Abstract
Ovalbumin (OVA), a non-inhibitory member of the serpin superfamily, forms fibrillar aggregates upon heat-induced denaturation. Recent studies suggested that OVA fibrils are generated by a mechanism similar to that of amyloid fibril formation, which is distinct from polymerization mechanisms proposed for other serpins. In this study, we provide new insights into the mechanism of OVA fibril formation through identification of amyloidogenic core regions using synthetic peptide fragments, site-directed mutagenesis, and limited proteolysis. OVA possesses a single disulfide bond between Cys73 and Cys120 in the N-terminal helical region of the protein. Heat treatment of disulfide-reduced OVA resulted in the formation of long straight fibrils that are distinct from the semiflexible fibrils formed from OVA with an intact disulfide. Computer predictions suggest that helix B (hB) of the N-terminal region, strand 3A, and strands 4–5B are highly β-aggregation-prone regions. These predictions were confirmed by the fact that synthetic peptides corresponding to these regions formed amyloid fibrils. Site-directed mutagenesis of OVA indicated that V41A substitution in hB interfered with the formation of fibrils. Co-incubation of a soluble peptide fragment of hB with the disulfide-intact full-length OVA consistently promoted formation of long straight fibrils. In addition, the N-terminal helical region of the heat-induced fibril of OVA was protected from limited proteolysis. These results indicate that the heat-induced fibril formation of OVA occurs by a mechanism involving transformation of the N-terminal helical region of the protein to β-strands, thereby forming sequential intermolecular linkages.
Keywords: Amyloid, Peptides, Protein Conformation, Protein Denaturation, Protein Structure, Aggregation, Ovalbumin
Introduction
The aggregation of misfolded proteins is of significant importance in biology because this phenomenon is closely related to numerous human diseases, including neurodegenerative diseases, such as Alzheimer and Parkinson diseases (1, 2). Amyloid fibril formation of denatured proteins and polymerization of the serpin family of serine protease inhibitors provide well defined structural models of neurodegenerative diseases. The serpins possess a metastable conformation that can undergo a unique conformational change involving the insertion of a proteolytically cleaved reactive center loop into the central β-sheets (3, 4), which is required for their inhibitory function. Mutations of serpins lead to their polymerization and intracellular aggregation. Polymer formation of serpins, such as α1-antitrypsin, is associated with liver cirrhosis (5), whereas that of neuroserpin (6) results in a cumulative loss of neurons and the onset of Alzheimer-like dementia.
The mechanism of serpin polymerization is currently being investigated (7–10). The best described case is provided by the polymerization of the Z variant of α1-antitrypsin (11); the Z mutation has a minimal effect on the activity or stability of the native protein (10), and the defect in secretion is due to a perturbation of the folding pathway. A recent structural study of antithrombin indicated that the serpin polymerization is well explained by a domain swap model, two long antiparallel β-strands inserting in the center of the β-sheet of the neighboring monomer (12, 13).
The egg white protein ovalbumin (OVA),2 a non-inhibitory member of the serpin superfamily (14, 15), is a useful model for the investigation of the serpin aggregation because its denaturation and folding has been extensively studied. OVA is a glycoprotein with a molecular mass of 45 kDa and contains ∼35% α-helix and ∼45% β-sheet, similar to the percentages in serpin family members. A five-stranded β-sheet runs parallel to the long axis of the molecule, whereas the reactive center loop of OVA takes the form of an exposed α-helix (16, 17) (Fig. 1a). OVA has a single heterogeneous carbohydrate chain covalently linked to Asn293. The N-glycosylated carbohydrate moiety consists of 4–6 mannose residues and 2–4 N-acetyl-β-d-glucosamine residues with total molecular mass ranging from 1.3 to 2.1 kDa. OVA possesses one solvent-accessible disulfide bond between Cys73 and Cys120 (orange spheres in Fig. 1) as well as four free sulfhydryl groups that are buried in the interior of the protein. The denaturation and refolding of OVA have been characterized in detail using disulfide rearrangement analysis (18–22). In the absence of salt, OVA conformation exhibits a nearly reversible two-state heat-induced transition with a midpoint temperature of 76 °C and reaches an almost completely unfolded state with a significant degree of secondary structure at 80 °C (23). In the presence of salt, OVA undergoes irreversible heat denaturation with the formation of semiflexible fibrillar types of aggregates (23–28).
FIGURE 1.
Schematic illustration of three-dimensional ribbon model of OVA drawn with PyMOL. a, OVA (Protein Data Bank code 1OVA (17)); b, loop-inserted structure of P1-P1′-cleaved OVA mutant R339T (Protein Data Bank code 1JTI (36)). The regions predicted to have high β-aggregation propensity are colored red, and the region included in the anti-parallel β-sheet formed in the loop-inserted conformation is colored cyan. Val41, Val175, Val327, Val342, and Val376 are shown as yellow spheres. Cys73 and Cys120 are shown as orange spheres, and Arg199 is shown as a green sphere. Numbering is based on the sequence of whole OVA.
Unlike the other serpins, the aggregation mechanism of OVA has been suggested to resemble that of amyloid fibril formation (25, 27). The typical amyloid fibril possesses the cross-β-structure (29), and the kinetics of fibril formation is often approximated to a nucleation-growth model, where the slow formation of the nucleus is the rate-limiting step of the reaction, followed by a rapid fibril extension step (30, 31). The proposed amyloid type aggregation of OVA is based on the formation of β-conformation as monitored by CD spectroscopy as well as an increase in thioflavin T fluorescence (25, 27). However, amyloid fibril formation of OVA has not been definitively confirmed because x-ray fiber diffraction did not validate the cross-β-structure, and the kinetics of fibril formation of OVA is non-nucleation-dependent (23).
The partial loop-inserted model has previously been proposed to explain the formation of a thermostabilized form of OVA (S-OVA) from native conformation under elevated pH conditions (15, 32, 33). Although this model was subsequently excluded by analyzing the crystal structure of S-OVA (34), the P1-P1′-cleaved and thermostabilized OVA mutant has been shown by crystallography to assume the fully loop-inserted conformation (35, 36). Therefore, OVA also possesses a metastable conformation like other serpins, and the intermolecular partial loop insertion model has not been completely ruled out as the mechanism for heat-induced fibril formation (37). These results suggest that the heat-induced OVA fibril is formed by a unique mechanism. Indeed, this phenomenon constitutes an intriguing model to study the aggregation mechanism of serpins.
Here, we provide new insights into the mechanism of heat-induced aggregation of OVA by identifying the core regions for the formation of fibrillar aggregates. Amyloidogenic proteins contain local regions with high β-aggregation (aggregation with β-sheet conformation) propensity, which act as a core for the formation of amyloid fibrils (38, 39). The identification of such regions facilitated the elucidation of the aggregation mechanism. Detailed characterization of the core of β2-microgloblin (β2m) amyloid fibrils at single-residue resolution has been performed by the hydrogen/deuterium exchange of amide protons combined with NMR analysis (39). However, this method is limited to proteins whose three-dimensional structure in solution can be solved by NMR and, therefore, not suitable for OVA, which has a higher molecular weight than β2m and whose NMR solution structure is unsolved. To identify the core regions for the heat-induced fibril of OVA, we carried out a systematic mapping of the core regions using synthetic peptide fragments, site-directed mutagenesis, and limited proteolysis. In the heat-denatured states of OVA, thiol-disulfide exchanges between the single disulfide bond Cys73 and Cys120 and the other four free cysteines proceed extensively (23). To avoid the thiol-disulfide exchanges and simplify analysis of the results, fibril formation of OVA was performed under disulfide-reduced conditions. The conformational property of disulfide-reduced OVA (SH-OVA) has been reported to be similar to that of intact OVA except that the heat transition temperature was 6.8 °C lower and the N-terminal helical region of SH-OVA is more flexible than that of intact OVA (40). By identifying the core regions for heat-induced aggregation of SH-OVA, we discuss the molecular mechanism for the fibril formation of OVA in the light of the aggregation of serpins.
