messer A. E., Gallon C. E., Jalilzadeh S., Garcia E., Kikonda-Kanda G., Goddard J., Harding S. E., Watkins H., Esteban M. T., Tsang V. T., McKenna W. J., Marston S. B. (2008) Cardiovasc. Res. 79, 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uchida K., Murakami U., Hiratsuka T. (1977) J. Biochem. 82, 469–476 [PubMed] [Google Scholar]
- 20. Chen Y., Chen W., Cobb M. H., Zhao Y. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollard T. D. (1982) Methods Cell Biol. 24, 333–371 [DOI] [PubMed] [Google Scholar]
- 22. Fiske C. H., Subbarow Y. (1925) J. Biol. Chem. 66, 375–400 [Google Scholar]
- 23. Nagy S., Ricca B. L., Norstrom M. F., Courson D. S., Brawley C. M., Smithback P. A., Rock R. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9616–9620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahm A., Paolini C., Pallaoro M., Nardi M. C., Jones P., Neddermann P., Sambucini S., Bottomley M. J., Lo Surdo P., Carfí A., Koch U., De Francesco R., Steinkühler C., Gallinari P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 17335–17340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Longworth M. S., Laimins L. A. (2006) Oncogene 25, 4495–4500 [DOI] [PubMed] [Google Scholar]
- 26. Fischle W., Dequiedt F., Fillion M., Hendzel M. J., Voelter W., Verdin E. (2001) J. Biol. Chem. 276, 35826–35835 [DOI] [PubMed] [Google Scholar]
- 27. Fischle W., Dequiedt F., Hendzel M. J., Guenther M. G., Lazar M. A., Voelter W., Verdin E. (2002) Mol. Cell 9, 45–57 [DOI] [PubMed] [Google Scholar]
- 28. Nakao K., Minobe W., Roden R., Bristow M. R., Leinwand L. A. (1997) J. Clin. Invest. 100, 2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krenz M., Sadayappan S., Osinska H. E., Henry J. A., Beck S., Warshaw D. M., Robbins J. (2007) J. Biol. Chem. 282, 24057–24064 [DOI] [PubMed] [Google Scholar]
- 30. Kamitomo M., Onishi J., Gutierrez I., Stiffel V. M., Gilbert R. D. (2002) J. Soc. Gynecol. Investig. 9, 335–341 [PubMed] [Google Scholar]
- 31. Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. (1993) Science 261, 58–65 [DOI] [PubMed] [Google Scholar]
- 32. Geeves M. A., Holmes K. C. (1999) Annu. Rev. Biochem. 68, 687–728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.