EXPERIMENTAL PROCEDURES
Peptides and Proteins
OVA (S-OVA free) was purchased from Sigma-Aldrich, and 20 mm Tris, pH 7.5, with 20 mm NaCl was used as solvent. The disulfide-reduced OVA (SH-OVA) was prepared by incubation of intact OVA in 15 mm DTT at 37 °C for 2 h. All peptides used in this study were synthesized and purified by Genescript (Piscataway, NJ). The purities of the peptides were >95% according to HPLC and mass spectroscopy. Wild-type and mutant OVA were expressed in Escherichia coli using the expression vector pET/OVA constructed and purified as described previously (41). A single carbohydrate chain of authentic OVA is absent in recombinant OVA, but its secondary structure and biophysical properties have been shown to be the same as those of authentic OVA except for a lower Tm of heat-induced unfolding (41). A QuikChangeTM site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce mutations and to amplify the full-length plasmid. The secondary structures of the mutants have been confirmed to be identical to that of intact OVA using CD spectroscopy.
Turbidity Measurement of the Heat-induced Aggregation
A 5 mg/ml solution of authentic OVA was diluted in buffer preheated to 80 °C in the optical cell of the spectrophotometer so that the final OVA concentration was 0.5 mg/ml. The kinetics of aggregation at 80 °C was monitored through turbidity change of the solution using absorbance readings at 320 nm. The kinetics of aggregation of recombinant OVA was measured at the final concentration of 0.1 mg/ml using absorbance at 420 nm.
GPC Assay of the Concentration of Non-denatured OVA
The decrease in the concentration of non-denatured OVA under heat-denaturing conditions was determined by GPC assays as reported previously (42). A 0.2 mg/ml OVA solution was incubated at 80 °C for various periods of time and then cooled down to room temperature. The solution was then centrifuged at 15,000 × g for 5 min to remove large aggregates so that the proteins in the supernatant consisted of non-denatured OVA and small aggregates. The non-denatured OVA in solution was separated by gel filtration chromatography using a Superdex 200 column (GE Healthcare, Piscataway, NJ). The absorbance of the eluate was monitored at 280 nm. Concentration of non-denatured OVA in the supernatant was determined from the intensity of its peak in the chromatogram after normalizing peak intensity against solutions of native OVA of known concentration.
CD Spectroscopy
The secondary structure of OVA was monitored by CD spectroscopic measurement using a Jasco J-720 (Tokyo, Japan). An optical cell with a 1-mm path length was used. The far-UV spectrum at 25 °C was measured with a scan speed of 20 nm/min.
Transmission Electron Microscope (TEM)
TEM images of OVA aggregates and amyloid fibrils of peptides were acquired with a JEM-1200EX II transmission electron microscope (JEOL, Tokyo, Japan) with an acceleration voltage of 85 keV. The samples were negatively stained with 1.5–2.0% phosphotungstate adjusted to pH 7.5 using sodium hydroxide.
FTIR Spectroscopy
FTIR spectra were recorded using a Spectrum GX (PerkinElmer Life Sciences). Spectral resolution was 2 cm−1, and the spectra of 64 scans were averaged. The intact OVA and SH-OVA were dissolved in D2O, and the pD was adjusted to 7.5. The peptides were first dissolved in HFIP and then dispersed in D2O. The solution was heat-treated at 80 °C for 1 h, and the precipitates were washed twice with D2O. The spectra of protein and peptide samples dried on a CaF2 window were measured.
DSC
Calorimetric measurements were performed using Nano-DSC II Model 6100 (Calorimetry Science Co., Lindon, UT). All experiments were carried out at a scan rate of 1.0 °C/min and protein concentrations of 0.5 mg/ml in 150 mm sodium phosphate buffer, pH 7.5, 60 mm DTT. Data analysis (base-line subtraction and concentration normalization) was performed using software from Calorimetry Science.
Dynamic Light Scattering (DLS)
The size of heat-induced aggregates of OVA in solution was measured by a DLS 7000 instrument (Otsuka Electronics, Osaka, Japan) at 25 °C. The helium-neon laser light with a 630-nm wavelength was used as the light source at a set angle of 45°. OVA solution at 1.0 mg/ml was diluted 10 times with 20 mm Tris, pH 7.5, 20 mm NaCl and incubated at 80 °C for 1 h. Experimental data were analyzed using the NNLS algorithm provided by the manufacturer.
Limited Proteolysis
The core region involved in the fibril was mapped by identifying the local region that is not susceptible to proteolytic cleavage as reported previously (22, 40, 43). A 2 mg/ml solution of OVA was heat-treated at 80 °C for 1 h and then digested with trypsin for 30 min at 37 °C. The enzyme/protein concentration ratio was 1:100 (w/w). The tryptic digestion of SH-OVA fibrils was performed after removing DTT from the aggregate by centrifugation. The digestion fragments were separated by 15% SDS-PAGE before transfer onto a PVDF membrane for sequencing. The N-terminal sequence analysis was performed by a Procise 492 sequencing system (Applied Biosystems, Foster City, CA).
Prediction of Aggregation Propensity
The prediction of core regions for amyloid fibril formation of OVA was performed using three different algorithms that are freely available on the World Wide Web (44–46). The prediction by the TANGO (44) and PASTA (45) algorithms is based on the physicochemical background (i.e. calculation of the energy stabilizing the conformation of β-aggregation). TANGO calculates the partition function of the phase space encompassing the random coil and the native conformations as well as other major conformational states, namely β-turn, α-helix, and β-aggregate. The frequency of population of each structural state for five segments is assumed to be relative to its energy, which is derived from statistical and empirical considerations. PASTA calculates the pairing energy of two residues in the parallel in-register arrangement of β-strands. Two different propensity sets were extracted depending on the orientation of the neighboring strands from a data base of known native structures of globular proteins. Distinct protein molecules involved in the fibril formation are assumed to adopt the minimum energy β-pairings in order to better stabilize the cross-β-core. AGGRESCAN (46) predicts highly aggregation-prone regions based on the aggregation propensity of individual amino acids derived from in vivo experiments for amyloid β. The highly aggregation-prone regions in the sequence were identified as those protein regions at least five residues in length in which the aggregation propensity is above the average aggregation propensity of the complete sequence.
RESULTS
Effects of Disulfide Reduction on Heat-induced Fibril Formation of OVA
Effects of disulfide reduction on the heat-induced fibril formation of OVA were investigated by monitoring the progress of heat-induced aggregation of intact OVA and SH-OVA. The thioflavin T fluorescence is commonly used for the detection of amyloid fibril. However, the fluorescence intensity of thioflavin T bound to OVA fibril is very low at 80 °C, and the kinetic measurement of OVA fibril formation at elevated temperature is difficult. Therefore, we monitored the aggregation of OVA at 80 °C by turbidity measurements using a spectrophotometer. An OVA solution was diluted in buffer preheated to 80 °C in the optical cell of the spectrophotometer so that the final OVA concentration was 0.5 mg/ml. The kinetics of aggregation was monitored by measuring the turbidity change of the solution using absorbance at 320 nm. As shown in Fig. 2a, the turbidity of SH-OVA increased to a greater extent compared with that of intact OVA. This finding suggests that the heat-induced aggregate of OVA grew faster when the single disulfide bond was reduced.
FIGURE 2.
The kinetics of aggregation formation by OVA. a, the turbidity increases upon aggregation at 80 °C, as monitored by the absorbance at 320 nm. Open circle, intact OVA; closed circle, SH-OVA; open square, intact OVA in the presence of 32IAIMSA37 (1:15 molar ratio). OVA solution was diluted in 20 mm Tris, pH 7.5, 20 mm NaCl preheated to 80 °C. The final concentration of OVA was 0.5 mg/ml. b, the concentration of non-denatured OVA decreases with the process of aggregation. The relative change of non-denatured OVA concentration was estimated from the peak of native OVA by GPC analysis of heat-treated OVA solution. Open circle, intact OVA; closed circle, SH-OVA; open square, recombinant wild-type OVA under reduced conditions.
The progress of heat-induced aggregation was also monitored by the concentration change in non-denatured OVA solution as estimated from the GPC chromatogram (42). Initially, the large aggregates of OVA were separated from non-denatured protein and small aggregates by centrifugation of the heat-treated OVA solution. The non-denatured OVA in the supernatant was separated from OVA aggregates by gel filtration chromatography, and its concentration was determined from the peak intensity in the chromatogram. The decrease in the concentration of non-denatured OVA with heating time was plotted in Fig. 2b. As shown in this figure, the rate of decrease of non-denatured OVA is much faster than the turbidity increase, with the progress of the OVA aggregation shown in Fig. 2a. These results indicate that small aggregates of protein are formed in the early stage of OVA aggregation, followed by slow association between these aggregates to form larger aggregates that pellet upon centrifugation. The concentration of non-denatured SH-OVA decreased faster than that of intact OVA, indicating that reduction of the single disulfide bond accelerated the heat-induced unfolding of OVA, which is consistent with the previous report that the conformational stability of OVA is lowered by the reduction of the disulfide bond (40).
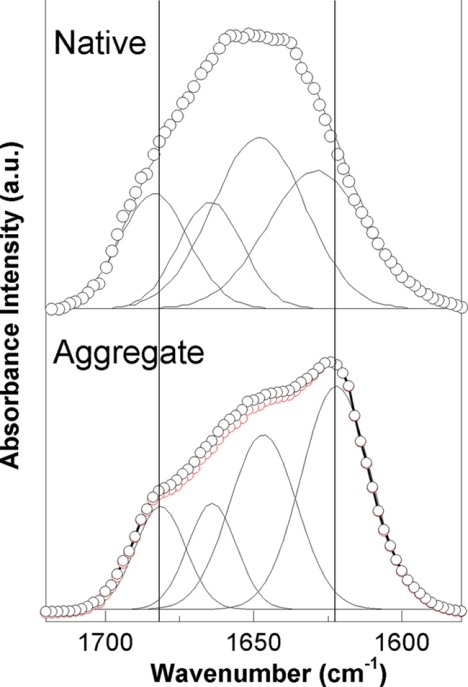
The effect of the disulfide bond reduction on the heat-induced aggregation of OVA was further examined by monitoring the morphology of heat-induced aggregates by TEM (Fig. 3). Intact OVA formed semiflexible fibrillar aggregates from heat treatment at 80 °C. Interestingly, heat treatment of SH-OVA resulted in the formation of long straight fibrils, which were distinct from the semiflexible fibrils formed by intact OVA. The secondary structures of these OVA aggregates were analyzed by measuring FTIR (Fig. 4). Native OVA has a broad amide I band with a maximum at 1650 cm−1. In contrast, heat-induced OVA aggregates produce an amide I band with a maximum at 1625 cm−1, which indicates higher β-sheet content. Curve fitting of the spectra shows that the amide I band of OVA aggregates contains β-sheet components that can be detected at 1625 and 1680 cm−1, which are different from those of native OVA that absorb at 1631 and 1684 cm−1. The different positions of the β-sheet components indicate that the aggregates do not have the same β-sheet structure as that of the native OVA conformation. The β-sheet content of OVA aggregates was estimated to be 55% from the areas of these components, which is more than that of the native conformation (45%). The profile of the amide I band of aggregates formed from SH-OVA (Fig. 4, red open circles) is almost identical to that formed from intact OVA despite its distinct morphology.
FIGURE 3.
TEM images of heat-induced fibrillar aggregates of OVA. Shown are the semiflexible fibrillar aggregates of intact OVA and the long straight fibrillar aggregates of SH-OVA. Heat-induced aggregates were obtained by heat treatment of 0.5 mg/ml OVA solution in 20 mm Tris, pH 7.5, 20 mm NaCl at 80 °C for 1 h.
FIGURE 4.
The secondary structure of native OVA and OVA aggregates monitored by FTIR spectroscopy. Top, the amide I band of native OVA; bottom, the amide I band of heat-induced aggregates obtained from intact OVA (black open circle) and SH-OVA (red open circle). Individual components from curve fitting are displayed using thin lines. The sum of the individual fittings closely overlaps with the experimental data.
Prediction of the Core Regions of OVA Fibril Formation
Amyloidogenic proteins contain local regions with high β-aggregation (aggregation with β-sheet conformation) propensity, which act as a core for the formation of amyloid fibrils (38, 39). The prediction of core regions for amyloid fibril formation has been successfully performed using algorithms that take into account the physicochemical properties of sequences and their environments (44–47). We analyzed the amino acid sequence of OVA using three different algorithms, which are all available on the World Wide Web, to identify high β-aggregation propensity regions. TANGO (44) predicted that 32IAIMSALAMVYL43, 172MVLVNAIVFK181, and 376VLFFGR381 constitute high β-aggregation propensity regions (Fig. 5). AGGRESCAN (46) also predicted that these segments of sequence represent high β-aggregation propensity regions. Similarly, PASTA (45) predicted that 172MVLVNAIVFK181 and amino acid sequence Phe364–Val383, which also included 376VLFFGR381, are β-aggregation-prone regions, whereas 32IAIMSALAMVYL43 was not predicted to have a high β-aggregation propensity. The positions of the high β-aggregation propensity regions predicted by TANGO in the three-dimensional structure of OVA are shown as red ribbons in the schematic illustrations in Fig. 1. 32IAIMSALAMVYL43 corresponds to helix B (hB) in the N-terminal region, whereas 172MVLVNAIVFK181 corresponds to strand 3A (s3A) in the central β-sheets, and amino acid sequence Phe364–Val383 includes strand 5B (s5B; 376VLFFGR381) and strand 4B (s4B; 364FLFCIK369) in the C-terminal region. It should be noted here that 32IAIMSALAMVYL43 forms a α-helix in the native conformation of OVA, whereas the other high β-aggregation propensity regions are β-strands.
FIGURE 5.
Prediction of aggregation propensity in OVA. The β-aggregation propensity according to TANGO and PASTA is plotted against the sequence of OVA. Black, TANGO prediction at 25 °C; red, TANGO prediction at 80 °C; blue, PASTA prediction.
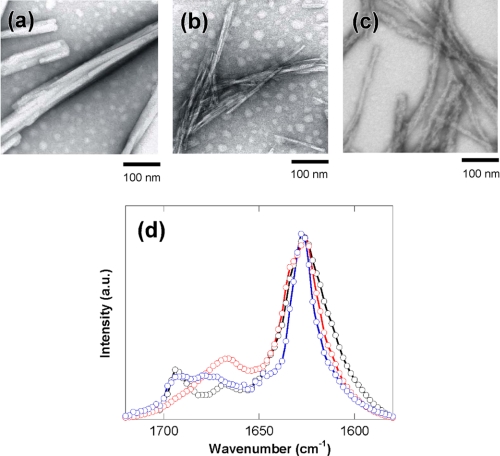
Peptide fragments predicted to have high β-aggregation propensity tend to form amyloid fibrils by themselves (48–51). Thus, a test of amyloid fibril formation of these peptides may help to verify whether the sequences are genuine core regions for fibril formation. We prepared synthetic peptides that corresponded to the predicted high β-aggregation region (Table 1) and examined the formation of fibrils. The peptide fragments were first dissolved in HFIP and then dispersed in 20 mm Tris-HCl, pH 7.5, 20 mm NaCl and heat-treated at 80 °C for 1 h. Because 32IAIMSALAMVYL43 was highly insoluble in HFIP, its half fragments 32IAIMSA37 and 38LAMVYL43 were examined instead. TEM measurements revealed that 38LAMVYL43 of hB, 172MVLVNAIVFK181 of s3A, and 364FLFCIK369 of s4B formed amyloid fibrils (Fig. 6, a–c). The FTIR measurements of these fibrillar aggregates showed the appearance of a narrow amide I band with a maximum at 1625 cm−1, which serves as a clear indicator for extensive β-sheet structure typical for amyloid-type fibrils (Fig. 6d).
TABLE 1.
Sequence of OVA peptide variants and the morphology of aggregates obtained by incubation at 80 °C
| Region | Sequence | Solubility and amyloid fibril formation |
|---|---|---|
| Amyloidogenic core regions | ||
| hB | 32IAIMSALAMVYL43 | Highly insolublea |
| 32IAIMSA37 | Soluble,b NDc | |
| 38LAMVYL43 | Amyloid | |
| s3A | 172MVLVNAIVFK181 | Amyloid |
| 172MVLV175 | Soluble, ND | |
| 178IVFK181 | Soluble, ND | |
| s4B | 364FLFCIK369 | Amyloid |
| s5B | 376VLFFGR381 | ND |
| Reactive center loop | 338GREVVGSAEAGVDA351 | Soluble, ND |
| 346EAGVDA351 | Soluble, ND | |
| 338GTEVVGSAEAGVDA351 | Soluble, ND | |
a Insoluble in aqueous solution, DMSO, and HFIP.
b Soluble, soluble in aqueous solution.
c ND, amyloid was not detected.
FIGURE 6.
Structures of amyloid fibrils of the peptide fragments predicted to have high β-aggregation propensity. Shown are TEM images of the fibrillar aggregates of 38LAMVYL43 of hB (a), 172MVLVNAIVFK181 of s3A (b), and 364FLFCIK369 of s4B (c). d, secondary structures as monitored by FTIR spectroscopy. The amide I bands of 38LAMVYL43 (black line), 172MVLVNAIVFK181 (red line), and 364FLFCIK369 (blue line) are shown in the same graph. The peptide was first dissolved in HFIP; diluted in 20 mm Tris, pH 7.5, 20 mm NaCl at a concentration of 0.5 mg/ml; and heat-treated at 80 °C for 1 h.
Although we did not find fibrillar aggregates for the peptide fragment from s5B, this result does not indicate that s5B is not a high β-aggregation propensity region. Interestingly, the s5B sequence of 376VLFFGR381 is similar to the sequence of the core region of amyloid β protein KLVFF (52). These sequences share the diphenylalanine motif, which can self-assemble into highly ordered β-sheet structures (48, 49). Although there has been some controversy as to whether KLVFF itself forms amyloid fibrils, a recent study showed that KLVFF formed amyloid fibrils under certain conditions (51). Therefore, 376VLFFGR381 may also have the potential to form amyloid fibrils under appropriate conditions. Hence, the local regions of hB, s3A, and s4–5B of OVA are β-aggregation-prone regions, as predicted by the computer algorithms.
Mutational Analysis of the Core Region of OVA Fibril
To investigate whether the predicted sequences act as the core for heat-induced fibril formation in OVA, we examined the effect of amino acid substitution in these regions. The heat-induced aggregation of recombinant OVA overexpressed in E. coli (WT-OVA) and its mutants in the reduced condition was studied. The valine residues, which are commonly found in hB, s3A, and s5B, were substituted with alanine to give V41A, V175A, and V376A, respectively. In addition, mutational analysis was also performed for the region of Leu321–Ala351 (cyan region in Fig. 1a), which includes s5A and the reactive center loop and forms anti-parallel β-sheet in the loop-inserted conformation of the P1-P1′-cleaved R339T mutant of OVA (Fig. 1b). Therefore, amino acid substitution in this region would affect fibril formation if OVA fibrils are formed by the partial loop-inserted mechanism. To investigate the contribution of this region to fibril formation, Val327 in s5A and Val342 in the reactive center loop were also substituted to alanine (V327A and V342A).
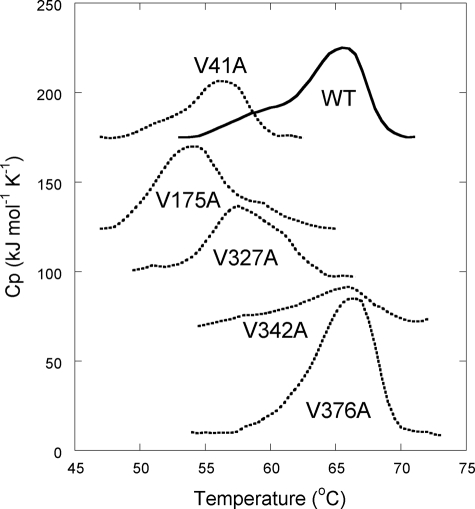
The positions of the five mutations on the three-dimensional structures of OVA are shown as yellow spheres in Fig. 1. The secondary structure of WT-OVA and the five OVA mutants in reduced conditions were confirmed to be the same as SH-OVA by CD spectroscopy. The thermal stabilities of WT-OVA and its mutants under reduced conditions were monitored by DSC (Fig. 7). The WT-OVA displayed a denaturation temperature (Tm) of 65.7 °C, ∼6 °C lower than that of SH-OVA. The Tm of V342A and V376A were the same as that of WT-OVA, whereas mutants V41A, V175A, and V327A had lower Tm values.
FIGURE 7.
Temperature dependence of the partial heat capacity of OVA mutants. For illustrative purposes, the data sets have been offset against the V376A data set. Measurements were performed at a 1 mg/ml protein concentration in 20 mm Tris, pH 7.5, 20 mm NaCl, 60 mm DTT.
The progress of heat-induced aggregation of WT-OVA was monitored by measuring the concentration of non-denatured protein estimated from the GPC chromatogram. As shown in the square plots in Fig. 2b, the monomer concentration of WT-OVA decreased at almost the same rate as that of SH-OVA, indicating that the elimination of a single carbohydrate side chain in WT-OVA did not significantly affect the rate of heat-induced unfolding of OVA. The kinetics of aggregation under reduced conditions at 80 °C was monitored by the change in turbidity of the solution. The turbidity of recombinant WT-OVA was first measured at 0.5 mg/ml (i.e. the same concentration as that used in Fig. 2a). However, the turbidity increased too rapidly and was over the range of the spectrophotometer. Therefore, the turbidity of recombinant WT-OVA was then measured at a lower concentration of 0.1 mg/ml using an absorbance at 420 nm. However, even at the lower concentration, the turbidity of WT-OVA (Fig. 8a) increased faster and to a greater extent than that of SH-OVA, as shown in Fig. 2a. Thus, the elimination of the single carbohydrate side chain significantly increased the heat-induced aggregation of OVA at elevated temperature, indicating that the carbohydrate side chain has a role in preventing the molecular association of unfolded OVA as reported previously (43).
FIGURE 8.
Effect of amino acid substitution on the heat-induced aggregation of OVA. a, kinetics of aggregation formation of the reduced recombinant OVAs. The increase in turbidity with the process of aggregation of the reduced recombinant OVAs at 80 °C was monitored by absorbance at 420 nm. b, number-weighted diameter size distribution from DLS measurements of the heat-induced aggregate of WT and V41A. The OVA solutions were first incubated in 20 mm Tris, pH 7.5, 20 mm NaCl, 15 mm DTT and then diluted in 20 mm Tris, pH 7.5, 20 mm NaCl preheated to 80 °C. The final concentration of OVA was 0.1 mg/ml.
The kinetics for turbidity of WT-OVA had a maximum at 12 min, and then the turbidity gradually decreased. The decrease in turbidity may be due to sedimentation of large aggregates formed in a later stage of the aggregation process. The kinetics of the turbidity changes in the heat-induced aggregation of V175A, V327A, V342A, and V376A were similar to that of WT-OVA, suggesting that these mutations did not significantly affect the heat-induced aggregation process of OVA. However, the turbidity change of V41A reached its maximum earlier than the others (Fig. 8a, red open circles). The size of aggregates of recombinant OVA obtained by heat treatment at 80 °C was analyzed by DLS measurements. The size of the aggregates formed by WT-OVA and all of the other mutants, except V41A, were in the range of 2–5 μm, whereas the size of heat-induced aggregates of V41A was in the range of 2–3 μm (Fig. 8b).
The morphology of heat-induced aggregates of OVA mutants formed at 80 °C under reduced conditions was monitored by TEM (Fig. 9a). Despite examining a large number of grids, fibrillar aggregates of V41A were not found; instead, amorphous aggregates were observed. In contrast, WT-OVA and the other mutants formed long straight fibrillar aggregates. These findings are consistent with the observation that relatively small aggregates were generated by V41A, as shown by DLS. To further examine the effect of V41A on OVA aggregation, secondary structures of the aggregates were analyzed by FTIR measurements (Fig. 9b). The profile of the amide I band of the V175A aggregate displayed a maximum at ∼1625 cm−1, indicating increased content of β-strand, as shown for the SH-OVA aggregate. Indeed, except for V41A, all of the other mutants showed the same amide I profile. By contrast, the intensity of the maximum at 1625 cm−1 was reduced in V41A, indicating that the β-sheet content of V41A is lower than for the other proteins. Therefore, the V41A mutation affected the conformational transition to β-strands at elevated temperature to interfere with the formation of long straight fibrils.
FIGURE 9.
Structures of amyloid fibrils of the recombinant OVAs. a, TEM images of aggregates of the recombinant OVA and its mutants V41A, V175A, and V342A. The mutants were preincubated with 15 mm DTT and then heat-treated at 80 °C for 1 h. V327A and V376A formed long straight fibrils (data not shown). b, amide I band of FTIR spectroscopy of aggregates of OVA and WT and V41A formed by heat treatment at 80 °C for 1 h in D2O in the presence of 15 mm DTT.
Effects of Peptide Fragments of OVA on Fibril Formation
It has been reported that soluble peptides corresponding to the amyloidogenic core region can prevent or trigger the formation of amyloid fibrils of proteins (52, 53). Therefore, the mechanism of heat-induced aggregation of OVA may be analyzed through investigating the effects of soluble peptide fragments. Hence, soluble fragments from the predicted core regions of OVA were designed. The solubility of several peptide fragments from the predicted amyloidogenic region of OVA was examined, and 32IAIMSA37 of hB and 172MVLV175 and 178IVFK181 of s3A were found to be soluble in aqueous solution (Table 1). We confirmed that these peptides did not form amyloid fibrils themselves when they were heat-treated in aqueous solution. The effect of these peptides on the fibril formation of OVA at 80 °C under non-reduced conditions was investigated.
As shown in the TEM image in Fig. 10, long straight fibrils were formed when OVA was co-incubated with 32IAIMSA37 (1:15 molar ratio), and they were distinct from semiflexible fibrils formed in the absence of this peptide fragment. In addition, 32IAIMSA37 of hB had a slight effect on the aggregation kinetics of OVA (Fig. 2a, open square plot). By contrast, 172MVLV175 and 178IVFK181 of s3A did not affect the morphology of OVA fibrils. The turbidity measurements showed that these peptides did not affect the aggregation kinetics of OVA at 80 °C. 32IAIMSA37 of hB induced the formation of long straight fibrils of OVA without significantly changing the aggregation kinetics, an effect on OVA fibril formation similar to that caused by reduction of the disulfide bond between Cys73 and Cys120. Therefore, the effect of 32IAIMSA37 on OVA aggregation could be explained by the interaction of 32IAIMSA37 and hB to promote a conformational change.
FIGURE 10.
TEM image of heat-induced fibrillar aggregates of OVA in the presence of the peptide fragment from hB. Heat-induced aggregates were obtained by heat treatment of 0.2 mg/ml OVA solution with the peptide 32IAIMSA37 (1:15 molar ratio) in 20 mm Tris, pH 7.5, 20 mm NaCl at 80 °C for 1 h.
A previous study on the loop insertion of serpin showed that serpin polymerization may be blocked by peptide fragments of the reactive center loop (54). We examined the effect of the peptide fragments of the reactive center loop 338GREVVGSAEAGVDA351, 346EAGVDA351, and R339T variant 338GTEVVGSAEAGVDA351 on the heat-induced fibril formation of OVA (Table 1), but the fibril morphology as monitored by TEM was unaffected by these peptides. Our results indicate that 32IAIMSA37 has a unique effect on the heat-induced fibril formation of OVA compared with other soluble peptide fragments, suggesting that the hB region is the most important core region for the heat-induced fibril formation of OVA.
Proteolytic Mapping of the Core Region of the OVA Fibril
The role of the hB region in the heat-induced fibril formation of OVA was further assessed by limited proteolysis, which has been exploited successfully to analyze aspects of fibril formation (55). Proteolytic cleavage generally occurs at flexible regions of a polypeptide chain, and the structured regions of the amyloid fibril correspond to the most highly protease-resistant region. In this study, the protease-resistant region of the OVA fibril was assessed using trypsin, which is a particularly appropriate protease for these studies because the native conformation of OVA is highly resistant to trypsin but not in its non-native conformation (22, 40, 43). The heat-induced aggregates of intact OVA and SH-OVA were treated with trypsin, and the cleavage products were separated by 15% SDS-PAGE. As shown in Fig. 11, both aggregates gave a major band with a molecular mass of ∼23 kDa. N-terminal analysis of this band gave the sequence VTEQESK, which corresponded to residues 200–206 of OVA. The calculated molecular mass of the OVA fragment from Val200 to C terminus Pro385, including the N-glycosylated carbohydrate chain, is ∼23 kDa. Thus, the major band on the SDS-PAGE can be assigned to the fragment of OVA(200–385). The molecular mass of another N-terminal fragment OVA(1–199) is calculated to be 22 kDa, which would overlap with the same band. The sequence of this peptide fragment is not detectable because the N-terminal glycine residue of OVA is acetylated. These results indicate that OVA fibrils are extensively cleaved by trypsin at Arg199, implying that the local region of s4C (Fig. 1) is in a flexible conformation in the OVA aggregates.
FIGURE 11.
Trypsin cleavage of the heat-induced fibrillar aggregate of OVA. Shown is SDS-PAGE analysis (15% gel) of the trypsin digestion products of heat-induced OVA. Lane 1, molecular mass markers (kDa); lane 2, undigested OVA; lane 3, heat-induced aggregate of intact OVA incubated with trypsin; lane 4, heat-induced aggregate of SH-OVA incubated with trypsin.
As shown in lane 4 of Fig. 11, trypsin cleavage of the SH-OVA fibril gave additional fragments, indicating that the fibril from OVA with an intact disulfide is less susceptible to proteolysis. This is presumably because thiol-disulfide exchanges arise extensively during the aggregation process of the disulfide intact OVA (23), and the conformation of the fibril is relatively rigid compared with that of SH-OVA. Blotting and N-terminal analysis of bands of 10–20 kDa generally gave a mixture of sequences, except a band of sequence MKEEKY. This sequence corresponds to residues 285–290 of OVA, indicating that Arg284 of OVA was cleaved. Comparison of all of the possible combinations of the obtained sequences of the other bands with the original sequence of OVA facilitated identification of the sequences of a band as NVLQP and ISQAV. These sequences correspond to residues 159–163 and 323–327 of OVA, indicating that Arg158 and Lys322 were cleaved. Blotting of bands in the region of 30–43 kDa gave no signal by N-terminal analysis, suggesting that the N termini of the peptides were acetylated. Hence, these bands arise from cleavage at the C-terminal region of OVA. In this experiment, trypsin cleavage at the N-terminal region of heat-induced OVA fibrils was not detected. By contrast, Arg58, Arg104, and Arg110 at the N-terminal region of OVA are extensively cleaved by trypsin in the urea-denatured state (40). The absence of trypsin cleavage at the N-terminal helical region indicates that this portion of OVA is protected in the heat-induced fibril, which supports our conclusion that this part of the molecule forms the core region during heat-induced fibril formation.
DISCUSSION
In this study, we have revealed the detailed molecular mechanism of heat-induced fibril formation of a non-inhibitory serpin OVA. The fibril formation of proteins with extensive β-sheet structures, such as serpins, raises the question as to whether native β-sheet proteins can form amyloid-type fibrils and, if so, whether their structure is retained during fibril formation (56). Our results did not provide support for the partial loop insertion mechanism proposed for OVA, in which native conformation may be retained (15, 33, 37). Mutations in s5A and the reactive center loop did not affect heat-induced aggregation, and co-incubation of OVA with peptide fragments from the reactive center loop did not change the morphology of the aggregates. In addition, deconvolution of the FTIR amide I band of OVA aggregates showed different positions of the β-sheet components from those of native OVA, suggesting that the β-sheet structure of the OVA aggregates is different from that of the native conformation. Taken together, the results obtained in our study suggest that the heat-induced fibril formation of OVA would be best explained by the amyloid-type mechanism.
The morphology of the heat-induced aggregate of OVA was significantly affected by reducing the single disulfide bond between Cys73 and Cys120. Heat treatment of SH-OVA resulted in formation of long straight fibrils, which are distinct from the semiflexible fibrils formed by intact OVA. A similar effect of single disulfide reduction on the morphology of amyloid fibril formation has been reported previously for β2m (57, 58). The disulfide-intact β2m formed twisted long straight fibrils at lower pH and in the absence of NaCl. Reduction of the single disulfide bond of β2m led to formation of thinner and more flexible filaments. The distinct morphology of β2m amyloid fibril formation can be explained by switching between competitive pathways; the reversible non-nucleated assembly was observed for formation of semiflexible fibrils, whereas the formation of long straight fibrils is approximated by a nucleation-dependent reaction where the slow formation of the nucleus is the rate-limiting step, which is followed by a rapid irreversible fibril extension step. Nonetheless, the kinetics of heat-induced aggregation of both intact OVA and SH-OVA are non-nucleation-dependent, and the change in the morphology of OVA cannot be explained by switching the pathways.
Site-directed mutagenesis of various residues in the proposed amyloidogenic core regions of OVA showed that only the V41A mutation in hB has an inhibitory effect on fibril formation. In addition, a soluble peptide fragment of hB also changed the morphology of the heat-induced aggregate of OVA upon co-incubation. Hence, the hB region acts as the most important core region for the heat-induced fibril formation of OVA. We propose that the α-to-β conformational transition of the N-terminal region triggers formation of fibrillar aggregates because we found that hB exhibits high β-aggregation propensity. Protein fibrils may then be formed through the β-strands as they form sequential intermolecular linkages. Similar results have been reported for several amyloid-forming proteins that harbor an α-helix in a polypeptide segment that should form a β-strand according to secondary structure predictions (59). For example, the transformation of an α-helical segment of a monomeric prion to a β-strand conformation is the key event in the formation of aggregates (60), and an α-to-β transition is also a key step in the amyloid β-protein fibrillogenesis (61). The change in fibril morphology of OVA may be explained by the fluctuating conformation of the N-terminal helical region in SH-OVA (40), and as the single disulfide bond between Cys73 and Cys120 is reduced, the conformational α-to-β transition in this region accelerates. When intact OVA with the disulfide bond between Cys73 and Cys120 was heat-treated, the α-to-β transition decelerates and interferes with the formation of long straight fibrils. The 32IAIMSA37 peptide of hB induces the formation of long straight fibrils of OVA without significantly changing the aggregation kinetics, which is similar to the effect of reducing the disulfide bond between Cys73 and Cys120. The effect of 32IAIMSA37 on OVA aggregation is well explained by the interaction of 32IAIMSA37 and hB of OVA to promote the α-to-β transition in this region.
Our experimental data indicated that the native conformation of OVA is altered in the heat-induced fibrils of OVA, which is in contrast to the serpin polymers, where native conformation may be retained. Therefore, the mechanism of fibril formation in OVA seems to be unique in the serpin superfamily. Because hB was found to play an important role in fibril formation, the sequence and structure of hB were compared with those of the other serpins to see how OVA might have a unique aggregation mechanism. Because the three-dimensional crystal structures of several serpins have already been known, conserved residues can be identified and compared for this superfamily within the same structural element. The crystal structures of these serpins (3fgq for human neoroserpin (62), 2znh for antithrombin III (12), 1qmb for α1-antitrypsin (63), and 2ach for α1-antichymotrypsin (64)) indicate that the conserved sequences are involved in the α-helix. We have performed TANGO analysis of these serpins, and the resulting β-aggregation propensity of the hB region at 25 °C was ∼10% for human neoroserpin, ∼10% for antithrombin III, ∼60% for α1-antitrypsin, and ∼60% for antichymotrypsin, values that are much lower than that of OVA (>95%). This result suggests that the high β-aggregation propensity of the hB region of OVA may account for the presence of an α-to-β transition in this region to promote the amyloid fibril formation, which is unique for OVA as a member of the serpin superfamily. Fig. 12a shows the alignment of the sequence of hB in OVA and the sequences of the corresponding regions in other serpins. The hB sequence of OVA contains more than 80% hydrophobic amino acids, which is higher than other serpins and may account for its high β-aggregation propensity. The hB sequence of OVA is conserved in other members of the serpin superfamily, but the amino acids corresponding to Tyr42 of OVA are not conserved. The significantly lower β-aggregation propensities of human neoroserpin and antithrombin III may be explained by the charged residues present in the region flanking the hydrophobic sequence. The presence of such an aggregation suppressor in the protein amino acid sequence has been proposed because the region flanking the aggregation hydrophobic sequence has been observed to be enriched in proline or charged residues, such as lysine, arginine, aspartic acid, and glutamic acid (44, 65). The charged residues of the hB region of human neoroserpin and antithrombin III may play a role in preventing amyloid-type aggregation in vivo. To study the role of Tyr42 in fibril formation of OVA, we performed TANGO analysis of relevant OVA mutants to examine the effect on fibril formation (Fig. 12b). The β-aggregation propensity of hB was significantly reduced by introduction of the V41A substitution, which is consistent with our experimental results. TANGO also predicted that the Y42A mutation does not significantly affect the high β-aggregation propensity of OVA. To examine this prediction, we prepared the Y42A mutant of OVA, and the morphology of the corresponding heat-induced aggregate was then monitored by TEM. We found that Y42A formed long straight amyloid fibrils, which were the same as that of WT (data not shown). Therefore, the role of Tyr42 in terms of β-aggregation is not significant compared with that of Val41. Tyr42 may have a smaller influence on fibril formation of OVA because its position is close to a region flanking the hydrophobic sequence.
FIGURE 12.
Analysis of the amino acid sequence of the hB region of serpins. a, alignment of the sequence of hB in OVA and the sequences of the corresponding regions in other serpins. The residues are colored according to type: nonpolar (yellow), charged (red), aromatic (blue), and polar uncharged (green). b, TANGO prediction for the effect of single amino acid substitution on the β-aggregation of the hB region of OVA.
In conclusion, we found that the heat-induced fibril formation of OVA is drastically changed upon reduction of the single disulfide bond between Cys73 and Cys120. Mutational analysis showed that the V41A substitution in the hB structural motif interfered with fibril formation, suggesting that the N-terminal region of OVA, where the single disulfide bond is located, plays an important role in the formation of fibrils. The peptide fragment from hB consistently promoted formation of long straight fibrils of disulfide intact OVA, and the N-terminal region of the heat-induced OVA fibril was protected from proteolytic cleavage. The results obtained in this study are best explained by a mechanism in which the N-terminal region, including hB, acts as the core for fibril formation. According to this mechanism, which is distinct from the serpin polymerization mechanism, the conformation of hB of OVA would be transformed to β-strands, thereby generating sequential intermolecular linkages to form amyloid-type fibrils. Further investigation into the detailed structure of fibril formed by OVA will serve as a promising approach for elucidating the general aggregation mechanism of serpins.
Acknowledgments
Yuko Imanishi, Yuki Kawachi, and Kimi Terasawa (Kyoto Institute of Technology) are acknowledged for technical assistance.
This work was supported by Japanese Ministry of Education, Culture, Sports, Science, and Technology Grants-in-aid for Scientific Research 20550111 and 21550154.
- OVA
- ovalbumin
- TEM
- transmission electron microscope
- HFIP
- hexafluoroisopropyl alcohol
- hB
- helix B
- s3A
- s4B, s4C, and s5B, strand 3A, 4B, 4C, and 5B, respectively
- β2m
- β2-microgloblin
- DLS
- dynamic light scattering.
REFERENCES
- 1. Dobson C. M. (2001) Philos. Trans. R. Soc. Lond. B. Biol. Sci. 356, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selkoe D. J. (2003) Nature 426, 900–904 [DOI] [PubMed] [Google Scholar]
- 3. Stein P. E., Carrell R. W. (1995) Nat. Struct. Biol. 2, 96–113 [DOI] [PubMed] [Google Scholar]
- 4. Silverman G. A., Bird P. I., Carrell R. W., Church F. C., Coughlin P. B., Gettins P. G., Irving J. A., Lomas D. A., Luke C. J., Moyer R. W., Pemberton P. A., Remold-O'Donnell E., Salvesen G. S., Travis J., Whisstock J. C. (2001) J. Biol. Chem. 276, 33293–33296 [DOI] [PubMed] [Google Scholar]
- 5. Sharp H. L., Bridges R. A., Krivit W., Freier E. F. (1969) J. Lab. Clin. Med. 73, 934–939 [PubMed] [Google Scholar]
- 6. Davis R. L., Shrimpton A. E., Holohan P. D., Bradshaw C., Feiglin D., Collins G. H., Sonderegger P., Kinter J., Becker L. M., Lacbawan F., Krasnewich D., Muenke M., Lawrence D. A., Yerby M. S., Shaw C. M., Gooptu B., Elliott P. R., Finch J. T., Carrell R. W., Lomas D. A. (1999) Nature 401, 376–379 [DOI] [PubMed] [Google Scholar]
- 7. Takehara S., Zhang J., Yang X., Takahashi N., Mikami B., Onda M. (2010) J. Mol. Biol. 403, 751–762 [DOI] [PubMed] [Google Scholar]
- 8. Yamasaki M., Sendall T. J., Harris L. E., Lewis G. M., Huntington J. A. (2010) J. Biol. Chem. 285, 30752–30758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ekeowa U. I., Freeke J., Miranda E., Gooptu B., Bush M. F., Pérez J., Teckman J., Robinson C. V., Lomas D. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 17146–17151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knaupp A. S., Levina V., Robertson A. L., Pearce M. C., Bottomley S. P. (2010) J. Mol. Biol. 396, 375–383 [DOI] [PubMed] [Google Scholar]
- 11. Lomas D. A., Evans D. L., Finch J. T., Carrell R. W. (1992) Nature 357, 605–607 [DOI] [PubMed] [Google Scholar]
- 12. Yamasaki M., Li W., Johnson D. J., Huntington J. A. (2008) Nature 455, 1255–1258 [DOI] [PubMed] [Google Scholar]
- 13. Huntington J. A., Sendall T. J., Yamasaki M. (2009) Prion 3, 12–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunt L. T., Dayhoff M. O. (1980) Biochem. Biophys. Res. Commun. 95, 864–871 [DOI] [PubMed] [Google Scholar]
- 15. Huntington J. A., Stein P. E. (2001) J. Chromatogr. B. Biomed. Sci. Appl. 756, 189–198 [DOI] [PubMed] [Google Scholar]
- 16. Stein P. E., Leslie A. G., Finch J. T., Turnell W. G., McLaughlin P. J., Carrell R. W. (1990) Nature 347, 99–102 [DOI] [PubMed] [Google Scholar]
- 17. Stein P. E., Leslie A. G., Finch J. T., Carrell R. W. (1991) J. Mol. Biol. 221, 941–959 [DOI] [PubMed] [Google Scholar]
- 18. Takahashi N., Hirose M. (1992) J. Biol. Chem. 267, 11565–11572 [PubMed] [Google Scholar]
- 19. Tatsumi E., Takahashi N., Hirose M. (1994) J. Biol. Chem. 269, 28062–28067 [PubMed] [Google Scholar]
- 20. Onda M., Tatsumi E., Takahashi N., Hirose M. (1997) J. Biol. Chem. 272, 3973–3979 [DOI] [PubMed] [Google Scholar]
- 21. Onda M., Hirose M. (2003) J. Biol. Chem. 278, 23600–23609 [DOI] [PubMed] [Google Scholar]
- 22. Onda M., Nakatani K., Takehara S., Nishiyama M., Takahashi N., Hirose M. (2008) J. Biol. Chem. 283, 17568–17578 [DOI] [PubMed] [Google Scholar]
- 23. Tani F., Shirai N., Onishi T., Venelle F., Yasumoto K., Doi E. (1997) Protein Sci. 6, 1491–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nemoto N., Koike A., Osaki K., Koseki T., Doi E. (1993) Biopolymers 33, 551–559 [DOI] [PubMed] [Google Scholar]
- 25. Azakami H., Mukai A., Kato A. (2005) J. Agric. Food Chem 53, 1254–1257 [DOI] [PubMed] [Google Scholar]
- 26. Broersen K., Weijers M., de Groot J., Hamer R. J., de Jongh H. H. (2007) Biomacromolecules 8, 1648–1656 [DOI] [PubMed] [Google Scholar]
- 27. Pearce F. G., Mackintosh S. H., Gerrard J. A. (2007) J. Agric. Food Chem 55, 318–322 [DOI] [PubMed] [Google Scholar]
- 28. Weijers M., Broersen K., Barneveld P. A., Cohen Stuart M. A., Hamer R. J., De Jongh H. H., Visschers R. W. (2008) Biomacromolecules 9, 3165–3172 [DOI] [PubMed] [Google Scholar]
- 29. Nelson R., Sawaya M. R., Balbirnie M., Madsen A. Ø., Riekel C., Grothe R., Eisenberg D. (2005) Nature 435, 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi N., Hasegawa K., Yamaguchi I., Okada H., Ueda T., Gejyo F., Naiki H. (2002) Biochim. Biophys. Acta 1601, 110–120 [DOI] [PubMed] [Google Scholar]
- 31. Padrick S. B., Miranker A. D. (2002) Biochemistry 41, 4694–4703 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi N., Onda M., Hayashi K., Yamasaki M., Mita T., Hirose M. (2005) Biosci. Biotechnol. Biochem. 69, 922–931 [DOI] [PubMed] [Google Scholar]
- 33. Huntington J. A., Patston P. A., Gettins P. G. (1995) Protein Sci. 4, 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamasaki M., Takahashi N., Hirose M. (2003) J. Biol. Chem. 278, 35524–35530 [DOI] [PubMed] [Google Scholar]
- 35. Arii Y., Hirose M. (2002) Biochem. J. 363, 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamasaki M., Arii Y., Mikami B., Hirose M. (2002) J. Mol. Biol. 315, 113–120 [DOI] [PubMed] [Google Scholar]
- 37. Shirai N., Tani F., Higasa T., Yasumoto K. (1997) J. Biochem. 121, 787–797 [DOI] [PubMed] [Google Scholar]
- 38. Chiti F., Taddei N., Baroni F., Capanni C., Stefani M., Ramponi G., Dobson C. M. (2002) Nat. Struct. Biol. 9, 137–143 [DOI] [PubMed] [Google Scholar]
- 39. Hoshino M., Katou H., Hagihara Y., Hasegawa K., Naiki H., Goto Y. (2002) Nat. Struct. Biol. 9, 332–336 [DOI] [PubMed] [Google Scholar]
- 40. Takahashi N., Koseki T., Doi E., Hirose M. (1991) J Biochem. 109, 846–851 [DOI] [PubMed] [Google Scholar]
- 41. Takahashi N., Orita T., Hirose M. (1995) Gene 161, 211–216 [DOI] [PubMed] [Google Scholar]
- 42. Weijers M., Barneveld P. A., Cohen Stuart M. A., Visschers R. W. (2003) Protein Sci. 12, 2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tani F., Shirai N., Nakanishi Y., Yasumoto K., Kitabatake N. (2004) Biosci. Biotechnol. Biochem. 68, 2466–2476 [DOI] [PubMed] [Google Scholar]
- 44. Fernandez-Escamilla A. M., Rousseau F., Schymkowitz J., Serrano L. (2004) Nat. Biotechnol. 22, 1302–11306 [DOI] [PubMed] [Google Scholar]
- 45. Trovato A., Seno F., Tosatto S. C. (2007) Protein Eng. Des. Sel. 20, 521–523 [DOI] [PubMed] [Google Scholar]
- 46. Conchillo-Solé O., de Groot N. S., Avilés F. X., Vendrell J., Daura X., Ventura S. (2007) BMC Bioinformatics 8, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pawar A. P., Dubay K. F., Zurdo J., Chiti F., Vendruscolo M., Dobson C. M. (2005) J. Mol. Biol. 350, 379–392 [DOI] [PubMed] [Google Scholar]
- 48. Tjernberg L. O., Callaway D. J., Tjernberg A., Hahne S., Lilliehöök C., Terenius L., Thyberg J., Nordstedt C. (1999) J. Biol. Chem. 274, 12619–12625 [DOI] [PubMed] [Google Scholar]
- 49. Reches M., Gazit E. (2003) Science 300, 625–627 [DOI] [PubMed] [Google Scholar]
- 50. Tanaka N., Tanaka R., Tokuhara M., Kunugi S., Lee Y. F., Hamada D. (2008) Biochemistry 47, 2961–2967 [DOI] [PubMed] [Google Scholar]
- 51. Krysmann M. J., Castelletto V., Kelarakis A., Hamley I. W., Hule R. A., Pochan D. J. (2008) Biochemistry 47, 4597–4605 [DOI] [PubMed] [Google Scholar]
- 52. Tjernberg L. O., Näslund J., Lindqvist F., Johansson J., Karlström A. R., Thyberg J., Terenius L., Nordstedt C. (1996) J. Biol. Chem. 271, 8545–8548 [DOI] [PubMed] [Google Scholar]
- 53. Kuroda Y., Maeda Y., Hanaoka H., Miyamoto K., Nakagawa T. (2004) J. Pept. Sci. 10, 8–17 [DOI] [PubMed] [Google Scholar]
- 54. Zhou A., Stein P. E., Huntington J. A., Sivasothy P., Lomas D. A., Carrell R. W. (2004) J. Mol. Biol. 342, 931–941 [DOI] [PubMed] [Google Scholar]
- 55. Frare E., Mossuto M. F., Polverino de Laureto P., Dumoulin M., Dobson C. M., Fontana A. (2006) J. Mol. Biol. 361, 551–561 [DOI] [PubMed] [Google Scholar]
- 56. Zandomeneghi G., Krebs M. R., McCammon M. G., Fändrich M. (2004) Protein Sci. 13, 3314–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith D. P., Radford S. E. (2001) Protein Sci. 10, 1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gozu M., Lee Y. H., Ohhashi Y., Hoshino M., Naiki H., Goto Y. (2003) J. Biochem. 133, 731–736 [DOI] [PubMed] [Google Scholar]
- 59. Kallberg Y., Gustafsson M., Persson B., Thyberg J., Johansson J. (2001) J. Biol. Chem. 276, 12945–12950 [DOI] [PubMed] [Google Scholar]
- 60. Harrison P. M., Bamborough P., Daggett V., Prusiner S. B., Cohen F. E. (1997) Curr. Opin. Struct. Biol. 7, 53–59 [DOI] [PubMed] [Google Scholar]
- 61. Kirkitadze M. D., Condron M. M., Teplow D. B. (2001) J. Mol. Biol. 312, 1103–1119 [DOI] [PubMed] [Google Scholar]
- 62. Takehara S., Onda M., Zhang J., Nishiyama M., Yang X., Mikami B., Lomas D. A. (2009) J. Mol. Biol. 388, 11–20 [DOI] [PubMed] [Google Scholar]
- 63. Huntington J. A., Pannu N. S., Hazes B., Read R. J., Lomas D. A., Carrell R. W. (1999) J. Mol. Biol. 293, 449–455 [DOI] [PubMed] [Google Scholar]
- 64. Baumann U., Huber R., Bode W., Grosse D., Lesjak M., Laurell C. B. (1991) J. Mol. Biol. 218, 595–606 [DOI] [PubMed] [Google Scholar]
- 65. Rousseau F., Serrano L., Schymkowitz J. W. (2006) J. Mol. Biol. 355, 1037–1047 [DOI] [PubMed] [Google Scholar]